Abstract
Purpose
Serotonin and norepinephrine reuptake inhibitors (SNRI) such as duloxetine have demonstrated clinical efficacy in the treatment of stress urinary incontinence (SUI). However, the therapeutic dose of duloxetine is often associated with unwanted side effects. In the present study, we examined whether the combined use of α2-adrenoceptor (AR) antagonists can reduce the dose of duloxetine because of synergistic effects.
Materials and Methods
Normal female rats and rats with SUI induced by vaginal distension (VD) were used. Urethral responses were measured using a microtransducer-tipped catheter. The effect of low-dose duloxetine (0.1 mg/kg), α2-AR antagonists (idazoxan or yohimbine) and sequential administration of drugs on the amplitude of urethral pressure responses during sneezing (A-URS) and urethral baseline pressure (UBP) was evaluated. Sneeze-induced leak point pressure (S-LPP) measurements were also performed.
Results
In normal and VD rats, low-dose duloxetine did not alter A-URS although it increased UBP significantly. However, when low-dose duloxetine was co-applied with α2-AR antagonists, A-URS was significantly increased. In all seven VD rats, leakage was observed during sneezing. After low-dose duloxetine administration, fluid leakage still occurred during sneezing; however, after co-administration of low-dose duloxetine and idazoxan, fluid leakage disappeared in two of seven rats, and S-LPP was significantly increased in the remaining incontinent VD rats.
Conclusions
The present findings support the concept that the combination therapy with α2-AR antagonists may provide an effective and novel strategy to reinforce the clinical efficacy of SNRI agents in the treatment of SUI.
Keywords: α2-adrenoceptor, stress urinary incontinence, SNRI, urethral continence reflex, urodynamics
Introduction
Stress urinary incontinence (SUI) is a one of the most common health problems defined as involuntary loss of urine secondary to an increase in abdominal pressure during events such as sneezing, coughing or laughing, in the absence of bladder contraction.1–3 This type of incontinence represents approximately a half of all urinary incontinence cases.4 There is increasing risk of SUI at or after the middle age, and the prevalence of SUI peaks during perimenopausal years.4 Based on the data of Diokno et al,5 SUI occurs in 37.7% of noninstitutionalized women older than 60 years of age.
Duloxetine, a serotonin and norepinephrine reuptake inhibitor (SNRI), has demonstrated clinical efficacy in the treatment of SUI.6 However, the side effects (nausea, constipation, dizziness, fatigue and somnolence) can be problematic especially in the elderly,7 and at present, duloxetine is not approved by the United States FDA for the treatment of SUI.
We previously established a rat model that can be used to examine sneeze-induced active urethral closure mechanisms, which are mediated by somatic nerve-induced reflex contractions of external urethral sphincter and pelvic floor striated muscle and reported that the active urethral closure during sneezing is different from other anti-SUI reflex mechanisms such as those during Valsalva-like maneuvers8. Because the urethral closure response during sneezing was not affected by opening the abdominal or by bilateral transection of pelvic and hypogastric nerves,8 the sneeze-induced continence mechanism is likely to be activated directly by sneezing, but not by activation of afferent pathways from the bladder. We have also reported that descending signals in the bulbospinal noradrenergic pathway can regulate the urethral continence reflex by facilitatory α1-adrenergic (AR) receptor and inhibitory α2-AR receptor,9,10 and that α2-AR receptor blockade can enhance the effects of duloxetine on the urethral EMG activity increased by abdominal compression.10 However, the synergistic effect of SNRI and α2-AR blockers has not been examined in the sneeze-induced urethral continence reflex. Therefore, we investigated whether a low-dose of duloxetine and α2-AR antagonists (idazoxan or yohimbine) have synergistic effects on the sneeze-induced continence reflex in rats. Moreover, from these two different types of α2-AR antagonists, we investigate the involvement of α2-AR controlling the sneeze-induced continence mechanism.
Material and Methods
Animals
Seventy-four adult female Sprague-Dawley rats weighing 236–306 g were studied with experimental protocols approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Experiments were performed in normal rats and rats with simulated birth trauma induced by vaginal distension (VD).11
Vaginal Distention
Under pentobarbital (30 mg/kg, IP) (Ovation, Deerfield, IL) anesthesia, a 10-Fr Foley balloon catheter (5 ml) (Rusch, Salt Lake, UT) with the tip cut off was inserted into the vagina and then the vaginal orifice was closed with suture. The balloon catheter was inflated with 4 ml of water to distend the vagina for 4 h.12 The experiments were conducted 4 days after the VD.
Surgical procedures for experiments
Under isoflurane anesthesia, a polyethylene catheter (PE-10) (Clay-Adams, Parsippany, NJ) was inserted into a jugular vein for drug injection. In some animals a PE-10 catheter was inserted into the intrathecal space at the level of L6-S1 spinal cord for intrathecal drug injection 1–2 days before the experiment. The urinary bladder was exposed through an abdominal incision, ureters were cut bilaterally, and their distal ends were ligated. The visceral branches of pelvic nerves were transected bilaterally near internal iliac vessels to prevent reflex bladder contractions.9 For the measurement of leak point pressure during sneezing (S-LPP), a PE-90 catheter connected to a pressure transducer (Transbridge 4M) (World Precision Instruments, Sarasota, FL) was inserted into the bladder through the dome for recording intravesical pressure. Feces were removed from the distal colon through a small incision in the colon wall and then a handmade, small 1-cm-diameter balloon catheter connected to a pressure transducer was inserted through the rectum and the colonic incision into the abdominal cavity to record abdominal pressure (Pabd) during sneezing. The abdomen was then closed with sutures. After the surgery, isoflurane anesthesia was turned off and replaced with urethane anesthesia (0.72 g/kg, IP) (Sigma Chemical, St. Louis, MO), and additional doses of anesthetic (0.1 g/kg per injection, IV) were administered as required before sneeze reflex testing started to obtain the sufficient level of anesthesia, which was confirmed by negative reflex responses to toe pinch. Rats were placed in a supine position for the experiments. The final dose of urethane ranged from 1.0–1.2 g/kg in the different animals.
Sneeze reflex
As reported previously,8,9 the sneeze reflex was induced by a rat’s whisker cut and inserted gently into the nostril under urethane anesthesia.
Urethral responses during sneezing
Normal and VD rats were examined after the bladder was emptied, and a 3.5-Fr nylon catheter with a side-mounted microtransducer located 1 mm from the catheter tip (SPR-524) (Millar Instruments, Houston, TX) was inserted into the urethra from the urethral orifice. The microtransducer-tipped catheter was then connected to a pressure transducer (Transbridge 4M) (World Precision Instruments), and urethral responses were recorded with a data-acquisition software (sampling rate 400 Hz) (Chart, AD Instruments, Castle Hill, NSW, Australia) on a computer system equipped with an analog-to-digital converter (Power Lab) (AD Instruments, Colorado Springs, CO). The catheter position was monitored throughout the experiments to confirm that the location of the transducer had not changed. The amplitude of urethral pressure responses during sneezing (A-URS) and urethral baseline pressure (UBP) were measured. A-URS represents the maximal pressure change (cmH2O) from the baseline. The averaged UBP was obtained from a plateau section of pressure recordings just before the sneeze response according to our previous reports.9 To evaluate the intensity of the induced sneeze, which varied with each sneeze event, pressure increases in Pabd during sneezing were also measured via an intra-abdominal balloon catheter. Sneeze-induced responses were compared before and after injection of drugs.
Leak point pressure during sneezing
In another group of normal and VD rats, after the bladder was emptied, 0.4 ml of saline solution containing Evans blue (100 μg/ml) (Sigma Chemical) was injected into the bladder. The sneeze reflex was induced to examine whether fluid leakage from the urethral orifice was induced by sneezing. Intravesical pressure changes were recorded to monitor the increase in abdominal pressure during sneezing with data acquisition software (sampling rate 400 Hz) (Chart, AD Instruments) on a computer system equipped with an analog-to-digital converter (Power Lab) (AD Instruments). The maximal intravesical pressure was measured during each sneeze event, and the lowest pressure value that induced fluid leakage from the urethral orifice was defined as the S-LPP. After control S-LPPs were obtained, sneeze reflexes were induced again following administration of duloxetine with or without idazoxan to evaluate the effect of the drug on sneeze-induced fluid leakage and S-LPP.
Application of drugs
Duloxetine and idazoxan were dissolved in distilled water and administered in doses based on our preliminary and previous experiments.9,10 Intravenous duloxetine and idazoxan were administered in a volume of 0.1 ml/100 g body weight. For intrathecal application, 1 μl of drug solution was given via the implanted intrathecal catheter and flushed by 10 μl of saline. Yohimbine hydrochloride (Tocris Bioscience, Ellisville, MO) was also tested intravenously and intrathecally in the same manner.
Data Analysis
All Data are expressed as means ± SE. Excessively large sneezes that induced increases in Pabd greater than +2 SD above the average and very small responses inducing increases in Pabd less than 3 cmH2O were excluded from data analyses. The values of the A-URS and UBP as well as increases in Pabd during sneezing were averaged in each rat. The mean ± SE in a group of animals was then calculated from the averaged value in each rat. A paired t-test was used to compare A-URS, UBP, and increases in Pabd during sneezing before and after duloxetine administration. Repeated measures analysis of variance (ANOVA), followed by Tukey’s multiple comparison test, were used to compare changes in A-URS and UBP as well as increases in Pabd during sneezing before and after sequential administration of duloxetine and idazoxan or yohimbine. Student’s t-test for unpaired data was used to compare A-URS and UBP as well as increases in Pabd during sneezing between normal and VD rats. In S-LPP experiments, one way ANOVA, followed by Tukey’s multiple comparison test, was used to compare changes in S-LPP during sneezing before and after drug administration. All data were analyzed using the statistical software package Prism (Graphpad Software, San Diego, CA). P values <0.05 were considered to be significant.
Results
In normal rats, a single injection of duloxetine (1 and 10 mg/kg, IV) significantly increased A-URS and UBP (table. 1). Low-dose (0.1 mg/kg) duloxetine increased UBP by 9.0% compared with pre-drug values (P<0.05), but did not alter A-URS significantly. The average value of sneeze-induced increases in Pabd measured by intra-abdominal catheters was not significantly changed by duloxetine.
Table 1.
Effects of Duloxetine on sneeze-induced pressure changes in normal rats
| Treatment | A-URS | UBP | Increase in Pabd | |
|---|---|---|---|---|
| Duloxetine 0.1mg/kg (n = 10) | Predrug control | 29.0±3.3 | 26.7±1.7 | 8.2±0.8 |
| After Duloxetine | 30.4±3.9 | 29.1±1.8* | 9.0±0.7 | |
| Duloxetine 1mg/kg (n = 10) | Predrug control | 30.3±3.2 | 25.4±1.9 | 9.0±2.1 |
| After Duloxetine | 37.5±4.6* | 31.2±2.1* | 9.8±2.0 | |
| Duloxetine 10mg/kg (n = 7) | Predrug control | 28.8±3.5 | 27.3±1.6 | 8.3±0.6 |
| After Duloxetine | 43.0±5.6* | 32.7±1.8* | 8.8±0.8 | |
Values (in cmH2O) are means ± SE. A-URS, amplitude of urethral pressure response during sneezing; UBP, urethral baseline pressure; increase in Pabd, increase in abdominal pressure during sneezing.
Significant difference from predrug values:
P < 0.05
Intravenous administration of either idazoxan (0.3mg/kg) or yohimbine (1mg/kg) produced some increase in UBP (data not shown), but almost totally suppressed the sneeze reflex. Therefore, we chose to use intrathecal administration of these compounds at the L6-S1 spinal cord level for further experiments. Single dose of idazoxan ranging from 4 to 40 nmol were administered intrathecally while recording urethral pressure using microtransducer-tipped catheters. These doses of idazoxan did not alter A-URS or UBP significantly (n=5, data not shown). However, when low-dose (0.1mg/kg, IV) duloxetine and idazoxan (4 nmol, IT) were co-applied, A-URS was increased by 69.1% in normal rats (P<0.01) and 58.9% in VD rats (P<0.01) compared with pre-drug control values, and by 52.8% in normal rats (P<0.01) and 43.5% in VD rats (P<0.05) compared with the value of the low-dose duloxetine (fig. 1, table 2) In addition, in both normal and VD rats, co-application of low-dose duloxetine (0.1 mg/kg) and yohimbine (30 nmol, IT) significantly (P<0.05) increased A-URS (46.3% and 37.3% increases in normal rats and 30.3% and 38.7% increases in VD rats) compared with pre-drug control and low-dose duloxetine values, respectively (table 3).
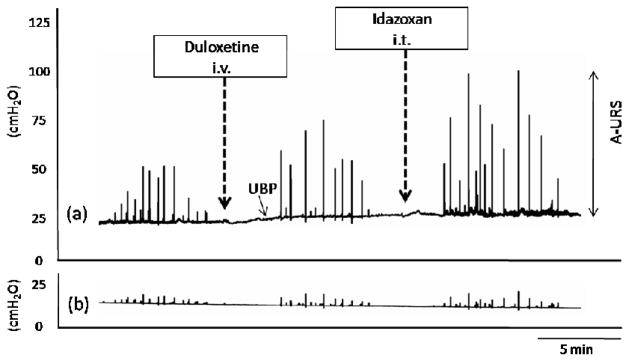
Figure 1.
Representative traces of urethral (a) and abdominal (b) pressure changes induced by sequential administration of duloxetine (0.1 mg/kg intravenously [i.v.]) and intrathecal (i.t.) idazoxan (4 nmol). Low-dose (0.1.mg/kg) duloxetine alone did not increase A-URS significantly, however additional administration of idazoxan increased A-URS. A-URS: the amplitude of urethral pressure responses during sneezing, UBP: urethral baseline pressure
Table 2.
Effect of sequential administration of low-dose duloxetine (0.1 mg/kg, i.v.) and idazoxan (4 nmol i.t.) on sneeze-induced pressure changes in normal and vaginal distended rats
 |
Values (in cmH2O) are means ± SE. VD, vaginal distension; A-URS, amplitude of urethral pressure response during sneezing; UBP, urethral baseline pressure; increase in Pabd, increase in abdominal pressure during sneezing.
P < 0.05 vs. predrug control,
P < 0.01 vs. predrug control,
P < 0.05 vs. control rats.
Table 3.
Effect of sequential administration of low-dose duloxetine (0.1 mg/kg, i.v.) and yohimbine (30 nmol i.t.) on sneeze-induced pressure changes in normal and vaginal distended rats
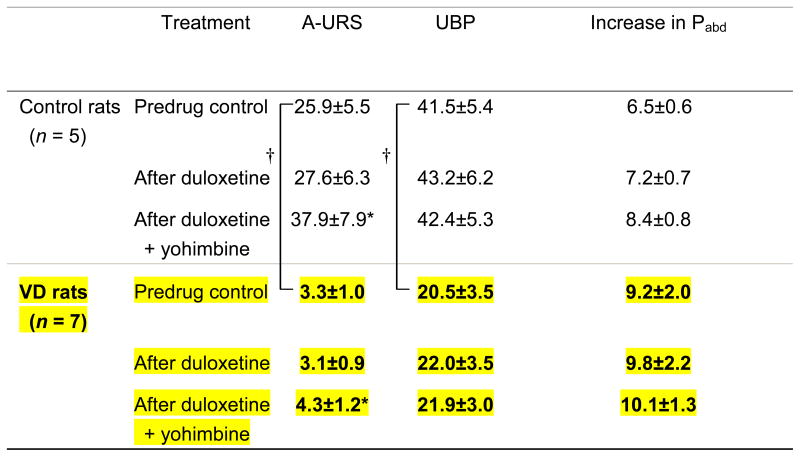 |
Values (in cmH2O) are means ± SE. VD, vaginal distension; A-URS, amplitude of urethral pressure response during sneezing; UBP, urethral baseline pressure; increase in Pabd, increase in abdominal pressure during sneezing.
P < 0.05 vs. predrug control,
P < 0.05 vs. control rats.
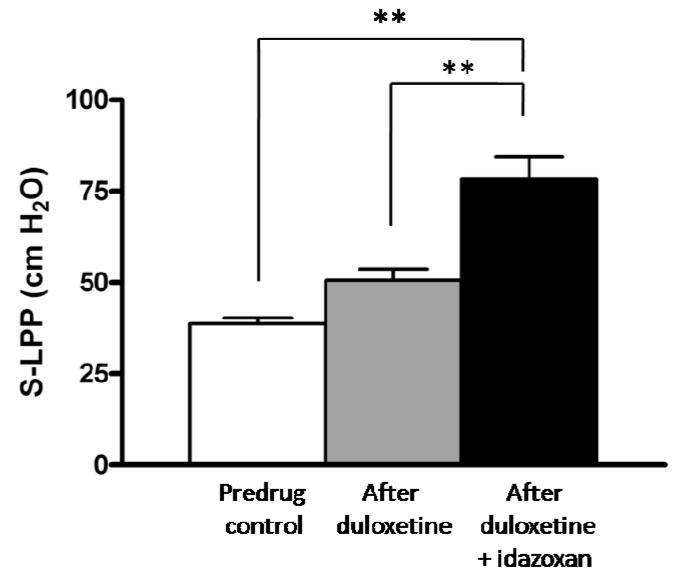
Sneezing increased intravesical pressure on average to 87.7 ± 5.7 cmH2O in six normal rats; however, no fluid leakage was observed during sneezing before and after drug administration. Duloxetine or idazoxan did not affect the peak intravesical pressure during sneezing. However, in all seven VD rats, leakage was observed during sneezing before drug administration. S-LPP in these VD rats averaged 38.7 ± 1.6 cmH2O. After low-dose duloxetine (0.1mg/kg, IV) administration, fluid leakage was still observed during sneezing in all VD rats; however, after co-administration of low-dose duloxetine and idazoxan (4 nmol, IT), fluid leakage disappeared in two of seven incontinent VD rats, and S-LPP was significantly increased to 78.5 ± 6.0 cmH2O (P<0.01) in the remaining five incontinent VD rats (fig. 2).
Figure 2.
Drug-induced changes in sneeze leak point pressure (S-LPP) in vaginal distended rats. Low-dose duloxetine (0.1 mg/kg, intravenously) alone did not increase S-LPP significantly, however additional intrathecal application of idazoxan (4 nmol) significantly increased S-LPP. **P < 0.01.
Discussion
The results of the present study indicate that co-administration of α2-AR antagonists can enhance the anti-SUI effects of SNRI medication. While low-dose duloxetine (0.1 mg/kg) did not alter A-URS or S-LPP significantly, additional administration of idazoxan or yohimbine, which alone had no detectable effects, increased A-URS and S-LPP to the level comparable to that of 1 mg/kg duloxetine alone in the present (table. 1) and previous studies9. We have previously reported that external urethral sphincter contractions during passive intravesical pressure rises, which are mediated by pelvic-to-pudendal reflex pathways, contribute to urinary continence and that the effects of duloxetine on external urethral sphincter contractions in Valsalva-like stress conditions induced by lower abdominal compression are potentiated by co-application of α2-AR antagonists in rats with acute spinal cord transection.10 In this study, we used spinal cord intact rats and investigated the spinal descending continence mechanism activated by sneezing. This reflex mechanism is more transient, but of greater magnitude than bladder-to-urethral continence reflexes induced by passive intravesical pressure rises.8 These findings, which represent the first demonstration of a synergistic effect of α2-AR antagonists and SNRI on sneeze-induced urethral continence reflex, raise the possibility that drugs targeting α2-ARs might be useful in the management of SUI.
SUI is a one of the most common health problems,1 and imposes substantial costs both on the individual and the society in the United States and worldwide.13–15 The basic concept of the SUI treatment is to increase the resistance of the sphincter and urethra. Although continual pelvic muscle exercises can be effective for SUI patients, compliance and long-term success have been low, and drop-out rates are high.16 Over the past decade, several types of midurethral sling procedures have become a first-line surgical therapy for SUI patients.17,18 For SUI surgery, age per se is not a contraindication;19 however, surgical interventions are irreversible, and elderly patients are at higher risk for surgical procedures and anesthetic complications. Also, elderly women tend to be suffering from persistent SUI, persistent or de novo urge symptoms and voiding difficulty after surgery.20 Thus pharmacotherapies are still in great demand in SUI patients, especially in an elderly population. Moreover, unlike younger women, elderly women are often treated with multiple drugs. Therefore drugs with fewer side effects or drug interactions are preferable.
Duloxetine, a dual reuptake inhibitor preventing the depletion of both serotonin and norepinephrine (NE) at neuronal synapses,21 is approved for the treatment of major depressive disorders and diabetic neuropathy.22 Duloxetine has also been reported to significantly reduce urine loss and improve quality of life in SUI women.23–26 Duloxetine is believed to act at serotonin and NE nerve terminal in the Onuf’s nucleus, to reduce reuptake of the monoamines, and enhance the excitatory effect of the monoamines on motoneurons innervating urethral striated muscle sphincter.6,27 However, at present, duloxetine is approved for SUI only in the EU, but not in the USA, in part because the therapeutic dose of duloxetine is often associated with unwanted side effects. However, the dose of SNRI could be reduced for the control of SUI when it is administered together with α2-AR antagonists, because the combined application of low-dose duloxetine and α2-AR antagonists significantly enhanced the sneeze-induced continence reflex. The reduction of therapeutic SNRI dose might decrease the unwanted side effects of SNRI although clinical investigation will be needed to clarify this point.
When applied alone, low-dose duloxetine increased UBP by 9.0% compared with pre-drug values (P<0.05) without affecting A-URS. Our previous study revealed that duloxetine can increase UBP by facilitating sympathetic activity carried through the hypogastric nerves to the urethral smooth muscles.9 However, a neurally-induced, striated muscle contraction (represented by A-URS) seems to be more important for the dynamic continence reflex phase and crucial for preventing urinary leakage during the sneeze-induced continence reflex.8 Therefore, a strategy to enhance A-URS might be preferable for the treatment of SUI during strong and phasic stress conditions such as sneezing or coughing.
In our previous study, intrathecally administered α1-AR antagonist prazosin or non-specific α-AR antagonist phentolamine decreased A-URS without affecting UBP28 during sneezing while in the present study intrathecally administered α2-AR antagonists idazoxan or yohimbine had no effect on any parameter. Thus it seems that the sneeze-induced urethral continence reflex is tonically enhanced by stimulation of α1-AR receptors, but not affected by α2-AR receptor activation when the bladder-to-external urethral sphincter reflex pathway has been interrupted by bilateral transection of the pelvic nerves. Evoked potential recording techniques in cats also revealed that inhibitory α2-AR receptors do not tonically control reflex mechanisms in the lower urinary tract.29 However, as shown in the present study the inhibitory effect of α2-AR receptors becomes significant when NE concentration is increased at the synapse after treatment with duloxetine because α2-AR receptor antagonists enhanced the facilitatory effect of duloxetine on sneeze-induced A-URS. We also previously reported that the facilitatory effect of duloxetine (1 mg/kg, IV) on A-URS during sneeze are mainly mediated by α1-AR receptor activation in the spinal cord, and that a reduction in A-URS, which was observed after duloxetine in the presence of an α1-AR receptor antagonist (terazosin), was negated by idazoxan.9 Taken together, it is likely that an increase in NE concentration at the synapse after duloxetine can stimulate both facilitatory α1-AR receptors and inhibitory α2-AR receptors in the spinal cord and that the α2-AR receptor blockade can remove the duloxetine-induced inhibition to enhance the effects of an SNRI drug on the sneeze-induced urethral continence reflex. In addition, both idazoxan and yohimbine administrated systemically almost abolished the sneeze reflex, suggesting that α2-AR receptors might modulate the threshold of sneeze reflex probably by acting at a supraspinal level.
It is known that some α2-AR receptor antagonists such as idazoxan also have varying affinity for imidazoline receptors.30 Therefore, it is difficult to determine if the pharmacological effects of idazoxan are due to block of α2-AR receptors and/or imidazoline receptors. Therefore in the current study, we also examined urethral responses during sneezing following administration of a pure α2-AR receptor antagonist, yohimbine, which does not interact with imidazoline receptors. Yohimbine enhanced the effects of low-dose duloxetine in not only normal but also VD rats, similarly to the effects of idazoxan (table. 3). Thus, the enhancing effects of idazoxan are likely to be due to α2-AR receptor blockade, even though idazoxan has equal affinity for imidazoline and α2-AR receptors.30
Conclusions
The present study indicates that the effects of duloxetine are enhanced by α2-AR blockade. Thus, co-administration with α2-AR antagonists could decrease the effective dosage of SNRI agents, thereby potentially reducing unwanted side effects. Thus we propose that combination therapy with α2-AR antagonists might be an effective and novel strategy to reinforce the clinical efficacy of SNRI agents in the treatment of SUI. Clinical studies examining the usefulness of the combination pharmacological therapy are required to test the hypothesis in patients with SUI.
Acknowledgments
This study was supported by grants from National Institutes of Health: DK067226, AR049398 and DK055387.
Abbreviation and Acronyms
- SUI
stress urinary incontinence
- AR
adrenergic
- VD
vaginal distension
- SNRI
serotonin norepinephrine reuptake inhibitor
- NE
norepinephrine
- A-URS
amplitude of urethral pressure responses during sneezing
- UBP
urethral baseline pressure
- S-LPP
sneeze leak point pressure
References
- 1.Swithinbank LV, Abrams P. The impact of urinary incontinence on the quality of life of women. World J Urol. 1999;17:225. doi: 10.1007/s003450050137. [DOI] [PubMed] [Google Scholar]
- 2.Hunskaar S, Burgio K, Diokno A, et al. Epidemiology and natural history of urinary incontinence in women. Urology. 2003;62:16. doi: 10.1016/s0090-4295(03)00755-6. [DOI] [PubMed] [Google Scholar]
- 3.Landefeld CS, Bowers BJ, Feld AD, et al. National Institutes of Health state-of-the-science conference statement: prevention of fecal and urinary incontinence in adults. Ann Intern Med. 2008;148:449. doi: 10.7326/0003-4819-148-6-200803180-00210. [DOI] [PubMed] [Google Scholar]
- 4.Hannestad YS, Rortveit G, Sandvik H, et al. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag. J Clin Epidemiol. 2000;53:1150. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 5.Diokno AC, Brock BM, Brown MB, et al. Prevalence of urinary incontinence and other urological symptoms in the noninstitutionalized elderly. J Urol. 1986;136:1022. [PubMed] [Google Scholar]
- 6.Shamliyan TA, Kane RL, Wyman J, et al. Systematic review: randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Ann Intern Med. 2008;148:459. doi: 10.7326/0003-4819-148-6-200803180-00211. [DOI] [PubMed] [Google Scholar]
- 7.Gahimer J, Wernicke J, Yalcin I, et al. A retrospective pooled analysis of duloxetine safety in 23,983 subjects. Curr Med Res Opin. 2007;23:175. doi: 10.1185/030079906X162719. [DOI] [PubMed] [Google Scholar]
- 8.Kamo I, Torimoto K, Chancellor MB, et al. Urethral closure mechanisms under sneeze-induced stress condition in rats: a new animal model for evaluation of stress urinary incontinence. Am J Physiol Regul Integr Comp Physiol. 2003;285:R356. doi: 10.1152/ajpregu.00010.2003. [DOI] [PubMed] [Google Scholar]
- 9.Miyazato M, Kaiho Y, Kamo I, et al. Effect of duloxetine, a norepinephrine and serotonin reuptake inhibitor, on sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol. 2008;295:F264. doi: 10.1152/ajprenal.90241.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuta A, Asano K, Egawa S, et al. Role of α2-adrenoceptors and glutamate mechanisms in the external urethral sphincter continence reflex in rats. J Urol. 2009;181:19. doi: 10.1016/j.juro.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin AS, Carrier S, Morgan DM, et al. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology. 1998;52:143. doi: 10.1016/s0090-4295(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 12.Kamo I, Kaiho Y, Canon TW, et al. Functional analysis of active urethral closure mechanisms under sneeze induced stress condition in a rat model of birth trauma. J Urol. 2006;176:2711. doi: 10.1016/j.juro.2006.07.139. [DOI] [PubMed] [Google Scholar]
- 13.McClish DK, Wyman JF, Sale PG, et al. Use and costs of incontinence pads in female study volunteers. Continence Program for Women Research Group. J Wound Ostomy Continence Nurs. 1999;26:207–8. 210. [PubMed] [Google Scholar]
- 14.Doran CM, Chiarelli P, Cockburn J. Economic costs of urinary incontinence in community-dwelling Australian women. Med J Aust. 2001;174:456. doi: 10.5694/j.1326-5377.2001.tb143374.x. [DOI] [PubMed] [Google Scholar]
- 15.Turner DA, Shaw C, McGrother CW, et al. The cost of clinically significant urinary storage symptoms for community dwelling adults in the UK. BJU Int. 2004;93:1246. doi: 10.1111/j.1464-410x.2004.04806.x. [DOI] [PubMed] [Google Scholar]
- 16.Morkved S, Bo K. Effect of postpartum pelvic floor muscle training in prevention and treatment of urinary incontinence: a one-year follow up. BJOG. 2000;107:1022. doi: 10.1111/j.1471-0528.2000.tb10407.x. [DOI] [PubMed] [Google Scholar]
- 17.Rezapour M, Ulmsten U. Tension-Free vaginal tape (TVT) in women with recurrent stress urinary incontinence--a long-term follow up. Int Urogynecol J Pelvic Floor Dysfunct 12 Suppl. 2001;2:S9. doi: 10.1007/s001920170004. [DOI] [PubMed] [Google Scholar]
- 18.Groutz A, Gold R, Pauzner D, et al. Tension-free vaginal tape (TVT) for the treatment of occult stress urinary incontinence in women undergoing prolapse repair: a prospective study of 100 consecutive cases. Neurourol Urodyn. 2004;23:632. doi: 10.1002/nau.20068. [DOI] [PubMed] [Google Scholar]
- 19.Gerten KA, Markland AD, Lloyd LK, et al. Prolapse and incontinence surgery in older women. J Urol. 2008;179:2111. doi: 10.1016/j.juro.2008.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon D, Gold R, Pauzner D, et al. Tension-free vaginal tape in the elderly: is it a safe procedure? Urology. 2005;65:479. doi: 10.1016/j.urology.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 21.Dugan SE, Fuller MA. Duloxetine: a dual reuptake inhibitor. Ann Pharmacother. 2004;38:2078. doi: 10.1345/aph.1E084. [DOI] [PubMed] [Google Scholar]
- 22.Detke MJ, Wiltse CG, Mallinckrodt CH, et al. Duloxetine in the acute and long-term treatment of major depressive disorder: a placebo- and paroxetine-controlled trial. Eur Neuropsychopharmacol. 2004;14:457. doi: 10.1016/j.euroneuro.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Voelker R. International group seeks to dispel incontinence “taboo”. JAMA. 1998;280:951. doi: 10.1001/jama.280.11.951. [DOI] [PubMed] [Google Scholar]
- 24.Norton PA, Zinner NR, Yalcin I, et al. Duloxetine versus placebo in the treatment of stress urinary incontinence. Am J Obstet Gynecol. 2002;187:40. doi: 10.1067/mob.2002.124840. [DOI] [PubMed] [Google Scholar]
- 25.Dmochowski RR, Miklos JR, Norton PA, et al. Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J Urol. 2003;170:1259. doi: 10.1097/01.ju.0000080708.87092.cc. [DOI] [PubMed] [Google Scholar]
- 26.Duckett J. Duloxetine as a treatment for stress incontinence--where are we now? Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1. doi: 10.1007/s00192-007-0463-0. [DOI] [PubMed] [Google Scholar]
- 27.Thor KB, Katofiasc MA. Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J Pharmacol Exp Ther. 1995;274:1014. [PubMed] [Google Scholar]
- 28.Kaiho Y, Kamo I, Chancellor MB, et al. Role of noradrenergic pathways in sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol. 2007;292:F639. doi: 10.1152/ajprenal.00226.2006. [DOI] [PubMed] [Google Scholar]
- 29.Danuser H, Bemis K, Thor KB. Pharmacological analysis of the noradrenergic control of central sympathetic and somatic reflexes controlling the lower urinary tract in the anesthetized cat. J Pharmacol Exp Ther. 1995;274:820. [PubMed] [Google Scholar]
- 30.Piletz JE, Zhu H, Chikkala DN. Comparison of ligand binding affinities at human I1-imidazoline binding sites and the high affinity state of alpha-2 adrenoceptor subtypes. J Pharmacol Exp Ther. 1996;279:694. [PubMed] [Google Scholar]