Abstract
Background
Body mass index (BMI), waist circumference (WC) and neck circumference (NC) are important screening tools for sleep disordered breathing (SDB). However, the utility of anthropometry for this purpose has not been evaluated among HIV-infected patients.
Methods
HIV-uninfected men (HIV−; n=60), HIV-infected men receiving highly active antiretroviral therapy (HIV+/HAART; n=58), and HIV-infected men not receiving HAART (HIV+/No HAART; n=41) from the Multicenter AIDS Cohort Study underwent a nocturnal sleep study and anthropomorphic assessment. Moderate-severe SDB was defined as an apnea/hypopnea event rate ≥15 episodes/hour. Receiver operating characteristic (ROC) curves were used to compare the ability of different anthropometric measurements to predict SDB within each group.
Results
Moderate-severe SDB was found in 48% (HIV−:57%; HIV+/HAART: 41%; HIV+/No HAART−: 44%). The performance of BMI, WC, and NC to predict SDB was excellent among the HIV− men (ROC areas-under-the curve (AUC): 0.83, 0.88, 0.88, respectively) and fair among the HIV+/HAART group (AUCs: 0.71, 0.77, 0.77, respectively). In contrast, these measurements had no predictive value in the HIV+/No HAART group (AUCs: 0.43, 0.41, 0.45, respectively). Moreover, in the HIV+/No HAART group, moderate-severe SDB was independently associated with serum C-reactive protein ≥3.0 mg/L (Odds Ratio (OR) 6.9; p=0.04) and HIV RNA > 10,000 copies/ml (OR 7.1; p=0.05).
Conclusions
BMI, waist circumference, and neck circumference had better predictive value for moderate-severe SDB in HIV-uninfected men compared to HIV-infected men, and had no value among HIV-infected men not receiving HAART. Among this latter group, systemic inflammation may contribute to the pathogenesis of SDB.
Keywords: obstructive sleep apnea, HIV, lipodystrophy, anthropometry, body composition, sleep disordered breathing
Introduction
Sleep disordered breathing (SDB), also known as obstructive sleep apnea, has a prevalence of 4% in men and 2% in women in the general population [1], and 40% in moderately overweight men [2]. It is associated with increased morbidity and mortality due to cardiovascular disease, stroke, and diabetes, as well as significant impairment in quality of life[3]. Obesity is the most important clinical risk factor for SDB, and measures of body fat composition, including body mass index (BMI), waist circumference, neck circumference, and visceral fat have all been used as predictors of clinically significant SDB [4–7].
Body composition abnormalities, including subcutaneous fat wasting (lipoatrophy) and central fat accumulation (lipohypertrophy), are common among HIV-infected patients and are attributable, at least in part, to the effects of highly active antiretroviral therapy (HAART). Exposure to thymidine analogue nucleoside reverse transcriptase inhibitors, such as stavudine and zidovudine, is closely associated with the development of lipoatrophy, whereas the pathogenesis of lipohypertrophy is not clear and is likely multifactorial [8]. Visceral fat is increased among non-obese HIV-infected patients compared to HIV-uninfected subjects of similar BMI [9], and in certain HIV-infected patients with a normal BMI, fat may accumulate in the cervical and dorsocervical areas [10]. Because of these body composition changes, the relationships between various measurements of adiposity and SDB may be different in HIV-infected patients compared to HIV− uninfected populations.
The goals of this study, therefore, were to examine the relationship between fat distribution and SDB in HIV-uninfected and HIV-infected men, and to test the predictive value of specific measures of anthropometry using a well-characterized cohort of men with or at risk for HIV infection. Because of the associations between SDB and cardiovascular disease, diabetes mellitus, and hypertension as well as reduced quality of life, it is important for HIV clinicians to know whether tools that are used to screen for this prevalent condition in the general population are adequate in HIV-infected patients. In this way, patients can be appropriately referred for overnight polysomnography and specific treatment can be instituted, if necessary.
Methods
Study Population
Participants were recruited from the Baltimore/Washington and Pittsburgh sites of the Multicenter AIDS Cohort Study (MACS) [11] into three groups: HIV-uninfected men (HIV−), HIV-infected men receiving HAART (HIV+/HAART), and HIV-infected men not receiving HAART (HIV+/No HAART). HIV-serostatus was assessed by enzyme-linked immunosorbent assay at each semi-annual MACS visit, with positive results confirmed by Western blot. HAART was defined according to the DHHS/Kaiser Guidelines[12] as: (a) two or more nucleoside reverse transcriptase inhibitors (NRTIs) in combination with at least one protease inhibitor (PI) or one non-nucleoside reverse transcriptase inhibitor (NNRTI); (b) one NRTI in combination with at least one PI and at least one NNRTI; (c) a regimen containing ritonavir and saquinavir in combination with one NRTI and no NNRTIs; and (d) an abacavir or tenofovir containing regimen of three or more NRTIs in the absence of both PIs and NNRTIs. Combinations of zidovudine (AZT) and stavudine (d4T) with either a PI or an NNRTI were not considered HAART. Men who had not received HAART in the previous 12 months were allowed into the HIV+/No HAART group.
Sampling methods
At the time of recruitment for the present study (2005–2008), 327 HIV− and 297 HIV+ men [243 receiving HAART and 54 not receiving HAART] were under observation at the Baltimore/Washington site. All HIV+/No HAART and random samples of HIV+/HAART and HIV− men of similar age and race were invited through a mailed letter to participate. Because of the scarcity of HIV+/No HAART men at the Baltimore/Washington site, additional such men (n=9) were recruited from the Pittsburgh site through 2008 and were studied in Baltimore. Interested candidates were screened for eligibility, with the following exclusion criteria: a) history of schizophrenia, b) acute infectious central nervous system disease, c) unstable cardiovascular disease in the past 3 months, d) uncontrolled hypertension, e) renal failure on dialysis, f) history of cirrhosis, g) use of supplemental oxygen, h) a history of upper airway surgery, including uvulopalatopharygoplasty, laser assisted uvuloplasty, and maxillomandibular advancement. The institutional review boards at each site approved study protocols and forms, and each participant provided written informed consent.
Study Protocol
Participants were admitted to the Johns Hopkins General Clinical Research Center between 6–8 pm and a polysomnography study to assess sleep and breathing was conducted between 11 pm and 7 am. On the following morning, participants underwent neck circumference measurement and dual-energy x-ray absorptiometry (DXA). Data and specimens from the MACS visit closest to the sleep study visit were used (median interval between MACS visit and sleep study visit: 4 days [Interquartile Range (IQR); 0, 39 days]); these data included demographic and HIV treatment information, HIV serostatus, plasma HIV RNA concentration (Amplicor HIV Monitor Assay, Roche Diagnostics, Nutley, NJ), T-cell subsets determined by flow cytometry, and clinician-assessed abnormalities in body composition, waist and hip circumference measurements as previously described [13]. A subset of the participants had serum levels of C-reactive protein (CRP) measured at a previous MACS visit (median duration between time of CRP measurement and the sleep study visit: 137 days [IQR: 14, 207 days]). CRP was assessed by the high sensitivity nephelometric method at Quest Diagnostics (Baltimore, MD), by which values > 3.0 mg/L are considered elevated.
Study Procedures
Polysomnography Testing
Continuous polygraphic recordings (EMBLA; Broomfield, CO) of a modified electrocardiographic (V6) lead, right and left electro-oculographic leads, submental and bilateral anterior tibialis surface electromyograms, and two electroencephalographic leads (C3-A2, O1-A2) were performed. Respiration was monitored by a nasal pressure transducer and oronasal thermocouples (Dymedix; Shoreview, MN) and breathing effort was recorded using thoracic and abdominal inductive plethysmography (EMBLA; Broomfield, CO). Continuous recording of the oxyhemoglobin saturation (SaO2) was obtained with an oximeter (XPOD; Nonin, Plymouth, MN).
Apnea was defined as complete cessation of airflow for at least 10 seconds. Hypopneic events were defined as a discernable reduction in airflow lasting for at least 10 seconds, which was accompanied by an electroencephalographic arousal or a drop in SaO2 of 4% or more. The respiratory disturbance index (RDI) was defined as the total number of apneic or hypopneic events per hour of total sleep time. Significant sleep disordered breathing was defined as an RDI ≥ 15 events/hour, which is considered moderate-severe SDB and represents a clinically important threshold at which treatment may be considered[14, 15].
Body Composition Measurements
Height was measured with a wall-mounted stadiometer and recorded to the nearest 0.5 cm. Body weight was measured to the nearest 0.1 kg with the participant wearing minimal clothing or an examination gown. Waist and hip circumferences were measured with the participant in a standing position using the protocol established in the 3rd National Health and Nutrition Examination Survey [16], as previously described [17, 18]. Neck circumference was measured inferior to the laryngeal prominence and perpendicular to the long axis of the neck, with the participant seated and the head in the Frankfurt horizontal plane[19].
Whole body DXA to assess whole-body and regional fat composition (trunk fat, extremity fat) was performed using a Hologic 4500A machine with QDA4500A software version 9.03 (Hologic Inc, Waltham, MA). Regional fat distribution was assessed using the ratio of trunk fat to extremity fat [20, 21].
Lipoatrophy of the face, buttocks, legs, and face and lipohypertrophy of the abdomen and dorsocervical region has been assessed by trained clinicians at each semi-annual MACS visit since April 1999, as previously described [13, 22]. “Mild” was defined as “only noted after close inspection”. “Moderate” was defined as fat changes “noticed by the clinician without specifically looking for them”. “Severe” was defined as fat changes “easily noted by a casual observer.” Data from the MACS visit closest to the sleep study visit were used for this analysis.
Statistical Analysis
ANOVA and chi-square testing were used to compare continuous and categorical variables, respectively, among the 3 groups. Pairwise comparisons of the groups using the Bonferroni test were performed if the overall ANOVA p-value was < 0.05. Between-group differences in RDI were assessed with Wilcoxon non-parametric testing because of the skewed distribution of this variable. Spearman’s correlation testing was performed between each body composition variable and RDI within each group to determine univariate associations. Receiver operating characteristic (ROC) curves [23] were used to determine the ability of each anthropometric variable to discriminate moderate-severe SDB from absent or mild SDB. The area-under the curve (AUC) was calculated for each ROC curve and compared with the AUCs of other body composition measurements within each group. In an exploratory analysis, multivariable logistic regression was used to identify factors associated with SDB in the HIV+/No HAART group. All analyses were conducted with STATA 8.2 (College Station, TX). Seven subjects were missing data on waist circumference, hip circumference, or neck circumference. These values were imputed using group-specific linear regression equations and the non-missing anthropometric variables for that individual. Similar results were obtained when these subjects were included or excluded from analysis (data not shown).
Results
Subject Characteristics, sleep findings, and body composition
One-hundred fifty-nine men participated in the study (Table 1). The HIV-uninfected men tended to be older than the HIV-infected men, whereas the racial distribution was similar among the groups. The HIV-infected groups had similar absolute CD4 T-cell counts, but, as expected, a larger proportion of the HIV+/HAART group had a viral load < 400 copies/mL compared to the No HAART group.
Table 1.
Subject Characteristics
| HIV− | HIV+ | |||
|---|---|---|---|---|
| HAART | No HAART | p | ||
| N | 60 | 58 | 41 | |
| Age (yrs)* | 54 (9) | 51 (8) | 49 (8)b | 0.02 |
| White, n (%) | 25 (42%) | 28 (58%) | 16 (39%) | 0.62 |
| HIV RNA < 400 copies/mL, (%) | 52 (91%) | 7 (17%) | <0.0001 | |
| CD4 (cells/mm3) | - | 583 (306) | 518 (287) | 0.27 |
| Hepatitis B Positive | 1 (2%) | 2 (3%) | 2 (5%) | 0.65 |
| Hepatitis C Positive | 13 (22%) | 12 (21%) | 15 (38%) | 0.12 |
| HIV Treatment Regimen | ||||
| PI-based, n (%) | - | 30 (53%) | 3 (7%) | |
| NNRTI-based, n (%) | - | 23 (40%) | 0 (0%) | |
| 3 NRTIs, n (%) | - | 3 (5%) | 0 (0%) | |
| Years from HAART initiation | - | 7.5 (2.8) | - | |
| HAART− naïve, n (%) | 21 (51%) | |||
| Polysomnography Variables | ||||
| RDI median (IQR) | 18 (6,29) | 11 (4,21) | 11 (4,24) | 0.09* |
| RDI ≥ 15 events/hour, n (%) | 34 (57%) | 24 (41%) | 18 (44%) | 0.19 |
| Anthropometric Measurements | ||||
| BMI (kg/m2) | 28.6 (7.2) | 25.5 (4.5)a | 25.4 (4.1)b | 0.003 |
| Waist circumference (cm) | 98.6 (16.9) | 93.8 (11.5) | 91.8 (12.8) b | 0.04 |
| Neck circumference (cm) | 40.8 (3.5) | 39.9 (3.2) | 39.0 (2.3) b | 0.02 |
| Waist:Hip Ratio | 0.96 (0.08) | 0.99 (0.06) | 0.95 (0.06)c | 0.02 |
| DXA Measurements | n=55 | n=56 | n=40 | |
| Total Fat (kg) | 22.8 (11.9) | 18.4 (9.2) a | 18.5 (10.1) | 0.05 |
| Trunk Fat (kg) | 13.3 (7.3) | 11.8 (5.7) | 10.8 (5.8) | 0.16 |
| Extremity Fat (kg) | 8.9 (4.6) | 6.0 (3.9)a | 7.1 (4.5) | 0.002 |
| Trunk: Extremity Fat Ratio | 1.5 (0.35) | 2.3 (0.95)a | 1.7 (0.67)c | < 0.001 |
| Biomarkers | n=24 | n=55 | n=36 | |
| CRP ≥ 3.0 mg/L, n (%) | 1 (4%) | 23 (42%) a | 13 (36%) b | 0.004 |
: HIV− vs HIV+/HAART+, p<0.05;
HIV− vs HIV+/HAART−, p<0.05,
HIV+/HAART+ vs HIV+/No HAART−, p<0.05, by Bonferroni test for continuous variables if p-value < 0.05 for overall ANOVA
by Kruskall-Wallis testing
Values represent means (standard deviation) unless otherwise noted
Respiratory Disturbance Index (RDI) is was defined as the total number of apneic or hypopneic events per hour of total sleep time
The median RDI was higher in the HIV-uninfected group compared to the two HIV-infected groups (p=0.09), as was the proportion of participants with moderate-severe SDB (RDI ≥ 15 events/hour). BMI was greater in HIV-uninfected men compared to the HIV-infected groups, as were waist and neck circumferences (Table 1). The waist:hip ratio was highest among the HIV+/HAART group, with the other two groups being similar.
DXA scans were performed in 152 (96%) of the participants. As was the case for BMI, average total fat was significantly higher in the HIV-uninfected group than in the two HIV-infected groups. A similar pattern was observed for trunk fat, but the differences among the groups were not statistically different. On average, the HIV+/HAART group had less extremity fat than the HIV-uninfected group, but a similar amount to the HIV+/No HAART group. Thus, the mean trunk to extremity fat ratio was higher in the HIV+/HAART men than in the other groups.
By examiner assessment, moderate-severe lipoatrophy was present in 29% (n=17) of the HIV+/HAART group, 12% (n=5) of the HIV+/No HAART group, and 5% (n=3) of the HIV-uninfected group (p=0.001). The proportion of men with moderate-severe lipohypertrophy was similar among the three groups in both the abdomen (HIV+/HAART: 34%; HIV+/No HAART: 28%; HIV-uninfected: 39%; p=0.50) and the dorsocervical area (7%, 3%, 5%, respectively; p=0.60).
Associations between Body Composition Measurements and Respiratory Disturbance Index
Among the HIV-uninfected men, waist circumference, neck circumference, and BMI showed excellent correlation with RDI (Spearman’s rho: 0.60–0.67) (Table 2). DXA variables showed similar correlations, with trunk fat having the highest coefficient (0.69). Among the HIV+/HAART group, correlations were of lesser magnitude and anthropometry was slightly more correlated than DXA (Table 2). Notably, DXA-derived extremity fat was positively associated with RDI in the HIV+/HAART group, providing evidence against an association between lipoatrophy and SDB. Among the HIV+/No HAART group, none of the body composition variables as assessed either by examiner or by DXA was significantly associated with RDI.
Table 2.
Univariate associations between Body Composition Measurements and Respiratory Disturbance Index (Spearman’s correlations (rho))
| HIV− | HIV+ | ||
|---|---|---|---|
| HAART | No HAART | ||
|
Anthropometric Measurements | |||
| BMI (kg/m2) | 0.60 c | 0.49 b | −0.08 |
| Waist (cm) | 0.67 c | 0.56 c | −0.09 |
| Neck (cm) | 0.62 c | 0.52 c | −0.17 |
| Waist:Hip Ratio | 0.55 c | 0.54 c | −0.01 |
| DXA Measurements | |||
| Total Fat (kg) | 0.67 c | 0.47 b | −0.09 |
| Trunk Fat (kg) | 0.69 c | 0.47 b | −0.06 |
| Extremity Fat (kg) | 0.64 c | 0.41 a | −0.14 |
| Trunk:Extremity Fat Ratio | 0.33 a | −0.03 | 0.05 |
p<0.05;
p<0.001;
p<0.0001
Prediction of Sleep Disordered Breathing using Anthropometry and Dual-Energy X-ray Absorptiometry
ROC analysis was used to determine the performance of body composition measurements in discriminating moderate-severe SDB from absent or mild SDB. Of the DXA-derived measurements, we selected trunk fat for analysis because of its strong association with RDI in the correlative analysis.
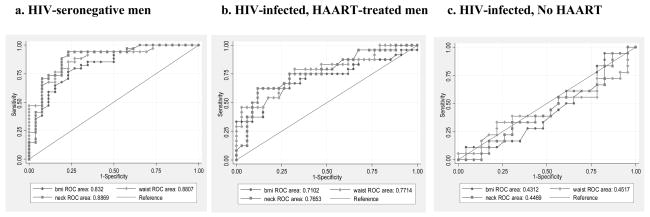
Among the HIV-uninfected men, body composition measurements predicted moderate-severe SDB very strongly, with AUCs between 0.80–0.89 (Table 3). Waist circumference, neck circumference, and DXA-derived trunk fat showed the best performance (AUC 0.88–0.89). Among the HIV+/HAART group, AUCs were lower (0.70–0.77), with waist circumference and neck circumference having the highest AUCs. In contrast, the performance of all body composition measurements in the HIV+/No HAART group was no better than chance (AUCs 0.41–0.54) (Figure 1). Similar patterns in the AUCs among the three groups were observed when lower RDI cutpoints were used: for 5 events/hour (HIV−: 0.68–0.80; HIV+/HAART: 0.69–0.72; HIV+/No HAART: 0.47–0.53) and for 10 events/hour (HIV−: 0.79–0.84; HIV+/HAART: 0.70–0.75; HIV+/No HAART: 0.43–0.52). Because of the differences in BMI between the HIV-uninfected and HIV-infected groups, we conducted sensitivity analyses including (1) only those with a BMI< 30 kg/m2 (n=127) and (2) only those with a BMI < 25 kg/m2 (n=71) and similar patterns in the AUCs among the three groups were observed (data not shown).
Table 3.
Receiver Operating Characteristic Areas Under the Curve (AUC) and 95% Confidence Intervals (CI) Evaluating the Performance of Body Composition Measurements to Discriminate Clinically Significant Sleep Disordered Breathing (Respiratory Disturbance Index ≥ 15 events/hour)
| HIV− | HIV+/HAART | HIV+/No HAART | |
|---|---|---|---|
| AUC (CI) | AUC (CI) | AUC (CI) | |
| BMI (kg/m2) | 0.83 (0.73, 0.94) | 0.71 (0.57, 0.86) | 0.43 (0.25, 0.61) |
| Waist (cm) | 0.88 (0.79, 0.97) | 0.77 (0.65, 0.90) | 0.41 (0.26, 0.64) |
| Neck (cm) | 0.89 (0.80, 0.98) | 0.77 (0.64, 0.89) | 0.45 (0.27, 0.63) |
| Waist: Hip | 0.80 (0.68, 0.93) | 0.75 (0.62, 0.88) | 0.54 (0.35, 0.7.3) |
| Trunk Fat by DXA (kg) | 0.88 (0.80, 0.98) | 0.70 (0.56, 0.84) | 0.47 (0.28, 0.65) |
Figure 1.
Receiver Operating Characteristic (ROC) Curves Demonstrating the Discriminatory Performance of Anthropometric Measures (BMI, waist circumference, neck circumference) to Predict Significant Sleep Disordered Breathing (Respiratory Disturbance Index (RDI) ≥ 15 events/hour) in HIV-seronegative men (a), HIV-infected, HAART-treated men (b), and HIV-infected, not receiving HAART (c)
Exploratory Analyses in the HIV+/No HAART Group
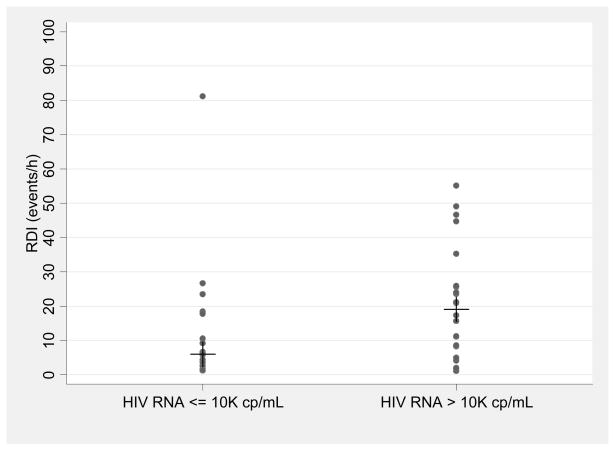
Because of the unexpected finding of the lack of association between adiposity and SDB in the HIV+/No HAART group, we undertook an exploratory analysis to characterize this group and identify predictors of SDB. Subject characteristics of the HIV+/No HAART group are presented in Table 5, stratified by the presence or absence of moderate-severe SDB. Those with moderate-severe SDB were of similar age, race, and BMI as those with absent or mild SDB. Despite similar CD4 cell counts, those with moderate-severe SDB were more likely to have HIV RNA > 10,000 copies/mL (p=0.04) and tended to have higher serum CRP concentrations (p=0.06). Conversely, the median RDI in those with HIV RNA > 10,000 copies/mL was greater than in those with HIV RNA ≤10,000 copies/mL (19.1 events/h (IQR:8.3, 25.9) vs 5.9 events/h (IQR:2.5, 17.8, p=0.03))(Figure 2). In the 20 men in the No HAART group who were previously HAART-exposed, the median (IQR) time since HAART exposure was 2.4 (1.1, 5.4) years and the median duration of HAART exposure was 3.7 (0.5, 6.2) years. Neither time since HAART, nor duration of previous HAART was associated with a high CRP (data not shown). Those with previous HAART exposure had similar median CRP levels to those without previous HAART exposure (1.9 vs 0.8 mg/L, p=0.27) and had a similar prevalence of high CRP (39% vs 33%, p=0.73).
Figure 2.
Respiratory Disturbance Index (apneic or hypopneic events/hour) in HIV-infected Men Not Receiving Highly Active Antiretroviral Therapy (n=41), stratified by HIV RNA ≤ or > 10,000 copies/mL (line represents median RDI in each group).
In a multivariable analysis among the 36 of 41 HIV+/No HAART subjects in whom all data were available, moderate-severe SDB was significantly associated with having a CRP ≥ 3.0 mg/L (Odds Ratio (OR) 6.9; 95% Confidence Interval (CI) 1.1, 43.1; p=0.04) and HIV RNA > 10,000 copies/ml (OR 7.1; 95% CI:1.0, 50.6; p=0.05), after adjustment for age, race, and BMI. Adjustment or stratification based on a previous history of HAART use yielded similar relationships between moderate-severe SDB and CRP ≥ 3.0 mg/L and HIV RNA > 10,000 copies/ml (data not shown).
With these findings, we investigated the possibility of association between moderate-severe SDB and CRP ≥ 3.0 mg/L or HIV RNA > 10,000 copies/ml in the HIV+/HAART group. While 42% of this group had a high CRP, the presence of CRP ≥3.0 mg/L was not related to moderate-severe SDB (p=0.66). Similarly, those with a HIV RNA > 10,000 copies/ml (n=3, 5%) were no more likely to have moderate-severe SDB, compared to those with lower HIV RNA concentrations (p=0.8), although the power of this analysis was limited.
Discussion
In this study of men in the MACS cohort, we found that anthropometric measurements, including BMI, waist circumference, and neck circumference, were excellent predictors of moderate to severe SDB among HIV-uninfected men, almost as predictive in HIV-infected men receiving HAART, and not at all predictive among HIV-infected men not being treated with HAART.
Among the HIV-uninfected men, the prevalence of moderate-severe SDB was high and closely related to measures of adiposity. In SDB, excess central adiposity leads to a more collapsible upper airway due to an increased mechanical load and a loss of neuromuscular control of the pharynx[24]. In previous studies of HIV-uninfected cohorts, ROC analysis of anthropometric variables yielded AUCs in the 0.88–0.94 range, indicating excellent discriminatory performance for RDI frequency> 5 events/hour[4, 25, 26]. In the present study, which used a cutoff of 15 events/hour, we found comparable AUCs ranging from 0.80–0.89 for anthropometric measures: however, AUCs using the cutpoints of 5 and 10 events/hour were lower. In addition, similar to other studies[7, 27, 28], the association of waist and neck circumference measurements to SDB severity was stronger predictor than BMI, and emphasizes the important influence of fat distribution on the association between adiposity and SDB.
The prevalence of SDB was high among the HIV-infected men receiving HAART, despite the relatively low average BMI in this group. Few studies have investigated the risk of SDB among HIV-infected patients. Lo Re et al reported a series of 12 HIV-infected patients who were found to have RDI ≥ 5 events/hour, most of whom had a neck circumference > 40 inches and BMI > 25 kg/m2, demonstrating the expected association between upper body obesity and SDB [29]. In our group of HAART-treated men, waist and neck circumference were the strongest predictors of SDB among the body composition parameters measured. Moreover, we found that relative fat distribution, as estimated by trunk:extremity fat ratio or waist:hip ratio, was less associated with SDB than absolute measurements. In contrast, lower extremity fat was not associated with SDB in HAART-treated men, providing evidence against the possibility that lipoatrophy is a risk factor for SDB.
The most striking finding in the present study was the lack of association between body composition measurements and SDB among the HIV-infected men not receiving HAART. Both simple anthropometry and DXA-derived measurements performed no better than chance for the prediction of moderate-severe SDB, even though the average BMI in this HIV-infected group was similar to that in the HAART-treated group. This finding suggests that other factors besides adiposity or fat distribution mediate SDB in this population. In an exploratory analysis, we found that high CRP and high HIV viral load were independently associated with moderate-severe SDB. Although preliminary, these findings may suggest that uncontrolled HIV-infection and the resulting systemic inflammation may contribute to the pathogenesis of SDB in this population.
In previous studies in HIV-uninfected subjects, higher levels of inflammatory markers, including CRP, IL-6, and TNF-α, have been associated with SDB[30–32]. It is unclear, however, whether systemic inflammation is a cause, consequence, or a confounded correlate of SDB. SDB is associated with increased sympathetic activation, hypoxia, and oxidative stress, all of which can amplify the inflammatory response[33]. The finding of reduced systemic inflammation after treatment of SDB with continuous positive airway pressure suggests that inflammation can be a consequence of SDB[34]. Conversely, there is evidence that inflammation may be causative. It has been proposed that the somnogenic effects of inflammatory cytokines[35, 36] may also compromise neuromuscular control of the upper airway and increase the likelihood of pharyngeal collapse[24]. Supportive clinical evidence comes from a randomized, placebo-controlled trial in which the TNF-α inhibitor, entanercept, was associated with reduced sleepiness and RDI, as well as decreased serum levels of IL-6 [37].
Another potential explanation for the lack of association between body composition and SDB in the HIV-infected men not receiving HAART is upper airway obstruction resulting from adenotonsillar hypertrophy. A cross-sectional study of 134 HIV-infected patients in the pre-HAART era used a sleep questionnaire to identify 14 cases of probable sleep disordered breathing, of whom SDB was confirmed in 9 patients on subsequent PSG testing[38]. Eight of these cases were found to have tonsillar hypertrophy on physical examination and in two of these cases the SDB improved after tonsillectomy. The prevalence of tonsillar hypertrophy in those patients who did not receive PSG testing was not reported. Nevertheless, the authors conclude adenotonsillar hypertrophy is a risk factor for sleep apnea. In our study, an otolaryngologic examination was not performed and it is possible that adenotonsillar hypertrophy contributed to the SDB in those not receiving HAART and that this also correlated with higher viral load and increased systemic inflammation. This possibility should be investigated in further studies.
Our study has several additional limitations. First, we used DXA to measure regional adiposity. Although DXA gives a precise measurement of fat, it cannot differentiate between visceral and subcutaneous adiposity. Further investigation of body composition and SDB in HIV-infected persons should include CT or MRI, which can measure visceral adiposity. Second, CRP measurements were available only in a subset of participants and were not concurrent with the sleep study. Next, because of the small sample size, our exploratory analysis of predictors of sleep disordered breathing in HIV-infected subjects not receiving HAART should be considered preliminary and requires confirmation in a larger cohort. Finally, our study included only men, which limits the generalizability of the findings.
Because of SDB is prevalent and is associated with an increased risk of cardiovascular disease and reduced quality of life, the identification of patients who have SDB and who may benefit from intervention is important in the clinical setting. Clinicians should be aware that HIV-infected patients with normal anthropomorphic measurements, particularly those not receiving HAART, may have clinically significant SDB. In addition, the association between SDB and systemic inflammation in the setting of uncontrolled HIV-disease may have important implications for the pathogenesis of SDB in HIV-infected patients. Further studies are required to clarify the relationship between adiposity, inflammation, and SDB in HIV-infected patients and to investigate whether antiretroviral therapy can impact parameters of SDB.
Table 4.
Characteristics of HIV-infected/No HAART group stratified by the presence of sleep disordered breathing (Respiratory Disturbance Index (RDI) ≥ 15 events/hour)
| RDI < 15 | RDI ≥ 15 | p | |
|---|---|---|---|
| n | 23 | 18 | |
| Age (years) | 47 (9) | 51 (7) | 0.13 |
| Race (n non-Black (%)) | 14 (56% ) | 11 (44%) | 0.99 |
| BMI (kg/m2) | 25.7 (4.2) | 25.1 (4.1) | 0.64 |
| CD4 cell count (cells/mm3) | 531 (321) | 503 (244) | 0.76 |
| HIV Duration (yrs) | 7.5 (7) | 9.1 (8.2) | 0.51 |
| HIV RNA > 100K copies/ml, n (%) | 3 (13%) | 5 (28%) | 0.24 |
| HIV RNA > 10K copies/ml, n (%) | 9 (41%) | 13 (59%) | 0.04 |
| HAART Naïve, n (%) | 13 (57%) | 8 (44%) | 0.44 |
| CRP: Median (IQR) | 0.8 (0.6, 1.7) | 3 (1, 4.5) | 0.06 |
| N=21 | N=15 |
Values represent means (standard deviation), unless otherwise noted
Acknowledgments
This work was supported in part by NIH (NCAAM) 5K23AT2862 (TTB), NIH (NHLBI) K23HL077137 (SPP), Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), NIH (NHLBI) HL79554 (PLS), (5-MO1-RR-00052) UO1-AI-35042, UO1-AI-35043, UO1-AI-35041
Reference List
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993 Apr 29;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002 Mar 1;165(5):677–82. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 3.Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007 Jul;132(1):325–37. doi: 10.1378/chest.07-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogretmenoglu O, Suslu AE, Yucel OT, Onerci TM, Sahin A. Body fat composition: a predictive factor for obstructive sleep apnea. Laryngoscope. 2005 Aug;115(8):1493–8. doi: 10.1097/01.mlg.0000172204.82314.c3. [DOI] [PubMed] [Google Scholar]
- 5.Santaolalla MF, Iriondo B, Jr, Aguirre LU, Martinez IA, Sanchez DR, Sanchez Fernandez JM. The predictive value of clinical and epidemiological parameters in the identification of patients with obstructive sleep apnoea (OSA): a clinical prediction algorithm in the evaluation of OSA. Eur Arch Otorhinolaryngol. 2007 Jun;264(6):637–43. doi: 10.1007/s00405-006-0241-5. [DOI] [PubMed] [Google Scholar]
- 6.Davies RJ, Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J. 1990 May;3(5):509–14. [PubMed] [Google Scholar]
- 7.Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep. 1995 Apr;18(3):158–66. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 8.Wohl DA, Brown TT. Management of morphologic changes associated with antiretroviral use in HIV-infected patients. J Acquir Immune Defic Syndr. 2008 Sep 1;49(Suppl 2):S93–S100. doi: 10.1097/QAI.0b013e318186521a. [DOI] [PubMed] [Google Scholar]
- 9.Joy T, Keogh HM, Hadigan C, et al. Relation of body composition to body mass index in HIV-infected patients with metabolic abnormalities. J Acquir Immune Defic Syndr. 2008 Feb 1;47(2):174–84. doi: 10.1097/QAI.0b013e31815b0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo JC, Mulligan K, Tai VW, Algren H, Schambelan M. “Buffalo hump” in men with HIV-1 infection. Lancet. 1998 Mar 21;351(9106):867–70. doi: 10.1016/S0140-6736(97)11443-X. [DOI] [PubMed] [Google Scholar]
- 11.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987 Aug;126(2):310–8. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 12.Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Ann Intern Med. 2002 Sep 3;137(5 Pt 2):381–433. doi: 10.7326/0003-4819-137-5_part_2-200209031-00001. [DOI] [PubMed] [Google Scholar]
- 13.Palella FJ, Jr, Cole SR, Chmiel JS, et al. Anthropometrics and examiner-reported body habitus abnormalities in the multicenter AIDS cohort study. Clin Infect Dis. 2004 Mar 15;38(6):903–7. doi: 10.1086/381684. [DOI] [PubMed] [Google Scholar]
- 14.Gould GA, Whyte KF, Rhind GB, et al. The sleep hypopnea syndrome. Am Rev Respir Dis. 1988 Apr;137(4):895–8. doi: 10.1164/ajrccm/137.4.895. [DOI] [PubMed] [Google Scholar]
- 15.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999 Aug 1;22(5):667–89. [PubMed] [Google Scholar]
- 16.Body Measurements (Anthropometry) Westat, Inc; 1988. National Health and Nutrition Survey III. [Google Scholar]
- 17.Brown TT, Chu H, Wang Z, et al. Longitudinal increases in waist circumference are associated with HIV-serostatus, independent of antiretroviral therapy. AIDS. 2007 Aug 20;21(13):1731–8. doi: 10.1097/QAD.0b013e328270356a. [DOI] [PubMed] [Google Scholar]
- 18.Brown T, Wang Z, Chu H, et al. Longitudinal anthropometric changes in HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr. 2006 Nov 1;43(3):356–62. doi: 10.1097/01.qai.0000243052.73321.8e. [DOI] [PubMed] [Google Scholar]
- 19.Heyward VH, Wagner DR. Applied Body Composition. Champagne, IL: Human Kinetics; 2004. [Google Scholar]
- 20.Brown TT, Ruppe MD, Kassner R, et al. Reduced bone mineral density in human immunodeficiency virus-infected patients and its association with increased central adiposity and postload hyperglycemia. J Clin Endocrinol Metab. 2004 Mar;89(3):1200–6. doi: 10.1210/jc.2003-031506. [DOI] [PubMed] [Google Scholar]
- 21.Hadigan C, Miller K, Corcoran C, Anderson E, Basgoz N, Grinspoon S. Fasting hyperinsulinemia and changes in regional body composition in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 1999 Jun;84(6):1932–7. doi: 10.1210/jcem.84.6.5738. [DOI] [PubMed] [Google Scholar]
- 22.Brown TT, Xu X, John M, et al. Fat distribution and longitudinal anthropometric changes in HIV-infected men with and without clinical evidence of lipodystrophy and HIV-uninfected controls: a substudy of the Multicenter AIDS Cohort Study. AIDS Res Ther. 2009;6:8. doi: 10.1186/1742-6405-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982 Apr;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008 Feb 15;5(2):185–92. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kushida CA, Efron B, Guilleminault C. A predictive morphometric model for the obstructive sleep apnea syndrome. Ann Intern Med. 1997 Oct 15;127(8 Pt 1):581–7. doi: 10.7326/0003-4819-127-8_part_1-199710150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Dixon JB, Schachter LM, O’Brien PE. Predicting sleep apnea and excessive day sleepiness in the severely obese: indicators for polysomnography. Chest. 2003 Apr;123(4):1134–41. doi: 10.1378/chest.123.4.1134. [DOI] [PubMed] [Google Scholar]
- 27.Davidson TM, Patel MR. Waist circumference and sleep disordered breathing. Laryngoscope. 2008 Feb;118(2):339–47. doi: 10.1097/MLG.0b013e3181587d7c. [DOI] [PubMed] [Google Scholar]
- 28.Deegan PC, McNicholas WT. Predictive value of clinical features for the obstructive sleep apnoea syndrome. Eur Respir J. 1996 Jan;9(1):117–24. doi: 10.1183/09031936.96.09010117. [DOI] [PubMed] [Google Scholar]
- 29.Lo Re V, III, Schutte-Rodin S, Kostman JR. Obstructive sleep apnoea among HIV patients. Int J STD AIDS. 2006 Sep;17(9):614–20. doi: 10.1258/095646206778113078. [DOI] [PubMed] [Google Scholar]
- 30.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000 Mar;85(3):1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 31.Trakada G, Chrousos G, Pejovic S, Vgontzas A. Sleep Apnea and its association with the Stress System, Inflammation, Insulin Resistance and Visceral Obesity. Sleep Med Clin. 2007 Jun;2(2):251–61. doi: 10.1016/j.jsmc.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep. 2007 Jan 1;30(1):29–34. doi: 10.1093/sleep/30.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008 Feb 15;177(4):369–75. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007 Oct 1;176(7):706–12. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 35.Krueger JM, Obal FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001 Mar;933:211–21. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi S, Tooley DD, Kapas L, Fang J, Seyer JM, Krueger JM. Inhibition of tumor necrosis factor in the brain suppresses rabbit sleep. Pflugers Arch. 1995 Dec;431(2):155–60. doi: 10.1007/BF00410186. [DOI] [PubMed] [Google Scholar]
- 37.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004 Sep;89(9):4409–13. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 38.Epstein LJ, Strollo PJ, Jr, Donegan RB, Delmar J, Hendrix C, Westbrook PR. Obstructive sleep apnea in patients with human immunodeficiency virus (HIV) disease. Sleep. 1995 Jun;18(5):368–76. doi: 10.1093/sleep/18.5.368. [DOI] [PubMed] [Google Scholar]