Abstract
Purpose:
This study evaluated the effects of phosphate-coated titanium on mineral apposition rate (MAR) and new bone-to-implant contact (BIC) in canines.
Materials and Methods:
2.2 mm × 4 mm electrolytically phosphated or non-phosphated titanium implants with acid-etched surfaces were placed in 48 mandibular sites in 6 foxhounds. Tetracycline and calcein dyes were administered 1 week after implant placement and 1 week before sacrifice. At twelve weeks following implant healing, animals were sacrificed. MAR and BIC were evaluated using fluorescence microscopy. Light microscopic and histological evaluation was performed on undecalcified sections.
Results:
Microscopic evaluation showed the presence of healthy osteoblasts lining bone surfaces near implants. Similar bone-to-implant contact was observed in phosphated and non-phosphated titanium implant sites. MAR was significantly higher near non-phosphated titanium implant surfaces than the phosphated titanium samples. No significant differences were found between dogs or implant sites.
Discussion and Conclusion:
Acid-etched only implants showed significantly higher mineral apposition rates compared to acid-etched, phosphate-coated implants.
Keywords: phosphate, titanium, acid-etched, mineral apposition rate, BIC
INTRODUCTION
Endosseous dental implants have drastically changed dentistry and periodontics in particular, becoming a widely accepted means of routine tooth replacement. Placement of dental implants has become a common option in comprehensive periodontal treatment plans for both fully and partially edentulous patients. Seckinger et al. estimated that 300,000 to 428,000 endosseous dental implants are placed annually [1].
The success of endosseous dental implants has been attributed to osseointegration [2] or functional ankylosis [3]. Osseointegration is defined as a direct structural connection at the light microscopic level between bone and the surface of a load-carrying implant [4]. The osseointegrated, endosseous implant functions without mobility and no soft interface is discernable between the implant and bone. At an electron microscopic level, the distance between the bone and implant surface is approximately 20 nm [5] or in contact with the implant surface [6].
Endosseous implants have reported rates of success over 90%; therefore, dental implants have become common for replacing missing teeth and for stabilizing dentures [7]. A recent study by Karoussis and associates [7] showed that over a ten-year period, sand-blasted, large grit, acid-etched (SLA Straümann®) implants showed a success rate of 96.5% in patients without a history of chronic periodontitis and 90.5% in patients with periodontitis. Implant success rates have proven to be consistently high for low-risk patients, but it is the patients at higher risk for implant failure that warrant improved implant surfaces to increase success rates. Higher-risk patients often include smokers, diabetics, osteoporotic women and patients with a history of aggressive periodontal disease. Implant failure rates in smokers have been shown to be twice as high as non-smokers [8, 9] and human studies have shown greater long-term implant failure rates in diabetic patients compared to patients without diabetes [10, 11].
Initial implant studies involved commercially pure titanium implants with a relatively smooth surface created by a machining process [4]. Buser and Steinemann demonstrated osseous integration between pure titanium and bone after healing occurred. Later studies demonstrated that implants made with rougher surfaces had more BIC and healed faster. Thus, many different surface textures and coatings of titanium implant substrates have been investigated in an effort to improve osseointegration [12]. Any surface that increases success in any patients would be beneficial. Examples of altered surfaces include: plasma-sprayed (Han 1994), hydroxyapatite (HA), sand-blasted, acid-etched (SBAE) [12] [13], and chemically modified SBAE [14].
Etching of the machined implant can be carried out using different acids, including hydrochloric acid, sulfuric acid, hydrofluoric acid, and nitric acid. Plasma sprayed titanium results in a six-fold increase in the crevices of the implant surface [15], producing crevices 30-50 um deep [16] and improving microretention.
These various surface treatments influence the growth and metabolic activity of cultured osteoblasts, with SBAE treatments yielding the most favorable results [12]. Anselme evaluated surface roughness and found that osteoblasts had better orientation and proliferation on titanium of micro-roughness (roughness below cell size) versus macro-roughness (roughness greater than cell size)[17]. Overall, varied surface coatings and roughness have shown superior bone-implant contact versus machined surfaces [13, 18].
The multi-functional polypeptide growth factor family of TGF is involved in inflammation, angiogenesis, embryogenesis, regulation of the immune response, wound healing, and extracellular matrix formation[19, 20]. Boyan et al. reported increasing surface roughness increased transforming growth factor-beta (TGF-β) production and directly increased osteoblast cell proliferation [21].
Recently, a new titanium surface consisting of electronically coating titanium with phosphate was developed and studied in medical implants [22]. Anodic oxidation in phosphoric acid increases the concentration of phosphate on the surface of titanium. By increasing the phosphate concentration, the nobility increases along with titanium corrosion resistance, and the surface hardness improves, facilitating biocompatibility [23]. A recent in vitro study found that osteoblasts on phosphated titanium produced significantly more TGF-ß than non-phosphated titanium [22]. In a canine study, which evaluated electrolytic phosphated titanium implants placed in the humerus, one month samples of the treated titanium had significantly enhanced BIC when compared to the non-treated group [23]. Phosphate treated titanium is currently being used in clinical trials in human hip transplants, hence the interest regarding its impact on dental applications. It is known that for bone formation to occur phosphate and calcium are required [24]. Therefore, osseointegration may be accelerated by the treatment of titanium with phosphate.
The study of a phosphate coating on acid-etched titanium implant in the dog model and evaluation by fluorochrome analysis of new bone to implant contact is unique [25, 26, 27, 28, 29]. Fluorochrome analysis is used to evaluate bone mineral apposition rate and new bone to implant contact percentage. Fluorescent dyes chelate to calcium ions, resulting in deposition of a double vital label on all actively mineralizing bone surfaces. Further research is necessary to evaluate the influence of electrolytic phosphating of titanium implant surfaces. The aim of this study was to evaluate the effect of phosphate-coated and acid-etched titanium on osseointegration of endosseous implants in a canine model.
MATERIALS AND METHODS
The experimental model used in this study involved the creation of standardized osteotomy sites (under surgical procedures) followed by implant placement in a canine model. This protocol was submitted for review and approved by the Institutional Animal Care and Use Committee at Baylor College of Dentistry - a member of The Texas A&M Health Science Center.
Study Animals
Six, male, American foxhounds (Canis familiaris) approximately 2 years old and weighing 25-30 kg were used in this study in an attempt to minimize interspecies variance. All animals were subjected to a physical and dental examination and were quarantined for ten days upon arrival. The protocols for anesthesia, post-operative care, and necropsy of the animal subject follow a model used in previous studies undertaken at this institution [30, 31]. For all procedures the animals received no food or water (NPO) for 12 hours prior to surgery. Monitoring of the animals heart rate, respiratory rate, end-tidal carbon dioxide level, pulse oximetry, percentage inspired vs. expired isoflurane, as well as observation of mucosal color and perfusion was performed by a certified animal technician throughout all surgical procedures.
Surgical Procedures
In order to create edentulous areas in the ridges of each animal, three premolars (P2, P3, P4) were extracted on each side of the mandible. Extractions were performed using a flap to facilitate sectioning of teeth. The flaps were closed with 4-0 sutures (polyglactin 910 Vicryl, Ethicon, Somerville, NJ). Post-operatively, the healing of the surgical sites was monitored. Radiographs were taken prior to and immediately following the extractions to verify complete removal of the teeth.
For post-operative pain control, butorphanol (0.2 to 0.4mg/kg) (Animal Health) was given subcutaneously (SQ) immediately post-operatively and every 12 hours subsequently as needed. Two hundred mg ibuprofen (Wyeth, Madison, WI) was administered by mouth (PO) by mixing into soft dog food (25% protein #8653 Laboratory Dog Diet, Harlan Teklad, Madison, WI) post-operatively for seven days. All animals were fed a soft diet twice daily until sacrifice.
General anesthesia was preceded by intramuscular administration of ketamine (Ketaset®) (2.2 mg/kg) and xylazine (Rompun®) (0.22 mg/kg) (Animal Health, Overland Park, KS) IM. Following onset of anesthesia, the animals were placed on a heating pad, intubated, and inhaled a 1.0 to 2% concentration of isoflurane in oxygen at a flow rate of 1 liter/minute during the surgical procedure. Respiration and electrocardiogram (EKG) were monitored during all procedures. Bilateral mandibular blocks were given with 2% lidocaine hydrochloride with 1:100,000 of epinephrine (Novocol Pharmaceutical of Canada, Cambridge, Ontario, Canada) for pain and hemostatic control (four carpules of 1.8 ml per dog). A bolus dose of penicillin (300,000 IU/10lb) was administered intramuscularly (IM) for antibiotic coverage approximately one hour pre-operatively (Animal Health) was administered to minimize possible post-operative infection.
Eight weeks following extractions of mandibular right and left P2, P3, and P4, the second surgical phase of the study commenced with implant placement. A periodontal probe (CP-12, Hu-Friedy, Chicago, IL, USA) was used to assure a minimum of three mm of spacing between implants at the crest of the ridge (edge to edge). Prior to surgery, random assignment was used to determine the distribution of experimental and control implants for each bilaterally paired site in each animal. Forty-eight, 2.2 mm × 4 mm, titanium implants were supplied by Lynntech, Inc. (College Station, Texas, USA). Twenty-four acid-etched implants and twenty-four implants with acid-etched and phosphate-coated surface were used.
Implants were prepared by the manufacturer by degreasing in acetone for 10 minutes, and deoxidized by immersing in 10% tetrafluoroboric acid for 3 minutes. The implants were subsequently washed with deionized water for 5-10 seconds and placed in an electrolytic cell. The desired phosphate surface was prepared by the anodic oxidation of titanium samples using 50 volts at room temp for 30 min in 1 M phosphoric acid. These parameters produced phosphorus concentrations on the titanium surfaces between 1.8 and 3.7% by energy dispersive X-ray (EDS) analysis, compared to undetectable levels on untreated implants (data not shown). These phosphate and acid-treated implants served as the phosphated group (PT) (Lynntech, Inc. treatment over the acid-etched surface). Twenty-four implants, with only acid-etching served as the control group (Con).
Each animal received four control and four experimental implants by random allocation. Implant placement was performed using a mucoperiosteal flap to allow for visualization of the edentulous ridges. Crestal incisions with full thickness buccal and lingual flap elevation were used. Each animal had eight implant sites and twenty-four sites per study group. A total of forty-eight implants were placed. Radiographs were taken prior to and after implant placement.
Osteotomy sites were prepared using a 2.2 mm (Straümann) twist drill to a depth of 4 mm such that the top edge of the implant was at the same level as the alveolar bone crest. A rubber drill stop (#085-8601 Patterson Dental, St. Paul, MN, USA) was attached to the twist drill to ensure a maximum drill depth of 4 mm. Following implant placement, periosteal releasing incisions were made as needed to assure minimal flap tension and mucoperiosteal flaps were sutured to obtain primary wound closure (4.0 polyglactin 910 Vicryl, Ethicon, Somerville, NJ). All animals were sedated and provided with the same post-operative pain control as previously described in the first surgery.
One week following implant placement and one week prior to animal sacrifice, all canines received an injection of fluorescent bone markers to incorporate into newly formed bone in the mandible. At one week after implant surgery, each canine received an intravenous injection of tetracycline HCl at a dose of 25 mg/kg. A second injection consisting of an additional dye, calcein green was administered intravenously one week prior to animal sacrifice at a dose of 10mg/kg.
Twelve weeks following the second surgical phase, all animals were sedated as previously described. Radiographs were taken prior to sacrifice. The common carotid arteries and external jugular veins were exposed and cannulated. Animals were euthanized under surgical plane general anesthesia with ketamine and administration of Beuthanasia D® 2 ml intracardiac (Schering-Plough). Mandibles were resected en bloc with an oscillating bone saw (Striker) with copious saline application, and immersed in 70% ethanol fixative solution and dehydrated through a series of ethanol concentrations [44] and submitted for undecalcified histological processing and analysis.
Histological Analysis
Individual implant sites were sectioned from the mandible and bisected with a rotary diamond blade with a thickness of 0.5 mm in a bucco-lingual orientation with the superior surface of the implant as reference points. Individual specimens were prepared for histological analysis through a series of steps. The specimens were processed according to the improved technique reported by Maniatopoulos [32] for sectioning of implants and peri-implant tissues. Briefly, processing of the specimens involved dehydration of the fixed, undecalcified tissues with an increasing concentration of 70%-100% ethanol baths, at which point the ethanol was exchanged for methylmethacrylate. Sectioning of the specimens was performed with a diamond saw (Isomet, Buehler Ltd., Lake Bluff, Illinois) to a thickness of 120 microns (μm). Five sections of each implant were obtained in a longitudinal axis from buccal to lingual. The most complete axial section was selected for analysis and luted to a microscope slide. The sections were ground down to approximately 60 μm through a series of polishers on a grinder (240 grit-320 grit-400 grit-600 grit) (Handimet2, Isomet, Buehler, Ltd.). The sections were polished (Buehler Alumina Micropolish, Isomet, Buehler Ltd.) with 0.3 μm and then 0.05 polishing paste. Each section was stained with Stevenel's blue (A: distilled H20 75 ml/methylene blue 1 gm B: distilled H20 75 ml/potassium permanganate 1.5 gm) to facilitate cell recognition.
Fluorochrome microscopic analysis was completed before the final staining with van Geison picro fuchsin and Stevenel's blue. Image acquisition of the entire section at 2× and 10× magnification was accomplished using a digital camera (Olympus) attached to a Nikon fluorescent microscope. Images were captured at 390 nm and 485 nm (tetracycline and calcein, respectively) and combined into a single image, which was saved as a TIF digital file using Metamorph® (MetaMorph Analytical Software, Downington, PA). All fluorescence analysis was done without investigator knowledge of the treatment and position of the implants. A 0.01 micrometer (Model MBM 11100, Nikon Corp, Japan) was used to calibrate the scale of the digital images captured for analysis using Bioquant software (R & M Biometrics, Nashville, TN). By measuring the amount of tetracycline labeled bone in contact with the implant, precise measurement of the percent of new BIC could be ascertained. After measuring the calcein labeled bone in contact with the implant, a ratio of old to new bone contact could be calculated. Sections taken at 2× magnification were used to trace the total implant outline vertically along the implant perimeter and to specifically identify areas of new bone to implant (BIC) contact, and data collected was used to calculate the percent of new BIC, the percent old BIC, and the total percent BIC.
Mineral apposition rate was obtained by measuring the linear distance between the tetracycline stain one week post implant placement and the calcein stain one week prior to sacrifice. Measurements were taken at 10 × magnification from 3 positions - buccal, apical, and lingual (Fig 1). The medial surfaces of a chosen pair of tetracycline and calcein fluorescent lines were traced on each of the three images. An average distance between the lengths of the traced lines was calculated using Bioquant and the average calculated from all three images measured.
Fig 1.
Photomicrograph of a section of bone showing tetracycline (red) and calcein green (green) uptake to illustrate how measurements for mineral apposition rate (MAR) were made. Thick white lines indicate the lines tracing the leading edges of the label. Thin white lines indicate the distances between the lines averaged by the Bioquant software to give MAR.
Methods of Analysis
Data were entered into a statistical software program (MiniTab® State College, Pennsylvania, USA) for analysis. Means and standard deviations were used to describe central tendencies and dispersion. Distributions were approximately normal (Tables 1 and 2). Due to small sample size, a Kruskal-Wallis test was used to compare implant groups (Table 3). One-way ANOVA was performed to determine if anterior or posterior implant location had any influence on the study outcome (Tables 4 and 5). Paired t-tests were used to determine intra-group differences and ANOVA for inter-groups (Table 6).
Table 1.
Mean values for mineral apposition rate
| Mean | SD | |
|---|---|---|
| Control | 0.99 | 0.37 |
| Experimental | 0.84 | 0.20 |
Values expressed μm per day. Control=Acid-etched titanium only; Experimental=Phosphated and Acid-Etched titanium.
Table 2.
Mean values for new bone-to-implant contact
| Mean | SD | |
|---|---|---|
| Control | 65.90 | 18.77 |
| Experimental | 61.64 | 18.67 |
Values expressed as percentages of new bone in contact with the implant perimeter. Control=Acid-etched titanium only; Experimental=Phosphated and Acid-Etched titanium.
Table 3.
Kruskal-Wallis test showing median values
| Mineral Apposition Rate (MAR) (P=0.096) |
% New Bone-To-Implant Contact (BIC) (P =0.439) |
|
|---|---|---|
| Control | 0.97 | 68.89 |
| Experimental | 0.86 | 62.29 |
MAR values expressed μm per day. Control=Acid-etched titanium only; BIC values expressed as percentages of new bone in contact with the implant perimeter. Control=Acid-etched titanium only; Experimental=Phosphated and Acid-Etched titanium.
Table 4.
Inferential statistics between experimental and control implants for MAR
| One-way ANOVA | General Linear Model | |
|---|---|---|
| Groups * Dog | P=0.007* | Dog P=0.002* Group P=0.034† |
| Groups * Position | P=0.268 | Group P=0.09 Position P=0.381 |
= Significant difference between dogs (p<0.002).
= Significant difference between control and experimental groups (p<0.034). No significant difference was detected between implant positions.
Table 5.
Inferential statistics between experimental and control implants for new bone-to-implant contact
| One-way ANOVA | General Linear Model | |
|---|---|---|
| Groups * Dog | P=0.916 | Dog P=0.643 Group P=0.540 |
| Groups * Position | P=0.692 | Dog P=0.431 Position P=0.441 |
No significant differences were detected between dogs, groups or implant position.
Table 6.
Paired T-Tests mean values
| Pairs | Mineral Apposition Rate (P=0.023)* |
% New Bone-To-Implant Contact (P=0.401) |
|
|---|---|---|---|
| Control | Mean | 1.03 | 67.73 |
| St. Dev | 0.34 | 18.22 | |
| Experimental | Mean | 0.83 | 63.54 |
| St. Dev | 0.21 | 18.25 | |
MAR values expressed μm per day. Control=Acid-etched titanium only; BIC values expressed as percentages of new bone in contact with the implant perimeter. Control=Acid-etched titanium only; Experimental=Phosphated and Acid-Etched titanium.
= Significant difference between control and experimental groups (p<0.002).
RESULTS
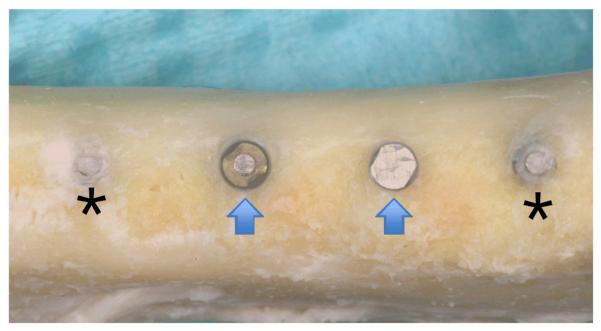
Both control and phosphated implants were placed flush with the alveolar crest level (Fig 2). Forty-seven implants were present for the duration of the study and 1 implant was lost at one week after implant surgery. Upon sacrifice at 12 weeks after implant placement, all sites exhibited clinically healthy tissues covering the implants. After full thickness flap reflection, forty-three implants were completely surrounded by bone up to the crest of the ridge. Four implants exhibited 2-3 mm of buccal dehiscence (two control and two experimental groups). In addition, two control and two experimental implants had bone growing over the top of the implant (Fig 2).
Fig 2.
Photo showing 4 implants 12 weeks after placement. Two implants have bone growth over the coronal aspect (asterisk) and two implants are level with the alveolar ridge (arrows).
Histology
Three implants were dislodged during histological processing and, thus, were not available for evaluation. No signs of infection were observed with any specimens (Fig 3). Histological processing confirmed the clinical findings of the four implants with 2-3mm buccal dehiscence as well as the bone present over the coronal surface of four implants (Fig 3E and 3F).
Fig 3.
Photographs of fluorescent labeled bone. Red color=Tetracycline dye, Green color=Calcein. (a) Acid-etched only titanium (control). (b) Phosphated and acid-etched titanium (experimental). (c) Acid-etched only titanium (control). (d) Phosphated and acid-etched titanium (experimental). (e) Buccal dehiscence. (f) Bone covered over top of implant. a, b, e, f magnification ×2; c, d magnification ×10.
Fluorochrome Analysis
Formation of large areas of new bone around the implant was generally noted at all sites, visible as tetracycline and calcein positive areas (Fig 3). Bone mineral apposition rate and new bone to implant contact was measured to compare bone healing around the control and test implant surfaces.
Mineral apposition rate (MAR)
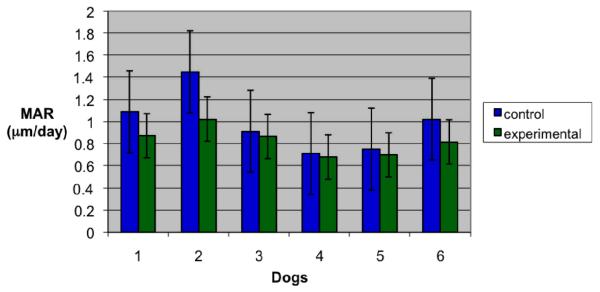
The mean MAR was 0.99 ± 0.84 μm/day for control implants and 0.84 ± 0.20 μm/day for phosphated implants (Table 1). A Kruskal-Wallis test showed MAR of phosphated implants was lower than MAR in controls, but this difference only approached statistical significance (p≤0.096)(Table 3). When comparing control and test implants between dogs, one-way ANOVA revealed a significant (p≤0.007) difference between dogs (Table 4, Fig 4). Further evaluation by a general linear model confirmed a significant difference between dog values (p≤0.002) and between the two implant groups (p≤0.034). Thus, both one-way ANOVA and general linear model showed phosphated and acid-etched implants with significantly lower MAR than control implants. Paired t-test confirmed a significantly (p≤0.023) higher MAR between control (1.03 ± 0.34 μm/day) and tests implants (0.83 ± 0.21 μm/day) within dogs and at similar sites in the mouth (Table 6). No statistical difference (p=0.268) in MAR was noted for control and test implants in relation to anterior or posterior position in the mouth.
Fig 4.
Mineral apposition rates for control and experimental implants. Significant differences were detected between the dogs. Lines represent standard deviation. (Control=Acid-etched titanium; Experimental= Phosphated and acid-etched titanium).
Bone to implant contact (BIC)
The total mean BIC was 95.0 % for control implants and 96.1 % for test implants (Table 6). The mean new BIC was 65.90 ± 18.77% for control implants and 61.64 ± 18.67% for test implants (Table 2). Old bone mean values were 29% for control implants and 34% for test implants. No statistical difference (p=0.439) was noted between implant groups by any of the statistical analyses performed (Table 3, 5, 6, Fig 5).
Figure 5.
New bone-to-implant contact for control and experimental groups. No significant differences were detected between the phosphate and non-phosphated groups between dogs. Lines represent standard deviation. (Control=Acid-etched titanium; Experimental=Phosphated and acid-etched titanium).
DISCUSSION
In many clinical situations, the standard of care for edentulous spaces and denture stabilization is based on placement of dental implants. New titanium implant surface technology has provided the means for accelerated osseointegration. This can shorten the healing phase after surgical implant placement, shortening the time to restoration. The increased number of studies reported in the dental literature indicates interest in the surface treatment of titanium implants with phosphate.
Nelson and associates, in a 6-month study, examined electrolytic phosphate-coated implants placed in a dog humerus model [23]. They evaluated pullout strength and the tissue interface and found no significant difference in pull out force between phosphate coated or pure titanium implants. Enhanced implant contact with bone and marrow was found along with less fibrous tissue at the implant interface. The electrolytically phosphated titanium surface studied by Nelson and associates is the same surface that was used in this study.
In the present study, clinical and radiographic examination of the implant sites demonstrated good overall gingival healing and absence of peri-implant infection or radiolucency in the presence of both control and phosphated implants. No signs of continuous peri-implant radiolucency were observed, which confirmed ankylotic stability of all 47 implants.
Tetracycline and calcein have a high affinity for calcium, rapidly binding to serum calcium within minutes of administration. By injecting dyes at selected points in the study timeline and measuring the distance between dyes, it was possible to identify histologically new bone that had formed following the implant placements in each group and visualization of bone growth patterns achieved during the healing period.
The mean new BIC values obtained in the current study are comparable with those reported in previous studies with osseointegrated implants in dog and porcine models [33, 16, 34]. However, the present study evaluated BIC values by measuring the fluorescently labeled bone against the implant. The tetracycline labeled bone was measured to give an indication of the amount of bone providing initial stability and the amount of new bone contributing to secondary stability. Phosphate added to the titanium surface did not alter either immediate or secondary stability. Few studies could be found that measure BIC by fluorescent microscopy. One study evaluated newly formed BIC by fluorescent microscopy in SLA Straümann palatal implants for orthodontic anchorage in dogs. A new BIC of 19% was noted at 4 weeks and 26% at 24 weeks [25]. These numbers are lower than noted in the present study. Nonetheless, the total BIC values of a recent study [35] and this study are comparable.
In this study, the mean mineral apposition rate was significantly higher in control than phosphated implants. In a preliminary study using implants in a rabbit model, Damien reported mineral apposition rates were much higher than those reported in this study [26], but the difference in mineral apposition rate between the two studies may be due to differences between species (dogs versus rabbits). The mineral apposition rate (MAR) reflects the rate at which new bone was deposited in a radial direction. Evidence in previous studies show that higher bone formation correlates with higher MAR; however, the lower MAR in the phosphate group of this study suggests the possibility of an increased lag time for enhanced recruitment and/or activation of osteoblasts along the bone surface [36].
Implants with surface treatments of phosphate may be significant due to the ability of calcium phosphate to upregulate platelet activation, increase fibrin retention, accelerate healing, and allow bone bonding. Phosphate is necessary to signal and provide a transient biological matrix for osteogenic cell migration to the implant surface that results in contact osteogenesis [37]. Both calcium and phosphate are required for mineral deposition during bone mineralization [38], and their presence on implants should be beneficial for accelerating osseointegration. However, some forms of phosphate such as pyrophosphates may delay or inhibit calcification and need to be broken down by alkaline phosphatase to remove this effect [38, 39]. It is unclear what form of phosphate is present on the implant surface in this study, and further examination of this is warranted.
A recent study showed that osteoblasts in the phosphated titanium group produced more TGF-ß1 than the non-phosphated group [22]. TGF-ß1 is known to suppress osteoblast proliferation and, conversely, induce maturation and differentiation of osteoblasts [40]. Interestingly, the effects of TGF-ß1 appear to be highly dependent on bone cell source and local environment as shown in studies that demonstrate either stimulation or inhibition of osteoblast proliferation [41]. In vivo animal models have shown both increased and decreased effects on MAR by TGF- ß1, depending on dose [42]. In this study, TGF-ß1 may have induced osteoblast proliferation at the expense of osteoblast differentiation (collagen production). Nucleation of the crystallization process requires the presence of a collagenous inducer [43], which may explain the reduced MAR of the phosphate group. The additional lag time of mineralization may result in more mineralization at a later time point. Thus, more information about mineralization could be obtained from a future study design involving multiple time points.
Other methods to phosphate implants are currently being used and studied. For example, discrete crystalline deposition (DCD) results in calcium phosphate nanocrystals approximately 70nm in size on the implant surface [37]. The adhesive property of this treatment is a concern; since, crystals may be sheared off and lose the intended effect or elicit an inflammatory reaction. Future studies would be beneficial to compare the shear force of various phosphated surfaces. Our study possibly should have examined different and earlier time points to look for peaks in the remodeling phase not found. Overall, the only significant difference in this study was a lower mineral apposition rate of the test implants between the dogs and between control/test groups. This difference may not be clinically relevant.
CONCLUSIONS
The results of this study demonstrated that implants with an acid-etched only and acid-etched, phosphate-coated titanium surface achieve comparable osseointegration. This study showed a 12-week survival rate of 97.9% for all implants. No statistical significant difference existed between the new BIC values of controls and experimental implants. Acid-etched only implants showed significantly higher mineral apposition rates compared to acid-etched, phosphate-coated implants (1.03 ± 0.34 μm/day and 0.83 ± 0.21 μm/day, respectively).
Figure 6.
Total, old, and new bone-to-implant contact for control and experimental implant groups. No significant differences were detected between the phosphate and non-phosphated groups between dogs. (Control=Acid-etched only titanium; Experimental=Phosphated and acid-etched titanium).
ACKNOWLEDGEMENTS
Support for this project was received from Baylor College of Dentistry (CHF), NIH grant # DEO15893-02 (CJN) and Straümann (LAO).
REFERENCES
- 1.Seckinger RJ, Barber HD, Phillips K, Saleh N, Ferarie J. A clinical study of titanium plasma sprayed (TPS)-coated threaded and TPS-coated cylindrical endosseous dental implants. Guide Impl Res. 1996;1:5–8. [Google Scholar]
- 2.Bränemark PI. Vital microscopy of bone marrow in rabbit. Diss Lund Scand J Lab Invest Suppl. 1959;3811:1–82. [PubMed] [Google Scholar]
- 3.Schroeder A, van der Zypen E, Stich H, Sutter F. The reactions of bone, connective tissue, and epithelium to endosteal implants with titanium-sprayed surfaces. J Maxillofac Surg. 1981;9:15–25. doi: 10.1016/s0301-0503(81)80007-0. [DOI] [PubMed] [Google Scholar]
- 4.Bränemark PI, Zarb GA, Albrektsson T. Introduction to Osseointegration. In: Bränemark PI, Zarb GA, Albrektsson T, editors. Tissue Integrated Prostheses: Osseointegration in Clinical Dentistry. Quintessence; Chicago: 1995. [Google Scholar]
- 5.Albrektsson T. In: Tissue Integrated Prostheses: Osseointegration in Clinical Dentistry. Bränemark PI, Zarb GA, Albrektsson T, editors. Quintessence; Chicago: 1985. [Google Scholar]
- 6.Listgarten MA, Lang NP, Schroeder HE, Schroeder A. Periodontal tissues and their counterparts around endosseous implants. Clin Oral Implants Res. 1991;2:1–19. doi: 10.1034/j.1600-0501.1991.020309.x. [DOI] [PubMed] [Google Scholar]
- 7.Karoussis IK, Salvi GE, Heitz-Mayfield LJA, Hammerle CHF, Lang NP. Long- term implant prognosis in patients with and without a history of chronic periodontitis: a 10-year prospective cohort study of the ITI dental implant system. Clin Oral Implant Res. 2003;14:329–339. doi: 10.1034/j.1600-0501.000.00934.x. [DOI] [PubMed] [Google Scholar]
- 8.De Bruyn H, Collaert B. The effect of smoking on early implant failure. Clin Oral Implants Res. 1994;5:260–264. doi: 10.1034/j.1600-0501.1994.050410.x. [DOI] [PubMed] [Google Scholar]
- 9.Bain CA. Smoking and implant failure - benefits of a smoking cessation protocol. Int J Oral Maxillofac Implants. 1996;11:756–759. [PubMed] [Google Scholar]
- 10.Olson JW, Shernoff AF, Tarlow JL, Colwell JA, Scheetz JP, Bingham SF. Dental endosseous implant assessments in a type 2 diabetic population: a prospective study. Int J Oral Maxillofac Implants. 2000;15:811–818. [PubMed] [Google Scholar]
- 11.Morris HF, Ochi S, Winkler S. Implant survival in patients with type 2 diabetes: placement to 36 months. Ann Periodontol. 2000;5:157–165. doi: 10.1902/annals.2000.5.1.157. [DOI] [PubMed] [Google Scholar]
- 12.Guizzardi S, Galli C, Martini D, et al. Different titanium surface treatment influences human mandibular osteoblast response. J Periodontol. 2004;75:273–282. doi: 10.1902/jop.2004.75.2.273. [DOI] [PubMed] [Google Scholar]
- 13.Novaes AB, Jr, Souza SL, de Oliveira PT, Souza AM. Histomorphometric analysis of the bone-implant contact obtained with 4 different implant surface treatments placed side by side in the dog mandible. Int J Oral Maxillofac Implants. 2002;17:377–383. [PubMed] [Google Scholar]
- 14.Buser D, Broggini N, Wieland M, et al. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83:529–533. doi: 10.1177/154405910408300704. [DOI] [PubMed] [Google Scholar]
- 15.Leimola-Virtanen R, Peltola J, Oksala E, Helenius H, Happonen RP. ITI titanium plasma-sprayed screw implants in the treatment of edentulous mandibles: a follow-up study of 39 patients. Int J Oral Maxillofac Implants. 1995;10:373–378. [PubMed] [Google Scholar]
- 16.Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991;25:889–902. doi: 10.1002/jbm.820250708. [DOI] [PubMed] [Google Scholar]
- 17.Anselme K, Bigerelle M, Noël B, Iost A, Hardouin P. Effect of grooved titanium substratum on human osteoblastic cell growth. J Biomed Mater Res. 2002;60:529–540. doi: 10.1002/jbm.10101. [DOI] [PubMed] [Google Scholar]
- 18.Bumgardner JD, Boring JG, Cooper RC, Jr, et al. Preliminary evaluation of a new dental implant design in canine models. Implant Dent. 2000;9:252–260. doi: 10.1097/00008505-200009030-00011. [DOI] [PubMed] [Google Scholar]
- 19.Skaleric U, Kramar B, Petelin M, Pavlica Z, Wahl SM. Changes in TGF-beta 1 levels in gingiva, crevicular fluid and serum associated with periodontal inflammation in humans and dogs. Eur J Oral Sci. 1997;105:136–142. doi: 10.1111/j.1600-0722.1997.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 20.Cornelini R, Rubini C, Fioroni M, Favero GA, Strocchi R, Piattelli A. Transforming growth factor-beta 1 expression in the peri-implant soft tissues of healthy and failing dental implants. J Periodontol. 2003;74:446–450. doi: 10.1902/jop.2003.74.4.446. [DOI] [PubMed] [Google Scholar]
- 21.Boyan BD, Lossdörfer S, Wang L, et al. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur Cell Mater. 2003;6:22–27. doi: 10.22203/ecm.v006a03. [DOI] [PubMed] [Google Scholar]
- 22.Dacy JA, Spears R, Hallmon WW, et al. Effects of phosphated titanium and enamel matrix derivatives on osteoblast behavior in vitro. Int J Oral Maxillofac Implants. 2007;22:701–709. [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson CJ, Minevski ZS, Urban RM, Turner TM, Jacobs JJ. Corrosion and wear resistant bioactive surgical implants; Proceedings from ASM Materials and Processes for Medical Devices Conference; Anaheim, CA. September 8-10, 2003. [Google Scholar]
- 24.Marieb EN. Human anatomy and physiology. In: Heyden SJ, Schaefer EM, editors. Bones and Bone tissue. 3rd Edition Benjamin/Cummings Publishing Company, Inc.; California: 1995. [Google Scholar]
- 25.Borbely P, Dunay MP, Jung BA, Wehrbein H, Wagner W, Kunkel M. Primary loading of palatal implants for orthodontic anchorage--a pilot animal study. J Craniomaxillofac Surg. 2008;36:21–27. doi: 10.1016/j.jcms.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Damien E, Hing K, Saeed S, Revell PA. A preliminary study on the enhancement of the osteointegration of a novel synthetic hydroxyapatite scaffold in vivo. J Biomed Mater Res A. 2003;66:241–246. doi: 10.1002/jbm.a.10564. [DOI] [PubMed] [Google Scholar]
- 27.Norrdin RW, Shih MS. Systemic effects of prostaglandin E2 on vertebral trabecular remodeling in beagles used in a healing study. Calcif Tissue Int. 1988;42:363–368. doi: 10.1007/BF02556354. [DOI] [PubMed] [Google Scholar]
- 28.Cope JB, Samchukov ML. Regenerate bone formation and remodeling during mandibular osteodistraction. Angle Orthodon. 2000;70:99–111. doi: 10.1043/0003-3219(2000)070<0099:RBFARD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Liu ZJ, King GJ, Herring SW. Condylar mineralization following mandibular distraction in rats. J Dent Res. 2006;85:653–657. doi: 10.1177/154405910608500714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodges NE, Perry M, Mohamed W, Hallmon WW, Rees T, Opperman LA. Distraction osteogenesis versus autogenous onlay grafting. Part II: biology of regenerate and onlay bone. Int J Oral Maxillofac Implants. 2006;21:237–244. [PubMed] [Google Scholar]
- 31.Sparks MS, Kerns DG, Wilson TG, Hallmon WW, Spears R, Haghighat N. Bone regeneration around implants in the canine mandible with cultured fibroblasts in polyglactin mesh. J Periodontol. 2007;78:1276–1287. doi: 10.1902/jop.2007.060056. [DOI] [PubMed] [Google Scholar]
- 32.Maniatopoulos C, Rodriguez A, Deporter DA, Melcher AH. An improved method for preparing histological sections of metallic implants. Int J Oral Maxillofac Implants. 1986;1:31–37. [PubMed] [Google Scholar]
- 33.Quinlan P, Nummikoski P, Schenk R, et al. Immediate and early loading of SLA ITI single-tooth implants: an in vivo study. Int J Oral Maxillofac Implants. 2005;20:360–370. [PubMed] [Google Scholar]
- 34.Cochran DL, Schenk RK, Lussi A, Higginbottom FL, Buser D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: a histometric study in the canine mandible. J Biomed Mater Res. 1998;40:1–11. doi: 10.1002/(sici)1097-4636(199804)40:1<1::aid-jbm1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 35.Nelson K, Ozyuvaci H, Bilgic B, Klein M, Hildebrand D. Histomorphometric evaluation and clinical assessment of endosseous implants in iliac bone grafts with shortened healing periods. Int J Oral Maxillofac Implants. 2006;21:392–398. [PubMed] [Google Scholar]
- 36.Sheng MH, Baylink DJ, Beamer WG, et al. Histomorphometric studies show that bone formation and bone mineral apposition rates are greater in C3H/HeJ (high-density) than C57BL/6J (low-density) mice during growth. Bone. 1999;25:421–429. doi: 10.1016/s8756-3282(99)00184-2. [DOI] [PubMed] [Google Scholar]
- 37.Mendes VC, Moineddin R, Davies JE. The effect of discrete calcium phosphate nanocrystals on bone-bonding to titanium surfaces. Biomaterials. 2007;28:4748–4755. doi: 10.1016/j.biomaterials.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Meyer JL, Reddi AH. Changes in pyrophosphatase activity during the de novo mineralization associated with cartilage and bone formation. Arch Biochem Biophys. 1985;242:532–539. doi: 10.1016/0003-9861(85)90240-1. [DOI] [PubMed] [Google Scholar]
- 39.Fleisch H, Neuman WF. Mechanisms of calcification: role of collagen, polyphosphates, and phosphatase. Am J Physiol. 1961;200:1296–1300. doi: 10.1152/ajplegacy.1961.200.6.1296. [DOI] [PubMed] [Google Scholar]
- 40.Alliston T, Choy L, Ducy P, Karsenty G, Derynck R. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001;20:2254–2272. doi: 10.1093/emboj/20.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centrella M, McCarthy TL, Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987;262:2869–2874. [PubMed] [Google Scholar]
- 42.Fujimoto R, Tanizawa T, Nishida S, et al. Local effects of transforming growth factor-beta1 on rat calvaria: changes depending on the dose and the injection site. J Bone Miner Metab. 1999;17:11–17. doi: 10.1007/s007740050057. [DOI] [PubMed] [Google Scholar]
- 43.Solomons CC, Neuman WF. On the mechanisms of calcification: the remineralization of dentin. J Biol Chem. 1960;235:2502–2506. [PubMed] [Google Scholar]