Abstract
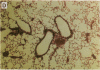
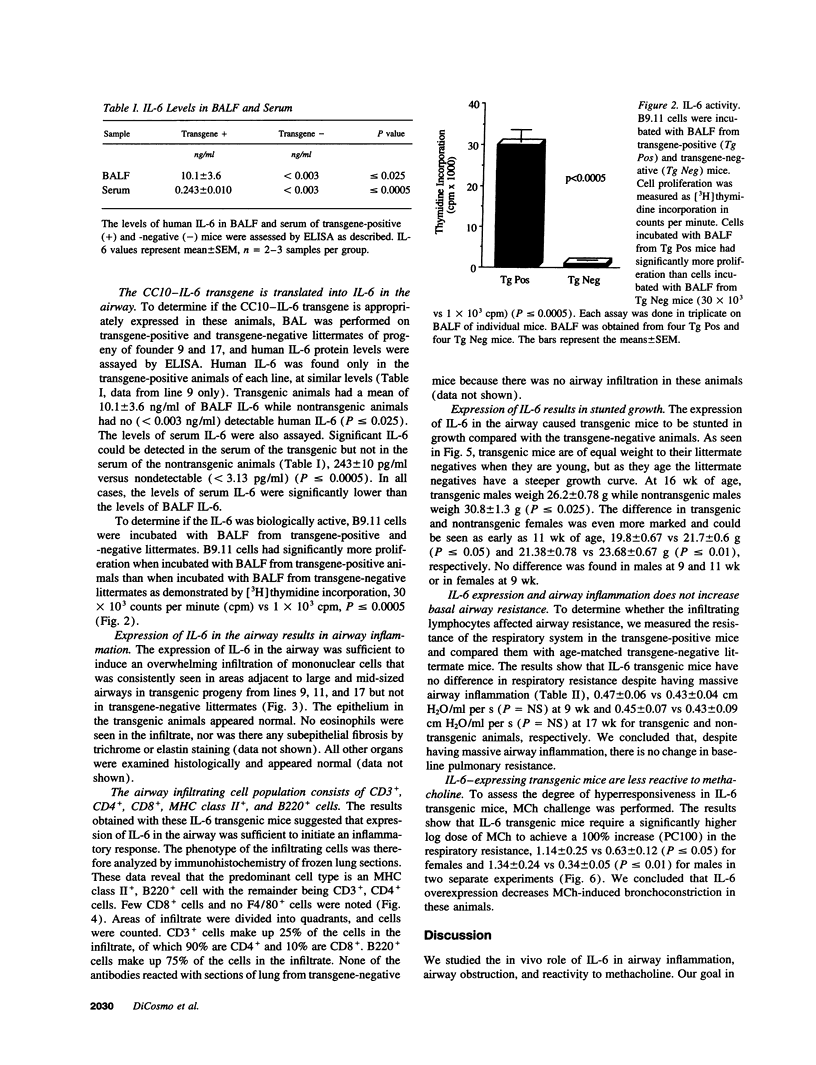
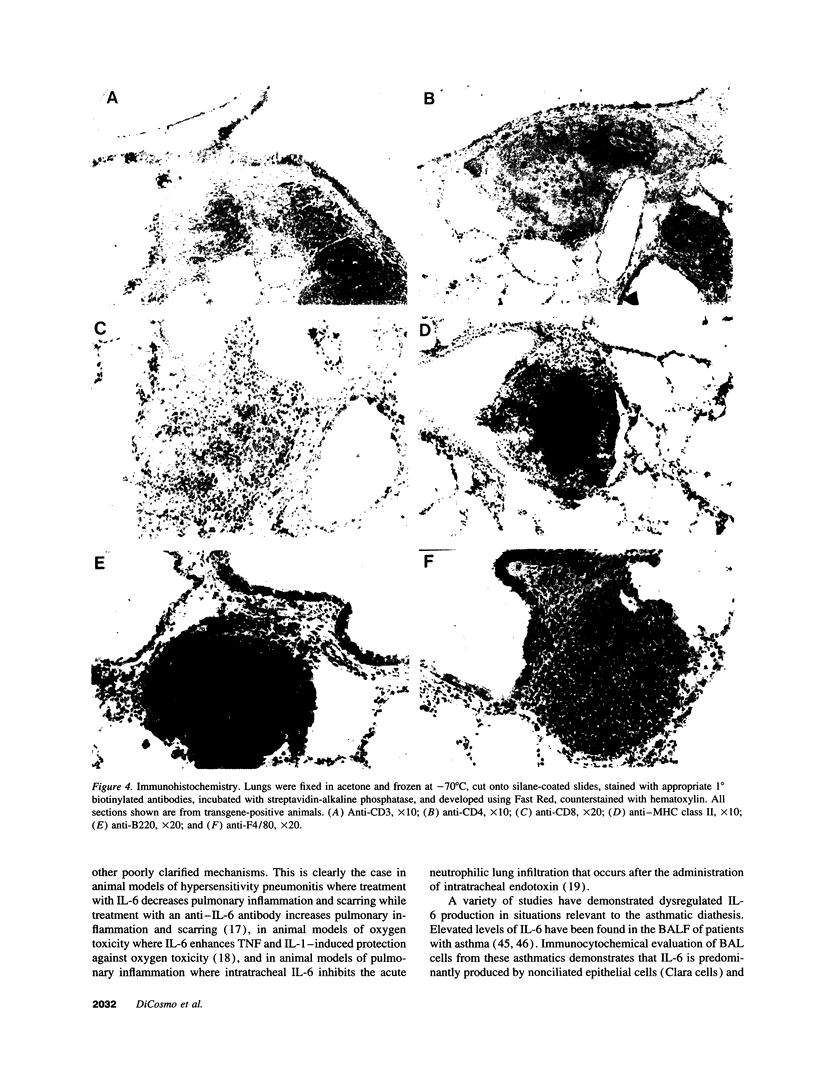
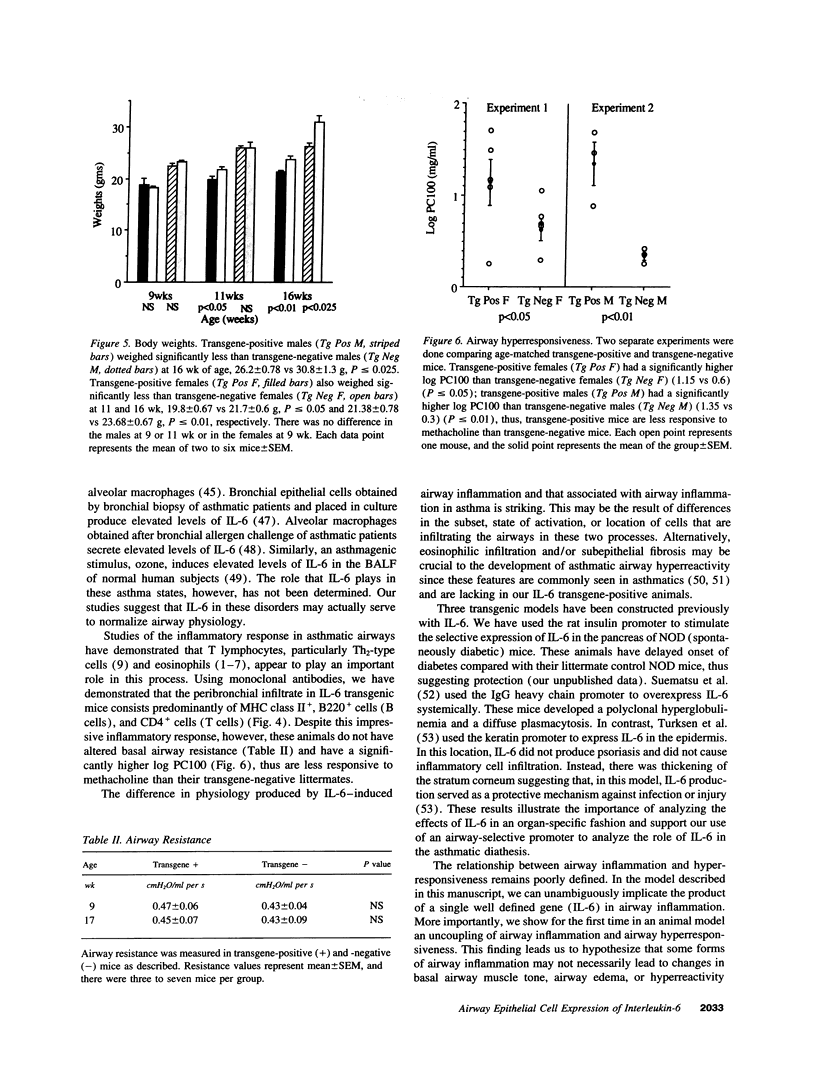
We produced transgenic mice which overexpress human IL-6 in the airway epithelial cells. Transgenic mice develop a mononuclear cell infiltrate adjacent to large and mid-sized airways. Immunohistochemistry reveals these cells to be predominantly CD4+ cells, MHC class II+ cells, and B220+ cells. Transgenic mice and nontransgenic mice had similar baseline respiratory system resistance (0.47 +/- 0.06 vs 0.43 +/- 0.04 cmH2O/ml per s at 9 wk of age, P = NS and 0.45 +/- 0.07 vs 0.43 +/- 0.09 cmH2O/ml per s at 17 wk of age, P = NS). Transgenic mice, however, required a significantly higher log dose of methacholine to produce a 100% increase in respiratory system resistance as compared with non-transgenic littermates (1.34 +/- 0.24 vs 0.34 +/- 0.05 mg/ml, P < or = 0.01). We conclude that the expression of human IL-6 in the airways of transgenic mice results in a CD4+, MHC class II+, B220+ lymphocytic infiltrate surrounding large and mid-sized airways that does not alter basal respiratory resistance, but does diminish airway reactivity to methacholine. These findings demonstrate an uncoupling of IL-6-induced airway lymphocytic inflammation and airway hyperresponsiveness and suggest that some forms of airway inflammation may serve to restore altered airway physiology.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMDUR M. O., MEAD J. Mechanics of respiration in unanesthetized guinea pigs. Am J Physiol. 1958 Feb;192(2):364–368. doi: 10.1152/ajplegacy.1958.192.2.364. [DOI] [PubMed] [Google Scholar]
- Aderka D., Le J. M., Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989 Dec 1;143(11):3517–3523. [PubMed] [Google Scholar]
- Bradley B. L., Azzawi M., Jacobson M., Assoufi B., Collins J. V., Irani A. M., Schwartz L. B., Durham S. R., Jeffery P. K., Kay A. B. Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. 1991 Oct;88(4):661–674. doi: 10.1016/0091-6749(91)90160-p. [DOI] [PubMed] [Google Scholar]
- Broide D. H., Lotz M., Cuomo A. J., Coburn D. A., Federman E. C., Wasserman S. I. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992 May;89(5):958–967. doi: 10.1016/0091-6749(92)90218-q. [DOI] [PubMed] [Google Scholar]
- Campbell I. L., Cutri A., Wilson A., Harrison L. C. Evidence for IL-6 production by and effects on the pancreatic beta-cell. J Immunol. 1989 Aug 15;143(4):1188–1191. [PubMed] [Google Scholar]
- Corrigan C. J., Hartnell A., Kay A. B. T lymphocyte activation in acute severe asthma. Lancet. 1988 May 21;1(8595):1129–1132. doi: 10.1016/s0140-6736(88)91951-4. [DOI] [PubMed] [Google Scholar]
- Corrigan C. J., Kay A. B. CD4 T-lymphocyte activation in acute severe asthma. Relationship to disease severity and atopic status. Am Rev Respir Dis. 1990 Apr;141(4 Pt 1):970–977. doi: 10.1164/ajrccm/141.4_Pt_1.. [DOI] [PubMed] [Google Scholar]
- Corrigan C. J., Kay A. B. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992 Dec;13(12):501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- DUNNILL M. S. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol. 1960 Jan;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Cameron R., Greengard P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. I. Its general distribution in synapses of the central and peripheral nervous system demonstrated by immunofluorescence in frozen and plastic sections. J Cell Biol. 1983 May;96(5):1337–1354. doi: 10.1083/jcb.96.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monchy J. G., Kauffman H. F., Venge P., Koëter G. H., Jansen H. M., Sluiter H. J., De Vries K. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis. 1985 Mar;131(3):373–376. doi: 10.1164/arrd.1985.131.3.373. [DOI] [PubMed] [Google Scholar]
- Denis M. Interleukin-6 in mouse hypersensitivity pneumonitis: changes in lung free cells following depletion of endogenous IL-6 or direct administration of IL-6. J Leukoc Biol. 1992 Aug;52(2):197–201. doi: 10.1002/jlb.52.2.197. [DOI] [PubMed] [Google Scholar]
- Desreumaux P., Janin A., Colombel J. F., Prin L., Plumas J., Emilie D., Torpier G., Capron A., Capron M. Interleukin 5 messenger RNA expression by eosinophils in the intestinal mucosa of patients with coeliac disease. J Exp Med. 1992 Jan 1;175(1):293–296. doi: 10.1084/jem.175.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin R. B., McDonnell W. F., Mann R., Becker S., House D. E., Schreinemachers D., Koren H. S. Exposure of humans to ambient levels of ozone for 6.6 hours causes cellular and biochemical changes in the lung. Am J Respir Cell Mol Biol. 1991 Jan;4(1):72–81. doi: 10.1165/ajrcmb/4.1.72. [DOI] [PubMed] [Google Scholar]
- Durham S. R., Ying S., Varney V. A., Jacobson M. R., Sudderick R. M., Mackay I. S., Kay A. B., Hamid Q. A. Cytokine messenger RNA expression for IL-3, IL-4, IL-5, and granulocyte/macrophage-colony-stimulating factor in the nasal mucosa after local allergen provocation: relationship to tissue eosinophilia. J Immunol. 1992 Apr 15;148(8):2390–2394. [PubMed] [Google Scholar]
- Elias J. A., Trinchieri G., Beck J. M., Simon P. L., Sehgal P. B., May L. T., Kern J. A. A synergistic interaction of IL-6 and IL-1 mediates the thymocyte-stimulating activity produced by recombinant IL-1-stimulated fibroblasts. J Immunol. 1989 Jan 15;142(2):509–514. [PubMed] [Google Scholar]
- Gleich G. J. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990 Feb;85(2):422–436. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- Gosset P., Tsicopoulos A., Wallaert B., Vannimenus C., Joseph M., Tonnel A. B., Capron A. Increased secretion of tumor necrosis factor alpha and interleukin-6 by alveolar macrophages consecutive to the development of the late asthmatic reaction. J Allergy Clin Immunol. 1991 Oct;88(4):561–571. doi: 10.1016/0091-6749(91)90149-i. [DOI] [PubMed] [Google Scholar]
- Hoang T., Haman A., Goncalves O., Wong G. G., Clark S. C. Interleukin-6 enhances growth factor-dependent proliferation of the blast cells of acute myeloblastic leukemia. Blood. 1988 Aug;72(2):823–826. [PubMed] [Google Scholar]
- Holgate S. T., Roche W. R., Church M. K. The role of the eosinophil in asthma. Am Rev Respir Dis. 1991 Mar;143(3 Pt 2):S66–S70. doi: 10.1164/ajrccm/143.3_Pt_2.S66. [DOI] [PubMed] [Google Scholar]
- Horii Y., Muraguchi A., Iwano M., Matsuda T., Hirayama T., Yamada H., Fujii Y., Dohi K., Ishikawa H., Ohmoto Y. Involvement of IL-6 in mesangial proliferative glomerulonephritis. J Immunol. 1989 Dec 15;143(12):3949–3955. [PubMed] [Google Scholar]
- Johnston S. L., Holgate S. T. Cellular and chemical mediators--their roles in allergic diseases. Curr Opin Immunol. 1989;2(4):513–524. doi: 10.1016/0952-7915(90)90004-z. [DOI] [PubMed] [Google Scholar]
- Jourdan M., Bataille R., Seguin J., Zhang X. G., Chaptal P. A., Klein B. Constitutive production of interleukin-6 and immunologic features in cardiac myxomas. Arthritis Rheum. 1990 Mar;33(3):398–402. doi: 10.1002/art.1780330313. [DOI] [PubMed] [Google Scholar]
- Kay A. B., Ying S., Varney V., Gaga M., Durham S. R., Moqbel R., Wardlaw A. J., Hamid Q. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med. 1991 Mar 1;173(3):775–778. doi: 10.1084/jem.173.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S., Min K., Sehgal P., Mizutani H., Birchall N., Ray A., May L. Production of IL-6 by keratinocytes. Implications for epidermal inflammation and immunity. Ann N Y Acad Sci. 1989;557:454–465. [PubMed] [Google Scholar]
- Li C. B., Gray P. W., Lin P. F., McGrath K. M., Ruddle F. H., Ruddle N. H. Cloning and expression of murine lymphotoxin cDNA. J Immunol. 1987 Jun 15;138(12):4496–4501. [PubMed] [Google Scholar]
- Marini M., Vittori E., Hollemborg J., Mattoli S. Expression of the potent inflammatory cytokines, granulocyte-macrophage-colony-stimulating factor and interleukin-6 and interleukin-8, in bronchial epithelial cells of patients with asthma. J Allergy Clin Immunol. 1992 May;89(5):1001–1009. doi: 10.1016/0091-6749(92)90223-o. [DOI] [PubMed] [Google Scholar]
- Martin T. R., Gerard N. P., Galli S. J., Drazen J. M. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol (1985) 1988 Jun;64(6):2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- Mattoli S., Mattoso V. L., Soloperto M., Allegra L., Fasoli A. Cellular and biochemical characteristics of bronchoalveolar lavage fluid in symptomatic nonallergic asthma. J Allergy Clin Immunol. 1991 Apr;87(4):794–802. doi: 10.1016/0091-6749(91)90125-8. [DOI] [PubMed] [Google Scholar]
- Metzger W. J., Hunninghake G. W., Richerson H. B. Late asthmatic responses: inquiry into mechanisms and significance. Clin Rev Allergy. 1985 May;3(2):145–165. doi: 10.1007/BF02992980. [DOI] [PubMed] [Google Scholar]
- Miles S. A., Rezai A. R., Salazar-González J. F., Vander Meyden M., Stevens R. H., Logan D. M., Mitsuyasu R. T., Taga T., Hirano T., Kishimoto T. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4068–4072. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Hirano T., Tang B., Matsuda T., Horii Y., Nakajima K., Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988 Feb 1;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N., Yoshizaki K., Tagoh H., Monden M., Kishimoto S., Hirano T., Kishimoto T. Elevation of serum interleukin 6 prior to acute phase proteins on the inflammation by surgical operation. Clin Immunol Immunopathol. 1989 Mar;50(3):399–401. doi: 10.1016/0090-1229(89)90147-5. [DOI] [PubMed] [Google Scholar]
- Okada S., Suda T., Suda J., Tokuyama N., Nagayoshi K., Miura Y., Nakauchi H. Effects of interleukin 3, interleukin 6, and granulocyte colony-stimulating factor on sorted murine splenic progenitor cells. Exp Hematol. 1991 Jan;19(1):42–46. [PubMed] [Google Scholar]
- Pack R. J., Al-Ugaily L. H., Morris G. The cells of the tracheobronchial epithelium of the mouse: a quantitative light and electron microscope study. J Anat. 1981 Jan;132(Pt 1):71–84. [PMC free article] [PubMed] [Google Scholar]
- Riedy M. C., Stewart C. C. Inhibitory role of interleukin-6 in macrophage proliferation. J Leukoc Biol. 1992 Jul;52(1):125–127. doi: 10.1002/jlb.52.1.125. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992 Jan 30;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990 Jan 1;75(1):40–47. [PubMed] [Google Scholar]
- Sehgal P. B. Interleukin-6: a regulator of plasma protein gene expression in hepatic and non-hepatic tissues. Mol Biol Med. 1990 Apr;7(2):117–130. [PubMed] [Google Scholar]
- Stripp B. R., Sawaya P. L., Luse D. S., Wikenheiser K. A., Wert S. E., Huffman J. A., Lattier D. L., Singh G., Katyal S. L., Whitsett J. A. cis-acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem. 1992 Jul 25;267(21):14703–14712. [PubMed] [Google Scholar]
- Suematsu S., Matsuda T., Aozasa K., Akira S., Nakano N., Ohno S., Miyazaki J., Yamamura K., Hirano T., Kishimoto T. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaak A. J., van Rooyen A., Nieuwenhuis E., Aarden L. A. Interleukin-6 (IL-6) in synovial fluid and serum of patients with rheumatic diseases. Scand J Rheumatol. 1988;17(6):469–474. doi: 10.3109/03009748809098809. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., White J. E., Del Vecchio P. J., Shaffer J. B. IL-6 enhances TNF-alpha- and IL-1-induced increase of Mn superoxide dismutase mRNA and O2 tolerance. Am J Physiol. 1992 Jul;263(1 Pt 1):L22–L26. doi: 10.1152/ajplung.1992.263.1.L22. [DOI] [PubMed] [Google Scholar]
- Turksen K., Kupper T., Degenstein L., Williams I., Fuchs E. Interleukin 6: insights to its function in skin by overexpression in transgenic mice. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5068–5072. doi: 10.1073/pnas.89.11.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulich T. R., Yin S., Guo K., Yi E. S., Remick D., del Castillo J. Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor beta inhibit acute inflammation. Am J Pathol. 1991 May;138(5):1097–1101. [PMC free article] [PubMed] [Google Scholar]
- Uyttenhove C., Coulie P. G., Van Snick J. T cell growth and differentiation induced by interleukin-HP1/IL-6, the murine hybridoma/plasmacytoma growth factor. J Exp Med. 1988 Apr 1;167(4):1417–1427. doi: 10.1084/jem.167.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercelli D., Jabara H. H., Arai K., Yokota T., Geha R. S. Endogenous interleukin 6 plays an obligatory role in interleukin 4-dependent human IgE synthesis. Eur J Immunol. 1989 Aug;19(8):1419–1424. doi: 10.1002/eji.1830190811. [DOI] [PubMed] [Google Scholar]
- Yoshimura N., Oka T., Kahan B. D. Sequential determinations of serum interleukin 6 levels as an immunodiagnostic tool to differentiate rejection from nephrotoxicity in renal allograft recipients. Transplantation. 1991 Jan;51(1):172–176. doi: 10.1097/00007890-199101000-00026. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K., Matsuda T., Nishimoto N., Kuritani T., Taeho L., Aozasa K., Nakahata T., Kawai H., Tagoh H., Komori T. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989 Sep;74(4):1360–1367. [PubMed] [Google Scholar]