Abstract
The clinical manufacture of antigen-specific cytotoxic T lymphocytes (CTL) for adoptive immunotherapy is limited by the complexity and time required to produce large numbers with the desired function and specificity. The culture conditions required are rigorous, and in some cases only achieved in 2cm2 wells in which cell growth is limited by gas exchange, nutrients and waste accumulation. Bioreactors developed to overcome these issues tend to be complex, expensive and not always conducive to CTL growth.
We observed that antigen-specific CTL undergo seven to ten divisions post-stimulation. However the expected CTL numbers were achieved only in the first week of culture. By recreating the culture conditions present during this first week - low frequency of antigen-specific T-cells and high frequency of feeder cells - we were able to increase CTL expansion to expected levels which could be sustained for several weeks without affecting phenotype or function. However, the number of 24-well plates needed was excessive and cultures required frequent media changes, increasing complexity and manufacturing costs. Therefore, we evaluated novel gas-permeable culture devices (G-Rex) with a silicone membrane at the base allowing gas exchange to occur uninhibited by depth of medium above. This system effectively supports the expansion of CTL and actually increases output by up to 20-fold while decreasing required technician time. Importantly, this amplified cell expansion is not due to more cell divisions but to reduced cell death. This bioprocess optimization increased T-cell output while decreasing the complexity and cost of CTL manufacture, making cell therapy more accessible.
INTRODUCTION
Infusion of antigen-specific cytotoxic T lymphocytes (CTLs) has proven safe and apparently effective for the prophylaxis and treatment of infections with cytomegalovirus (CMV), adenovirus (Adv), and Epstein-Barr virus (EBV) in transplant recipients(14;17;23;27;30;40). CTLs have also induced objective tumor responses and complete remissions in patients with advanced lymphoma, melanoma and nasopharyngeal carcinoma(6;7;10;12;13;26;32;35;41). The potential of T-cell therapy for cancer may be further improved through genetic modification to confer antigen specificity with recombinant T-cell receptors (TCRs) or chimeric antigen receptors (CARs), or by improving their homing and proliferative properties or their resistance to tumor immune evasion strategies(1;2;8;11;24;28;29;33;34;38;39).
Although promising, most current protocols for the activation and expansion of antigen-specific CTL ex vivo are complicated and labor intensive, limiting the broad application of this therapy. These problems could be overcome by optimization of conventional in vitro T-cell production to accelerate expansion and minimize cell handling, while ensuring that T effector functions are maintained, thereby increasing the general enthusiasm for, and feasibility of, antigen-specific T cell therapies.
Cell proliferation in culture is limited by requirements for nutrients and oxygen (O2), and by accumulation of waste products such as carbon dioxide (CO2) and lactic acid(19). The volume of medium used in conventional culture is restricted to the depth that allows sufficient O2 diffusion from the medium surface to the cells growing at the base of the vessel. Both O2 and nutrient requirements increase with cell concentration and rate of growth, so that cultures must be fed and split regularly. These frequent medium changes and cell manipulations are time consuming, expensive, reduce the reproducibility of the production process and the consistency of the resultant T-cell product, and increase the risk of contamination.
One way of overcoming these limitations is to use bioreactors that provide mechanical rocking or stirring, as well as medium and gas perfusion, thereby increasing the rate of cell expansion and maximum achievable cell density(5;9;15;18;20;21;36;37). These machines are expensive, complex and bulky however, so that the number of cultures that can be maintained in parallel is limited by space and availability. Further, although antigen non-specific T-cell cultures have been grown with great success in these bioreactors(18;36), antigen-specific CTL have strict requirements for cell-to-cell contact and have proved difficult to consistently adapt to moving cultures, since production of functional cells occurs best under static culture conditions.
Many groups, including our own, have found that optimal expansion of antigen-specific CTL lines occurs in the 2 cm2 wells of standard tissue culture-treated 24-well plates(25;30), in which the volume of medium in the wells is restricted by gas diffusion to 1mL/cm2. This volume in turn limits the supply of nutrients, which are rapidly consumed by proliferating T-cells. Consequent acidic pH and waste build-up rapidly impedes cell growth and survival, so that the maximum cell density that can be achieved is about 2 × 106 per cm2. Since the minimum seeding density is around 2.5 × 105 T-cells per cm2, the maximum weekly cell expansion is about 8 fold. Continued expansion of CTLs requires weekly re-seeding with antigenic restimulation, and twice weekly exchanges of medium and growth factors. Because the rate of expansion is slow, these manipulations must be repeated over a 4–8 week propagation period to obtain sufficient numbers for cell infusions, and sterility, identity and potency assays(16;23;30;31).
To maintain the desirable static culture conditions needed for antigen-specific CTL expansion whilst overcoming the obstacles of limited gas and nutrient supplies, we have evaluated a novel gas permeable rapid expansion cultureware (G-Rex) system in which O2 and CO2 are exchanged across a silicone membrane at the base of the flask(3). Gas exchange from below allows an increased depth of medium above, providing more nutrients and diluting waste. We now show that the G-Rex device supports the expansion of antigen-specific CTLs, as well as genetically modified T-cells and a range of suspension cell lines, without significantly altering cell phenotype or function. The system is scalable and suited to GMP-applications, and reduces the number of technician interventions approximately 4-fold while increasing the cell output by at least 3- and up to 20-fold when compared with conventional methods. Since these benefits are predominantly mediated by improved cell survival, the device decreases the number of cell divisions required to achieve a given cell number, an important consideration for the expansion and long term persistence of adoptively-transferred T cells(4).
MATERIALS AND METHODS
Generation of EBV-transformed B cell lines
After consent, we obtained peripheral blood from healthy donors. Then 5×106 peripheral blood mononuclear cells (PBMC) were infected with concentrated B95-8 EBV supernatant in the presence of cyclosporin A (Sandoz, Broomfield, CO) to establish an EBV-LCL(30).
EBV-CTL generation
Conventional CTL generation protocol (24 well plates- 2cm2 surface area and 2 mL volume)
Day 0
CTLs were initiated by co-culturing PBMCs from normal donors (1×106/mL) with gamma-irradiated (40 Gy) autologous EBV-LCLs at a 40:1 ratio (PBMC:LCLs) in a total volume/well of 2ml CTL medium [RPMI 1640 supplemented with 45% Click medium (Irvine Scientific, Santa Ana, CA), 2 mM GlutaMAX-I, and 10% FBS].
Day 9–12
CTLs were harvested, resuspended in fresh medium at 0.5 × 106 CTL per mL and restimulated with irradiated autologous EBV-LCLs at a ratio 4:1 (1×106:2.5×105 - CTL:LCL).
Day 13–16
CTL were fed with 1 ml of fresh medium containing recombinant human IL-2 (IL-2) (50 U/mL) (Proleukin; Chiron, Emeryville, CA)
Subsequent stimulations
CTL were restimulated weekly using a 4:1 CTL:LCL ratio with twice weekly addition of IL2.
Conventional CTL generation protocol (G-Rex)
Day 0
CTL were initiated by co-culturing 1 × 107 PBMC, (1×106/cm2), with gamma-irradiated (40 Gy) EBV-LCLs at a 40:1 PBMC to LCL ratio in a final volume of 30 ml of CTL medium in the G-Rex40 (10 cm2).
Day 9–12
The second stimulation was performed by removing 15 mL of medium, counting the T cells and adding 15ml of fresh CTL medium containing irradiated EBV-LCLs, resuspended in at an appropriate concentration to stimulate T cells at a ratio 4:1.
Day 13–16
IL2 (50U/ml – final conc.) was added it directly to the culture
Subsequent stimulations
Once the cells had expanded to a density of >5×106 per cm2 they were transferred to a G-Rex500 and stimulated with irradiated EBV-LCL (4:1) in a volume of 200ml CTL medium containing IL2 (50U/ml). Antigen stimulation was performed every 7 days thereafter using a 4:1 CTL:LCL ratio and cells were plated in 200ml of CTL medium and the cultures were supplemented twice weekly with 50U/ml of IL-2. This was repeated until the culture reach about 7×108 cells per G-Rex500. After this, they could be split among G-Rex500s at 5 × 107 T cells per G-Rex500 until sufficient T-cells were obtained.
Modifications to optimize this procedure were made as indicated in the results section and figure legends.
Cell lines and tumor cells
BJAB and K562 were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). All cells were maintained in culture with RPMI 1640 medium (GIBCO-BRL, Gaithersburg, MD) containing 10% heat-inactivated fetal calf serum (FCS), 2 mM L-glutamine, 25 IU/mL penicillin, and 25 mg/mL streptomycin (all from BioWhittaker, Walkersville, MD). Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Immunophenotyping
Cell surface
Cells were stained with Phycoerythrin (PE), fluorescein isothiocyanate (FITC), periodin chlorophyll protein (PerCP) and allophycocyanin (APC)-conjugated monoclonal antibodies (MAbs) to CD3, CD4, CD8, CD56, CD16, CD62L, CD45RO, CD45RA, CD27, CD28, CD25, CD44 from Becton-Dickinson (Mountain View, CA, USA). PE-conjugated tetramers (Baylor College of Medicine) and APC-conjugated pentamers (Proimmune Ltd, Oxford, UK), were used to quantify EBV-CTL precursor frequencies(23). For cell surface and pentamer staining 10,000 and 100,000 live events, respectively, were acquired on a FACSCalibur flow cytometer and the data analyzed using Cell Quest software (Becton Dickinson).
Carboxy-fluorescein diacetate, succinimidyl ester (CFSE) labeling
2 × 107 PBMC or EBV-specific CTLs (EBV-CTLs) were washed twice and resuspended in 850µl 1× phosphate-buffered saline (PBS) containing 0.1% Fetal Bovine Serum (FBS) (Sigma-Aldrich). Prior to staining, an aliquot of CFSE (10mM in dimethyl sulfoxide) (Celltracetm CFSE cell proliferation kit (C34554) Invitrogen) was thawed, diluted 1:1000 with 1× PBS and 150µl of the dilution was added to the cell suspension (labeling concentration was 1µM). Cells were incubated with CFSE for 10 minutes at room temperature. Subsequently 1ml FBS was added to the cell suspension followed by a 10 min incubation at 37°C. Afterwards cells were washed twice with 1× PBS, counted, and stimulated with antigen as described.
AnnexinV-7-AAD staining
To determine the percentage of apoptotic and necrotic cells in our cultures we performed Annexin-7-AAD staining as per manufacturers’ instructions (BD Pharmingentm #559763, San Diego, CA). Briefly, EBV-CTL from the 24-well plates and the G-Rex were washed with cold PBS, resuspended in 1× Binding Buffer at a concentration of 1 × 106 cells/ml, stained with Annexin V-PE and 7-AAD for 15 min at RT (25°C) in the dark. Following the incubation the cells were analyzed immediately by flow cytometry.
Chromium release assay
We evaluated the cytotoxic activity of EBV-CTLs in standard 4-hour 51Cr release assay, as previously described(31;38). As target cells we used autologous and HLA class I and II mismatched EBV-transformed lymphoblastoid cell line (EBV-LCL) to measure MHC restricted and unrestricted killing, as well as the K562 cell line to measure natural killer activity. Chromium-labeled target cells incubated in medium alone or in 1% Triton X-100 were used to determine spontaneous and maximum 51Cr release, respectively. The mean percentage of specific lysis of triplicate wells was calculated as follows: [(test counts – spontaneous counts)/(maximum counts – spontaneous counts)] × 100.
Enzyme-Linked Immunospot (ELIspot) assay
ELIspot analysis was used to quantitate the frequency and function of T cells that secreted IFNγ in response antigen stimulation(22;23). CTL lines expanded in 24 well plates or in the G-Rex were stimulated with irradiated LCL (40Gy) or LMP1, LMP2, BZLF1 and EBNA1 pepmixes (diluted to 1µg/ml) (JPT Technologies GmbH, Berlin, Germany), or EBV peptides HLA-A2 GLCTLVAML=GLC, HLA-A2 CLGGLLTMV=CLG, HLA-A2-FLYALALLL = FLY, and HLA-A29 ILLARLFLY=ILL (Genemed Synthesis, Inc. San Antonio, Texas), diluted to a final concentration of 2µM, and CTLs alone served as a negative controls. CTLs were resuspended at 1×106/ml in ELIspot medium [(RPMI 1640 (Hyclone, Logan, UT) supplemented with 5% Human Serum (Valley Biomedical, Inc., Winchester, Virginia) and 2-mM L-glutamine (GlutaMAX-I, Invitrogen, Carlsbad, CA)].
Ninety-six-well filtration plates (MultiScreen, #MAHAS4510, Millipore, Bedford, MA) were coated with 10µg/mL anti-IFN-γ antibody (Catcher-mAB91-DIK, Mabtech, Cincinnati, OH) overnight at 4°C, then washed and blocked with ELIspot medium for 1 hour at 37°C. Responder and stimulator cells were incubated on the plates for 20 hours, then the plates were washed and incubated with the secondary biotin conjugated anti-IFN-γ monoclonal antibody (Detector-mAB (7-B6-1-Biotin), Mabtech) followed by incubation with Avidin:biotinylated horseradish peroxidase complex (Vectastain Elite ABC Kit (Standard), #PK6100, Vector Laboratories, Burlingame, CA) and then developed with AEC substrate (Sigma, St. Louis, MO). Each culture condition was run in triplicate. Plates were sent for evaluation to Zellnet Consulting, New York, NY. Spot-forming cells (SFC) and input cell numbers were plotted.
Retrovirus production and transduction of T-lymphocytes
Retroviral supernatant was produced and T cells transduced as previously described(33;38). CAR expression on T-cells was measured 72 hours post-transduction and the cells maintained in culture in complete medium with the addition of rIL-2 (50 U/mL) every 3 days.
Statistical analysis
All in vitro data are presented as mean ± 1 SD. Student’s t test was used to compare the difference between two groups after appropriate log-transformation. A p-value less than 0.05 was accepted as indicating a significant difference.
RESULTS
Antigen-specific CTL expand inefficiently using conventional cell culture conditions
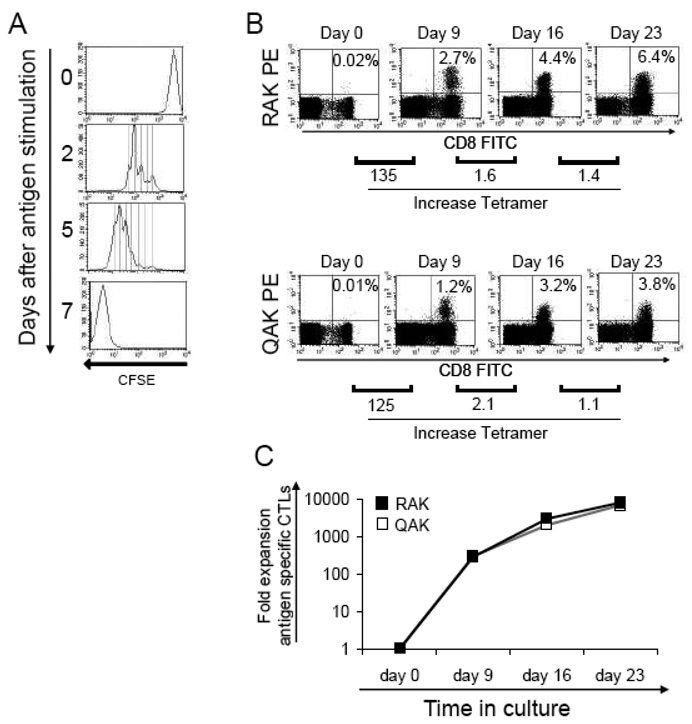
Antigen-specific CTL are traditionally activated and expanded by coculture with antigen presenting cells (APCs) in standard tissue-culture treated 24-well plates at fixed T-cell:APC ratios. For example, to activate and expand EBV-CTLs, PBMCs are stimulated with irradiated, autologous EBV-LCL at a responder to stimulator (R:S) ratio of 40:1. On day 9 and weekly thereafter, T-cells are restimulated with the EBV-LCLs at an R:S ratio of 4:1 with twice weekly medium change with the addition of IL-2 from day 14. Under these culture conditions, antigen-specific T-cells should undergo at least 7 cell doublings after each stimulation, as shown in Figure 1a. Thus we expect a weekly T-cell expansion of 128-fold (as measured by the frequency of antigen-specific T-cells × # of total cells). To test this assumption using EBV-CTLs as our model, we stimulated PBMC from EBV seropositive donors with autologous EBV-LCL and measured the frequency of tetramer positive cells after the first, second, and third stimulations. A representative experiment is shown in Figure 1b. On day 0 the frequency of T-cells reactive against two EBV tetramers, RAK and QAK was 0.02% and 0.01%, respectively. After a single stimulation we observed a 2.2 fold increase in total cell numbers (data not shown) on day 9, while the frequency of tetramer-positive T-cells had increased to 2.7% and 1.5%, respectively, representing a 135- and 125-fold increase in the frequency of antigen-specific tetramer positive T cells. Thus, the fold expansion of the antigen-specific components was around 280 during the first stimulation, as shown in Figure 1c. Unfortunately, however, this rate of antigen-specific T-cell expansion was not sustained during the 2nd and subsequent stimulations, after which the fold expansion of antigen-specific CTL was <5, even though the same number of cell doublings was observed during the first and subsequent weeks of culture, as measured by CSFE analysis. Table 1 illustrates the discrepancy between the expected and observed fold expansion of antigen-specific CTL (n = 3).
Figure 1. Expansion of antigen specific CTLs during the first week of culture in 24 well plates.
Panel A demonstrates that CTLs labeled with CFSE undergo for at least 7 doublings after antigenic stimulation, and this doubling rate was preserved through multiples rounds of stimulation. Panel B shows the enrichment of antigen-specific cells during the first week of culture, as demonstrated by pentamer analysis using the EBV-specific HLA-B8 restricted RAK and QAK pentamers. The increase in the frequency of antigen-specific cells was superior during the first week of culture (from day 0 to 9) that in the subsequent weeks (day 16 and Day 23). Fold increase in pentamer-positive cells is shown below the dot plots. This antigen-specific CTL enrichment, which preferentially occurs during the first week of culture, is reflected in the overall superior expansion of antigen-specific cells during the first week, as presented in Panel C.
Table 1.
| Cell doubling | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Expected fold expansion | 2 | 4 | 8 | 16 | 32 | 64 | 128 |
| Observed fold expansion (day 0–9) | 258 range (48 to 409) | ||||||
| Observed fold expansion (day 9–16) | 5.7 range (2.2 to 10.6) | ||||||
| Observed fold expansion (day 16–23) | 4.3 range (4.1 to 14.9) |
Minimal cell density promotes cell expansion
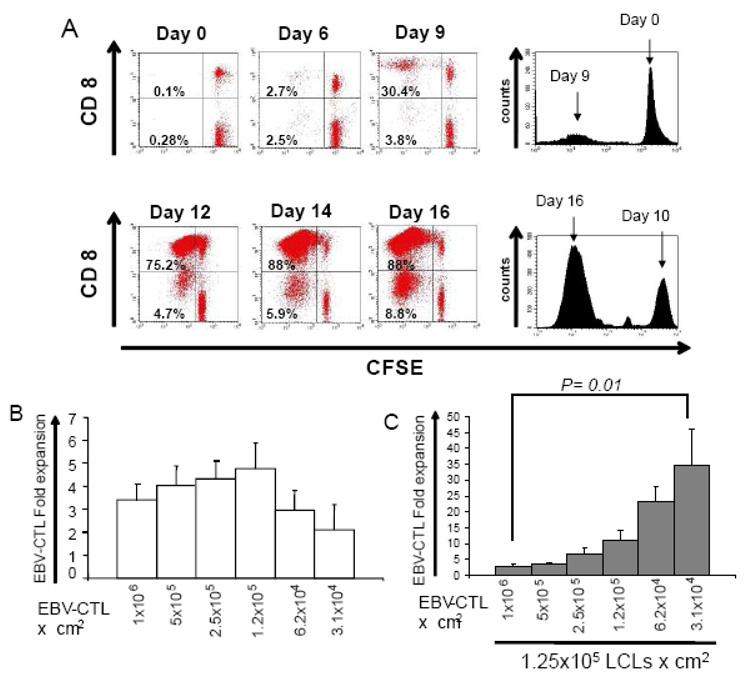
We hypothesized that the decreased cell numbers obtained following the second T-cell stimulation compared to the first stimulation was due either to the increased susceptibility of activated T-cells to activation induced cell death (AICD) or to limiting cell culture conditions. For example, at the first stimulation, the EBV antigen-specific T-cell component of PBMCs represents, at most, 2% of the population and so the antigen-specific responder T-cell seeding density is less than 2 × 104 per cm2, with the remaining PBMCs acting as non-proliferating feeder cells (seen as the CFSE positive cells in Figure 2a) that sustain optimal cell-to-cell contact and antigen-specific CTL expansion (Figure 2a – top panel). By contrast, at the second stimulation on day 9, the majority of T-cells are antigen-specific, resulting in a much higher seeding density (up to 5 × 105 cells per cm2) of responder cells. As a consequence, on restimulation the majority of cells proliferate (Figure 2a – bottom panel), and may therefore rapidly consume and exhaust their nutrients and O2 supply.
Figure 2. Superior antigen-specific CTL expansion can be optimized by modifying the culture composition.
As illustrated in panel A – top, during the first week of culture only a small fraction of the PBMCs labeled with CFSE proliferate after antigen stimulation while the majority of cells, which are not antigen-specific, act as a feeder cell layer. In contrast, during subsequent stimulations the majority of the cells in the culture are antigen-specific and proliferate following antigenic stimulation (panel A – bottom). Panel B, shows the proliferation of established EBV-CTLs can be affected by decreasing the traditional seeding cell density (1×106 CTL) to 1.2×105 CTL, while conserving the R:S (CTL:LCL) ratio of 4:1. However by increasing the density of non-proliferating feeder cells (irradiated LCL), the fold expansion of EBV-CTL can be dramatically increased by decreasing the initial seeding effector cell density to as low as 3.1×104 Panel C.
To determine if limiting culture conditions were responsible for sub-optimal T cell growth rates, we measured the expansion of activated T-cells plated at lower cell densities. We seeded activated EBV-CTL in 2 cm2 wells at doubling dilutions from 1×106 to 3.1×104 per cm2 while maintaining an R:S ratio of 4:1 (Figure 2b). The maximum CTL expansion (4.7±1.1 fold) was achieved with a starting CTL density of 1.25×105 per cm2, but further dilution decreased the rate of expansion (Figure 2b). Since this limiting dilution effect was likely due to lack of cell-to-cell contact, we cultured doubling dilutions of EBV-CTL from 1×106 to 3.1×104 with a fixed number of feeder cells (EBV-LCL plated at 1.25×105 per cm2) and assessed cell expansion over a 7 day period. We observed a significant increase in CTL expansion from 2.9±0.8 fold using the conventional cell numbers (1×106 CTL:1.25×105 LCL) to 34.7±11 fold expansion when 3.1×104 CTL/cm2 were incubated with 1.25×105 LCL/cm2, as presented in Figure 2c (p=0.01). Importantly, this modification of the culture conditions did not change the function or antigen specificity of the cells (See supplementary figure, Supplemental Digital content 1 http://links.lww.com/JIT/A29). Activated antigen-specific T cells are therefore potentially capable of greater expansion than we were able to achieve using our traditional culture conditions. Of note, the maximum cell number achieved after stimulation (1.7 to 2.5 × 106/cm2) was the same regardless of the starting cell density, suggesting that cell density is the limiting factor for cell growth.
Gas Permeable Rapid Expansion Cultureware (G-Rex) to promote gas exchange and decrease medium limitations
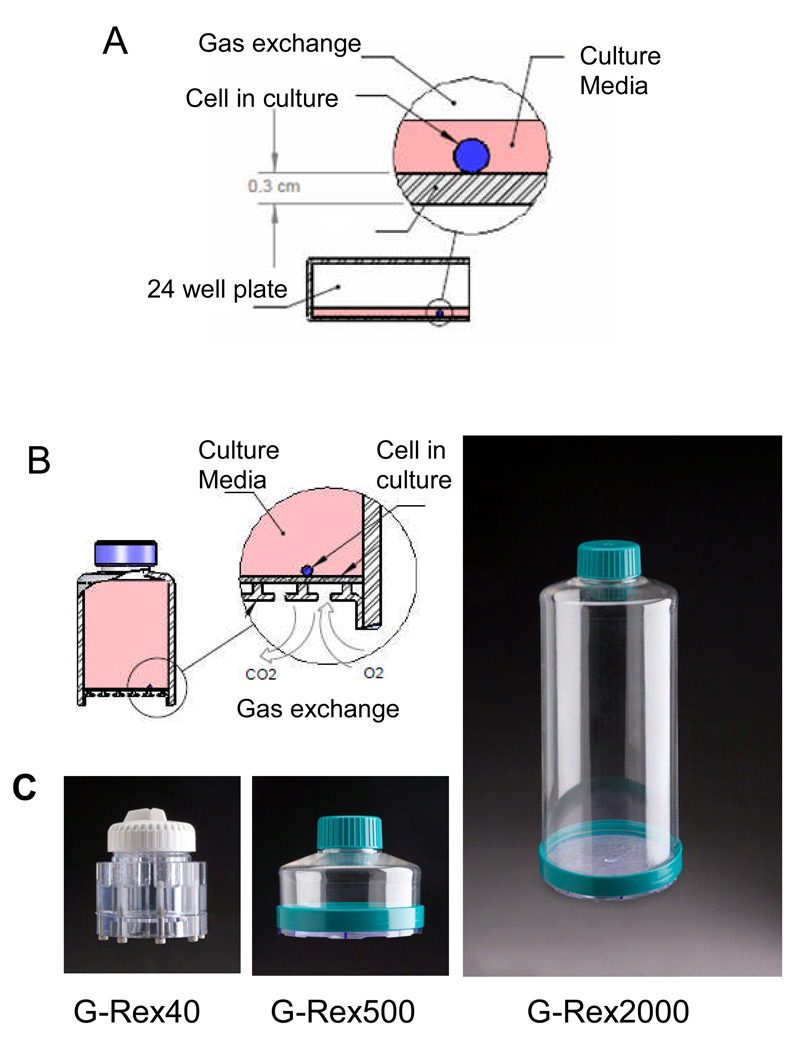
Although improved expansion (34 fold) of antigen-specific CTL can be obtained in tissue culture wells as described above, the number of wells required to support such expansion is excessive. For example 1 × 107 T-cells cultured in tissue culture wells at 3.1 × 104 per cm2 could produce circa 3.5 × 108 CTL in 1 to 2 weeks, but would require 161 wells of a 24-well plate (>6 plates), making this protocol impractical for routine use or for scale up, due to the technician time and culture manipulations required. To determine if the maximum T-cell cell density per cm2 could be increased, we evaluated novel gas permeable rapid expansion cultureware (G-Rex), developed by Wilson Wolf Manufacturing (New Brighton, MN). In conventional cultureware, gas exchange occurs across the surface of the medium, limiting medium depth to a maximum of only 1ml/cm2, (Figure 3a). This restricted medium-to-surface area ratio limits nutrient and growth factors and facilitates the rapid build-up of metabolites such as CO2 and lactic acid that increase the acidity of the culture. By contrast, in the G-Rex, O2 and CO2 are exchanged across a silicone membrane at the base of the device, removing the limitation of medium depth and allowing up to a 20-fold higher medium volume per unit area than in conventional cultureware. A schematic of the G-Rex flask is shown in Figure 3b. We evaluated three different flask sizes with surface areas and maximum media volumes of 10cm2 (40 mL or 4mL per cm2) for the G-Rex40, 100cm2 (500 mL or 5mL per cm2) for the G-Rex 500, and 100cm2 (2000 mL or 20mL per cm2) - G-Rex2000, respectively (Figure 3c).
Figure 3. G-Rex overcomes the limited volume of medium per surface area in conventional cultureware.
Panel A, illustrates that gas exchange in traditional cultureware is restricted to the surface area above the culture, which limits the depth of media. Panel B, shows a blueprint of the G-Rex showing that gas exchange occurs from the bottom of the device which allows for an increase in the volume of the media per surface area. Panel C, shows a photograph of the G-Rex 40, G-Rex 500 and G-Rex 2000.
T-cells cultured in the G-Rex have increased viability and expansion
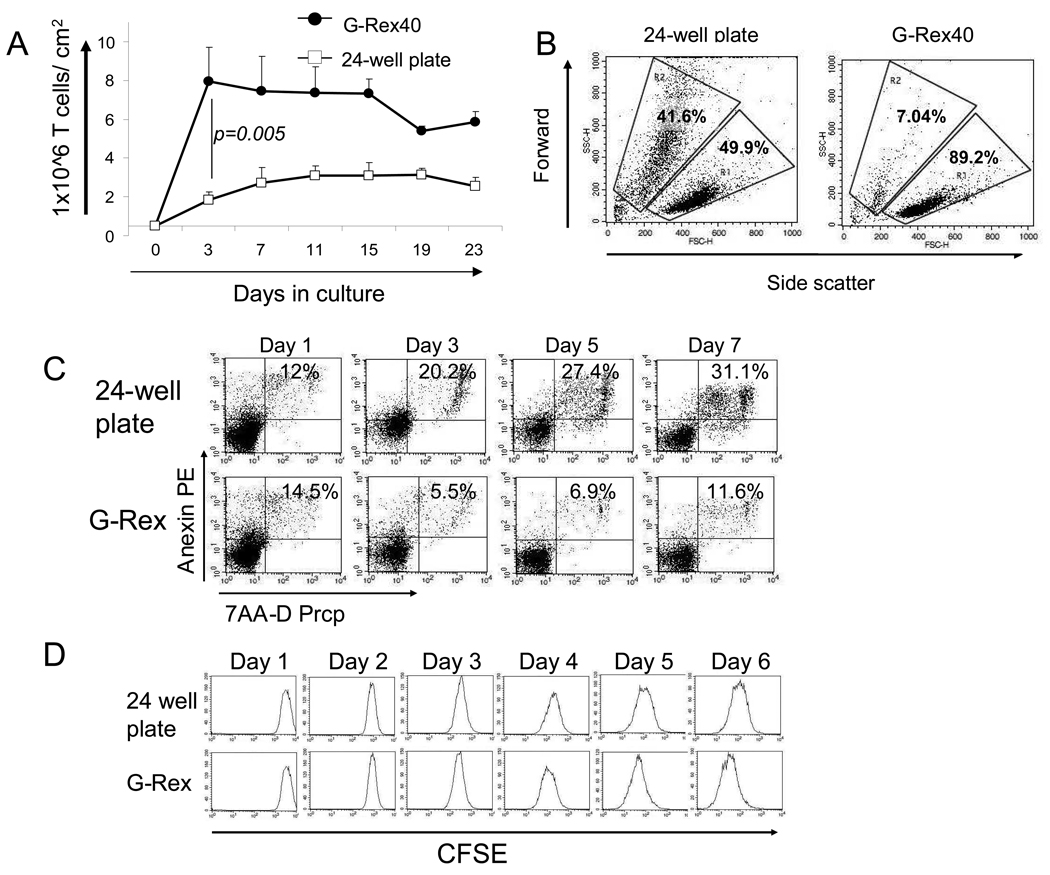
To determine if the maximum achievable cell density could be increased in G-Rex devices we cocultured activated EBV-CTL with autologous EBV-LCLs at the conventional 4:1 ratio of T-cell:LCL. CTL were seeded at 5×105 cells/cm2 in the G-Rex40 and expansion was compared with CTL seeded at the same density in a 24-well plate. After 3 days, the cell numbers in the G-Rex40 had increased from 5×105/cm2 to a median of 7.9×106/cm2 (range 5.7 to 8.1×106/cm2) without any medium exchange. This number further increased to 9.5×108 cells/cm2 (range 8.5 ×108 to 11.0 ×108/cm2) after replenishing the medium and IL-2 on day 7. In contrast, cells cultured for 3 days in conventional 24-well plates increased from 5×105/cm2 to a median of only 1.8×106/cm2 (range 1.7 to 2.5×106/cm2) by day 3 (Figure 4a) (p=0.005); cell density/numbers were not further increased by replenishing medium or IL-2. The G-Rex can also be used to culture virtually any human suspension cells that we evaluated, including activated T cells gene modified to express CAR molecules, EBV-LCLs and other hematopoietic cell lines as shown in (See Supplemental Figures 2 and 3, Supplemental Digital content 2 http://links.lww.com/JIT/A30 Supplemental Digital content 3 http://links.lww.com/JIT/A313).
Figure 4. The G-Rex increases the cell output by improving the viability and the cell density.
Panel A – Increased numbers of EBV-CTL per surface area can be attained in the G-Rex (solid circles) when compared with 24-well plates (open squares). This increase in the number of cells was achieved by an improvement in the cell viability as shown by the forward vs side scatter dot plot on day 7 and confirmed by daily Annexin-PI 7AAD flow cytometric analysis Panel B and C. The number of cell divisions in the cultures were evaluated by daily analysis of T cells labeled with CFSE as presented in Panel D.
To understand the mechanism behind the superior cell expansion in the G-Rex device, we assessed the viability of OKT3-stimulated peripheral blood T cells using flow cytometric forward vs side scatter analysis on day 5 of culture. EBV-CTLs could not be assessed in this assay due the presence of residual irradiated EBV-LCL in the cultures which would interfere with the analysis. As shown in Figure 4b cell viability was significantly higher than in the G-Rex40 cultures (49.9% viability vs 89.2% viability). We then analyzed the cultures daily for 7 days using Annexin-PI 7AAD to distinguish between live and apoptotic/necrotic cells, and observed consistently lower viability in T-cells expanded in 24 well plates (Figure 4c – top panel) compared to those in the G-Rex (Figure 4c – lower panel). These data suggest that the cumulative improved survival of proliferating cells contributed to the increased cell numbers in the G-Rex devices compared to the plates.
To determine if there was also a contribution from an increased number of cell divisions in the G-Rex versus the plates, T-cells were labeled with CFSE on day 0 and divided between a 40mL device and a 24 well plate. Daily flow cytometric analysis demonstrated no differences in the number of cell divisions from day 1 to day 3. From day 3 onwards, however, cells cultured in the G-Rex continued to divide, whereas cell divisions were reduced in the 2 mL wells, suggesting that the culture conditions had become limiting (Figure 4d). Thus, enhanced cell numbers in the G-Rex resulted from a combination of decreased cell death and prolonged proliferation.
CTL stimulation using optimized seeding cell density in the G-Rex induces maximum expansion of antigen-specific CTLs
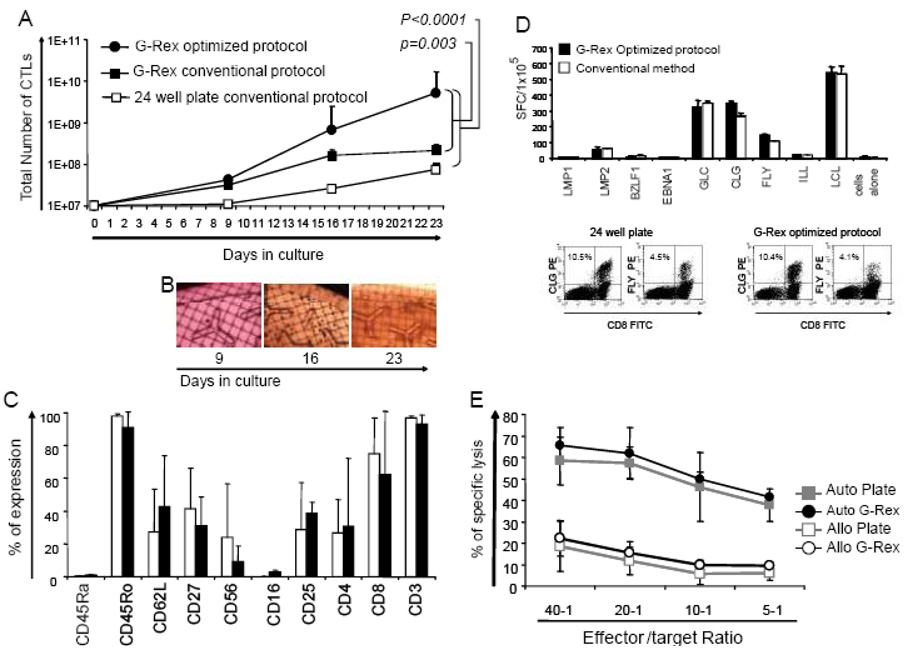
To simplify and shorten the manufacture of EBV-CTLs for clinical use, we established an optimized standard operating procedure (SOP) using the G-Rex for the initiation and expansion of antigen-specific T-cells. For EBV-CTL initiation we seeded PBMCs in the G-Rex40 at 1×106/cm2 (total = 107 PBMCs) and stimulated them with EBV-LCL using a 40:1 ratio of PBMC:EBV-LCL; This 40:1 ratio is critical in the first stimulation to maintain the antigen-specificity of the responder T-cells. However, on day 9, 1×107 responder T-cells were transferred to the G-Rex500 (1×105/cm2) with 5×107 LCL (5 × 105 per cm2) (1:5 ration of T-cells to LCLs) in a total of 200ml CTL medium. This seeding density produced consistent CTL expansion in all donors screened and simplified cell calculations for GMP staff. Four days later (day 13), IL-2 (50U/ml - final concentration) was added directly to the culture without medium change, and on day 16, the cells were harvested and counted. The median number of EBV-CTL obtained was 6.5×108 (range 2.4×108 to 3.5×109), which was approximately 4-fold more cells than would be achieved using our standard 4:1 ratio of T-cells:APCs in the G-Rex and 26 times more cells than in 24 well plates (our current standard protocol) (Figure 5a). The T-cells continued to divide until day 27–30 without requiring additional stimulation provided the cultures were split when cell density was >7×108, producing a median of 5.07×109 cells (range 1.1×109 to 2.5×1010), generating 23.7 fold and 68.4 fold more EBV-specific cells than the G-Rex with the conventional R:S ratio (p=0.003) and the 24 well plates (p<0.0001), respectively (Figure 5a).
Figure 5. The use of an optimized APC:CTL ratio in the G-Rex dramatically improves the cell output without modifying CTL phenotype or function.
Panel A shows the expansion of EBV-CTL using the conventional APC:CTL ratio and cell density in 24-well plates (open squares) in comparison with EBV-CTLs expanded in the G-Rex with the conventional and optimized APC:CTL ratio (solid squares and solid circles, respectively). Panel B shows the CTL growth over time in the G-Rex, as evaluated by microscopy. Panel C, shows the phenotypic comparison of EBV-CTLs cultured with the conventional method or with the optimized APC:CTLs ratio in the G-Rex. The antigen specificity of the expanded cells was evaluated by ELIspot and pentamer analysis and cytotoxicity assay, and a representative example is shown in Panel D and E.
Although in the G-Rex growing CTLs cannot be viewed clearly using light microscopy, clusters of CTLs can be visualized by eye or by inverted microscope and the appearance of the cells on days 9, 16, and 23 of culture is shown in Figure 5b. Culture in the G-Rex did not change the phenotype of the expanded cells (Figure 5c), with >90% CD3+ cells (96.7±1.7 vs 92.8±5.6; GPC vs 24-well), which were predominantly CD8+ (62.2% ± 38.3 vs 75% ± 21.7). Evaluation of the activation markers CD25 and CD27, and the memory markers CD45RO, CD45RA, and CD62L, demonstrated no substantive differences between CTL expanded under each culture condition. The antigen specificity was also unaffected by the culture conditions, as measured by ELIspot and pentamer analysis. Figure 5D shows a representative culture in which T-cells stimulated with EBV peptide epitopes from LMP1, LMP2, BZLF1 and EBNA1 and stained with HLA-A2-LMP2 peptide pentamers staining showed similar frequencies of peptide-specific T-cells. Further, the expanded cells maintained their cytolytic activity and specificity and killed autologous EBV-LCL (62% ± 12 vs 57% ± 8 at a 20:1 E:T ratio; G-Rex vs 24-well plate), with low killing of the HLA mismatched EBV-LCL (15% ± 5 vs 12% ± 7 20:1 ratio) as evaluated by 51Cr release assays (Figure 5E).
The G-Rex reduces the time, complexity and cost associated with CTL production for clinical use
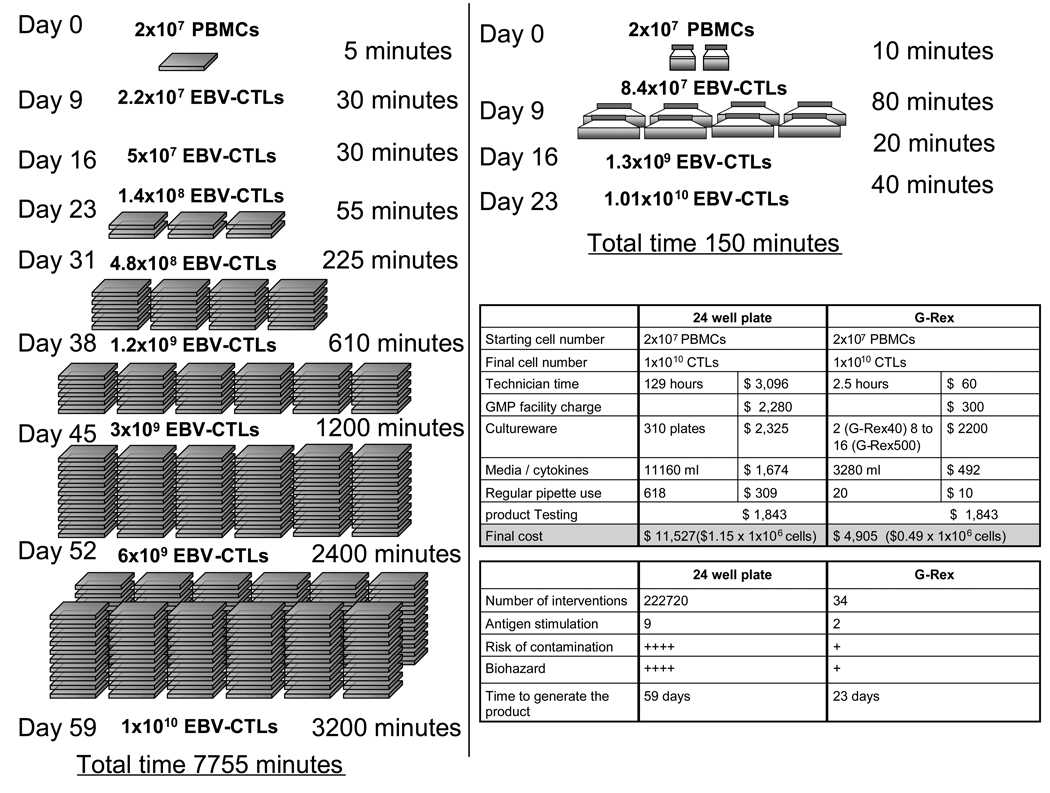
The broader implementation of T cell immunotherapy is limited by (i) the cost of production; (ii) the complexity of production, including repeat feeding of open culture systems, and multiple skilled “judgment calls”, thereby limiting scalability, and (iii) the time required for activation and expansion. Figure 6 shows the effects of the G-Rex on these obstacles. The “hands on” time for CTL manufacture is reduced because fewer cell manipulations are required, which consequently reduces the labor costs, decreases the complexity of manufacture and diminishes the risk of contamination. The duration of CTL manufacture is also reduced, by the increased rate of expansion. Hence production of 1×1010 CTLs, which would typically require approx. 60 days and 129 hours of technician time by conventional culture, is reduced to 23 days with 3 hours of labor and a reduction in the number of interventions from >200,000 to just 34, translating to a saving of >50% in CTL production costs (Figure 6).
Figure 6. Bioprocess optimization for CTLs production decrease the complexity, risk of contamination and cost of manufacture.
The conventional method of CTL culture using 24-well plates is convoluted, demanding frequent manipulation to sustain culture growth. This increases the manufacture cost and risk of contamination. In contrast, CTL culture using the optimized APC:CTL ratio in the G-Rex can produce the same number of cells in a shorter period of time thus decreasing the cost and complexity of manufacture.
DISCUSSION
We have described an improved manufacturing system for producing EBV-CTL, using optimized cell seeding densities and a novel gas permeable rapid expansion cultureware (G-Rex) to support the large-scale production of cells for clinical use. By using cultureware that promotes optimal O2 and CO2 exchange, the initial input volume of medium can be increased, which in turn increases the available nutrients and dilutes waste products without the need for culture agitation (which disrupts antigen-specific CTL expansion), frequent culture feeding, or continuous medium perfusion, facilitating large scale CTL production. The G-Rex can also support the expansion of CTL with other antigen specificities including cytomegalovirus as well as trivirus- (cytomegalovirus, EBV, adenovirus) specific CTLs (data not shown), and can be adapted to the culture of virtually any human suspension cells including gene-modified activated T-cells, EBV-LCLs and other hematopoietic cell lines as shown in Supplementary Figures 2 and 3.
In our initial series of experiments we measured the expansion of EBV-CTLs cultured in wells during the initial and subsequent stimulations using conventional APC:T cell ratios and observed that CTL expansion was far greater during the first stimulation of PBMC than during subsequent stimulations (Figure 1). This difference could not be ascribed to the propensity of activated T-cells to undergo AICD following T cell stimulation, but rather to differences in the composition of the cultures. In PBMCs the frequency of antigen-specific T cells is low (<2% of all T cells), thus activation and subsequent amplification of this small population of cells during the initial stimulation is not limited by the available nutrients and O2 in the 2ml well. However, during subsequent stimulations, the frequency of antigen-specific T-cells greatly increases to form >50% of the bulk population. Thus, upon antigenic restimulation the majority of specific cells are reactivated, and due to their high frequency, rapidly deplete O2 and nutrients to limiting levels, accounting for the high rate of apoptosis seen in the cultures (Figure 4b). By emulating the culture conditions present during the first week, we could successfully increase the expansion of antigen-specific CTLs during subsequent stimulation. This was achieved by simply reducing the seeding density of CTLs while preserving optimal cell-to-cell contact by introducing a constant number of feeder cells into the cell culture wells to support and promote cell expansion in a non-limiting environment.
The above optimization approach using 2 cm2 wells is not practical for large scale cultures for multiple individuals. Thus we determined whether optimal CTL activation and expansion could be achieved more simply and cost-effectively using the G-Rex device. When cells are grown in standard flasks or plates, the input volume of medium is restricted to the depth that permits O2 diffusion (normally 1ml/cm2). This in turn limits the available nutrients (glucose and amino acids) present in the medium(5;19). By augmenting O2 diffusion, the G-Rex device allows an increased volume of medium to be added above the growing cells. This improved culture system increased the maximum cell density that could be obtained in static cultures from 2–3 × 106 per cm2 of plastic in wells or plates, to about 107 CTL per cm2 of culture surface area in the G-Rex. This concentration of T-cells was supported by the increased volume of medium, about 4 mL/cm2 in the G-Rex40 and 5 mL/cm2 in the G-Rex500 compared to 1 mL/cm2 in wells. Although the volume of medium per cm2 could be as high as 20 mL/cm2 in the G-Rex2000, we found no increase in final cell density, although the viability of the cells was maintained even at the maximum densities, indicating that the cell concentration was governed by gas exchange rather than by exhaustion of medium.
While non-specific T cells are amenable to culture in plates, flasks and closed-system cell bioreactors(18;36), most groups report a requirement for either 2 cm2 wells of 24-well plates or cell culture bags for the growth of antigen-specific CTL, which have strict requirements for interaction with APCs and feeder cells since their growth and specificity is disrupted by moving cultures. Thus, bioreactors, which are commonly used to generate large numbers of suspension cells and use rocking, stirring and/or medium perfusion to increase cell density, are either not amenable to CTL generation or are prohibitively expensive to purchase and maintain and complex to run((5;15;18)). The requirement for 2 mL wells has made the preparation of CTL lines for adoptive immunotherapy time-consuming and complex, requiring 1 to 3 months to produce sufficient cells for therapeutic purposes and involving manipulations that increase the risk of contamination, and impede the broader clinical application of antigen-specific CTL. The G-Rex provides a substantial advance for the culture of CTL, requiring no mechanical shaking or stirring and no medium sampling, since medium requirements are fulfilled by the unlimiting volume allowed above the cells. The small footprint allows multiple devices to be cultured in a single incubator, so that the cultureware can efficiently produce antigen-specific CTL in a cost effective way for at least 1×1010 CTLs.
Central memory T cells appear to be required for long term in vivo repopulation, and there are concerns that excessive in vitro T-cell proliferation prior to infusion may lead to terminal differentiation and exhaustion. To determine whether the G-Rex would favor the unwanted production of exhausted, terminally differentiated effector cells(4), we measured both cell proliferation and cell death. We found that the increased cell numbers were a result of improved survival rather than of increased proliferation. In confirmation, phenotypic analysis of G-Rex-grown CTL revealed no apparent detrimental effects on the ratio of effector: memory phenotype.
Although the therapeutic significance of antigen-specific CTLs or genetically modified T-cells will depend on their effectiveness in the clinical setting, the ability to extensively apply these therapies will be determined in part by the complexity of the cell manufacturing process. Widespread provision will require optimized bioprocesses that are scalable, sterile, and safe, and that reproducibly make a potent cell product. Our data suggest that the culture device we describe will be able to significantly contribute to these ends.
Supplementary Material
Panel A shows that the rate of EBV-CTL expansion was preserved for several consecutive weeks by using a constant feeder layer of 1.25 × cm2 of LCLs and different dilutions of EBV-CTLs. After 4 weeks the EBV-CTLs antigen specificity and function was measured using IFNγ ELIspot and 51Cr release assay as presented in Panels B and C.
Panel A shows the transgene expression 72 hours after T cell transduction, detected by a monoclonal antibody that specifically recognizes the CH2CH3 region of the Kappa-CAR. Transduction efficiency ranged from 65.3% to 86.5%. Panel B shows the expansion of transgenic T cells over a 15 day period following transfer to the G-Rex vs 24-well plate. Panel C shows the cytolytic function of the expanded transgenic T cells in a 4 hour 51Cr release assay against a Kappa (+) tumor cell line (Daudi), and an irrelevant target K562. The phenotype of the T cells in the G-Rex or in 24 well plates was not significantly different (Panel D).
1×107 EBV-LCL were transferred to a G-Rex 2000 (1,000 ml of complete RPMI) or to a T175 (30 ml). As presented in panel A, the EBV-LCL cultured in G-Rex expanded more than those in the plates without requiring any manipulation or media change. This culture condition did not modify the final cell product as evaluated by Q-PCR for EBER and B cell marker CD20 as presented in Panels B and C.
Acknowledgements
This work was supported in part from NIH grants P50 CA126752 and PO1 CA094237, a Leukemia and Lymphoma Society Specialized Center of Research (SCOR; grant no. 7018), an Amy Strelzer Manasevit Scholar Award (to A.M.L) and a Dan L. Duncan Chair to H.E.H. G.D. is supported by the Doris Duke Charitable Foundation/Clinical Scientist development award and by a Leukemia and Lymphoma Society Translational Research grant. J.F.V. is supported by the When Everyone Survives (WES) foundation and an ASBMT New Investigator Award.
Footnotes
Financial Disclosure: the authors were funded by the NIH, The Leukemia + Lymphoma Society, ASBMT, WES Foundation, Dan L. Duncan Cancer Center, The Doris Duke Charitable Foundation, and an Amy Strelzer Manasevit Award.
Reference List
- 1.Ahmed N, Ratnayake M, Savoldo B, et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007;67:5957–5964. doi: 10.1158/0008-5472.CAN-06-4309. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed N, Salsman VS, Yvon E, et al. Immunotherapy for Osteosarcoma: Genetic Modification of T cells Overcomes Low Levels of Tumor Antigen Expression. Mol Ther. 2009 doi: 10.1038/mt.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avgoustiniatos ES, Hering BJ, Rozak PR, et al. Commercially available gas-permeable cell culture bags may not prevent anoxia in cultured or shipped islets. Transplant Proc. 2008;40:395–400. doi: 10.1016/j.transproceed.2008.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger C, Jensen MC, Lansdorp PM, et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohnenkamp H, Hilbert U, Noll T. Bioprocess development for the cultivation of human T-lymphocytes in a clinical scale. Cytotechnology. 2002;38:135–145. doi: 10.1023/A:1021174619613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bollard CM, Aguilar L, Straathof KC, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J Exp Med. 2004;200:1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bollard CM, Gottschalk S, Leen AM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bollard CM, Rossig C, Calonge MJ, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99:3179–3187. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 9.Carswell KS, Papoutsakis ET. Culture of human T cells in stirred bioreactors for cellular immunotherapy applications: shear, proliferation, and the IL-2 receptor. Biotechnol Bioeng. 2000;68:328–338. doi: 10.1002/(sici)1097-0290(20000505)68:3<328::aid-bit11>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Comoli P, Pedrazzoli P, Maccario R, et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol. 2005;23:8942–8949. doi: 10.1200/JCO.2005.02.6195. [DOI] [PubMed] [Google Scholar]
- 11.Di SA, De AB, Rooney CM, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley ME, Wunderlich J, Nishimura MI, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feuchtinger T, Matthes-Martin S, Richard C, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2006;134:64–76. doi: 10.1111/j.1365-2141.2006.06108.x. [DOI] [PubMed] [Google Scholar]
- 15.Foster AE, Forrester K, Gottlieb DJ, et al. Large-scale expansion of cytomegalovirus-specific cytotoxic T cells in suspension culture. Biotechnol Bioeng. 2004;85:138–146. doi: 10.1002/bit.10801. [DOI] [PubMed] [Google Scholar]
- 16.Heslop HE, Brenner MK, Rooney CM, et al. Administration of neomycin-resistance-gene-marked EBV-specific cytotoxic T lymphocytes to recipients of mismatched-related or phenotypically similar unrelated donor marrow grafts. Hum Gene Ther. 1994;5:381–397. doi: 10.1089/hum.1994.5.3-381. [DOI] [PubMed] [Google Scholar]
- 17.Heslop HE, Ng CYC, Li C, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nature Medicine. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 18.Hollyman D, Stefanski J, Przybylowski M, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother. 2009;32:169–180. doi: 10.1097/CJI.0b013e318194a6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirouac DC, Zandstra PW. The systematic production of cells for cell therapies. Cell Stem Cell. 2008;3:369–381. doi: 10.1016/j.stem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Klapper JA, Thomasian AA, Smith DM, et al. Single-pass, closed-system rapid expansion of lymphocyte cultures for adoptive cell therapy. J Immunol Methods. 2009;345:90–99. doi: 10.1016/j.jim.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knazek RA, Wu YW, Aebersold PM, et al. Culture of human tumor infiltrating lymphocytes in hollow fiber bioreactors. J Immunol Methods. 1990;127:29–37. doi: 10.1016/0022-1759(90)90337-u. [DOI] [PubMed] [Google Scholar]
- 22.Leen AM, Christin A, Khalil M, et al. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J Virol. 2008;82:546–554. doi: 10.1128/JVI.01689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 24.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–265. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 25.Leen AM, Sili U, Savoldo B, et al. Fiber-modified adenoviruses generate subgroup cross-reactive, adenovirus-specific cytotoxic T lymphocytes for therapeutic applications. Blood. 2004;103:1011–1019. doi: 10.1182/blood-2003-07-2449. [DOI] [PubMed] [Google Scholar]
- 26.Louis CU, Straathof K, Bollard CM, et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood. 2009;113:2442–2450. doi: 10.1182/blood-2008-05-157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 28.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintarelli C, Vera JF, Savoldo B, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rooney CM, Smith CA, Ng C, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 31.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 32.Roskrow MA, Suzuki N, Gan Y, et al. Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the treatment of patients with EBV-positive relapsed Hodgkin's disease. Blood. 1998;91:2925–2934. [PubMed] [Google Scholar]
- 33.Savoldo B, Rooney CM, Di SA, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110:2620–2630. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephan MT, Ponomarev V, Brentjens RJ, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 35.Straathof KC, Bollard CM, Popat U, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus--specific T lymphocytes. Blood. 2005;105:1898–1904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- 36.Tran CA, Burton L, Russom D, et al. Manufacturing of large numbers of patient-specific T cells for adoptive immunotherapy: an approach to improving product safety, composition, and production capacity. J Immunother. 2007;30:644–654. doi: 10.1097/CJI.0b013e318052e1f4. [DOI] [PubMed] [Google Scholar]
- 37.Trickett AE, Kwan YL, Cameron B, et al. Ex vivo expansion of functional T lymphocytes from HIV-infected individuals. J Immunol Methods. 2002;262:71–83. doi: 10.1016/s0022-1759(02)00018-2. [DOI] [PubMed] [Google Scholar]
- 38.Vera J, Savoldo B, Vigouroux S, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vera JF, Hoyos V, Savoldo B, et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol Ther. 2009;17:880–888. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 41.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Panel A shows that the rate of EBV-CTL expansion was preserved for several consecutive weeks by using a constant feeder layer of 1.25 × cm2 of LCLs and different dilutions of EBV-CTLs. After 4 weeks the EBV-CTLs antigen specificity and function was measured using IFNγ ELIspot and 51Cr release assay as presented in Panels B and C.
Panel A shows the transgene expression 72 hours after T cell transduction, detected by a monoclonal antibody that specifically recognizes the CH2CH3 region of the Kappa-CAR. Transduction efficiency ranged from 65.3% to 86.5%. Panel B shows the expansion of transgenic T cells over a 15 day period following transfer to the G-Rex vs 24-well plate. Panel C shows the cytolytic function of the expanded transgenic T cells in a 4 hour 51Cr release assay against a Kappa (+) tumor cell line (Daudi), and an irrelevant target K562. The phenotype of the T cells in the G-Rex or in 24 well plates was not significantly different (Panel D).
1×107 EBV-LCL were transferred to a G-Rex 2000 (1,000 ml of complete RPMI) or to a T175 (30 ml). As presented in panel A, the EBV-LCL cultured in G-Rex expanded more than those in the plates without requiring any manipulation or media change. This culture condition did not modify the final cell product as evaluated by Q-PCR for EBER and B cell marker CD20 as presented in Panels B and C.