Abstract
Background
There are limited randomized data on statins for primary prevention in older people, and the relative hazard of cardiovascular disease associated with elevated cholesterol weakens with advancing age.
Objective
To assess the efficacy and safety of rosuvastatin in individuals 70 years of age or older.
Design
Secondary analysis of the JUPITER trial, a randomized, double-blind, placebo-controlled trial.
Setting
1315 sites in 26 countries randomized subjects in JUPITER.
Participants
Among the 17802 randomized participants with low-density lipoprotein cholesterol (LDL) levels of less than 130 mg/dL and high-sensitivity C-reactive protein levels of 2.0 mg/L or higher, and without cardiovascular disease, 5695 were 70 years of age or older.
Intervention
Participants were randomly assigned in a 1:1 ratio to receive 20 mg rosuvastatin daily or placebo.
Measurements
The primary end point was the occurrence of a first cardiovascular event (myocardial infarction, stroke, arterial revascularization, hospitalization for unstable angina, or death from cardiovascular causes).
Results
The 32% of trial participants aged 70 years or older accrued 49% (N=194) of the 393 confirmed primary end points. The rates of the primary end point in this age group were 1.22 and 1.99 per 100 person-years of follow-up in the rosuvastatin and placebo groups, respectively (hazard ratio 0.61; [95% CI, 0.46 to 0.82]; P<0.001). Corresponding rates of all-cause mortality in this age group were 1.63 and 2.04 (hazard ratio 0.80; [95% CI, 0.62 to 1.04]; P=0.090). While there was no significant heterogeneity in treatment effects by age, absolute reductions in event rates associated with rosuvastatin were greater in older individuals. The relative rate of any serious adverse event among older people in the rosuvastatin group versus placebo was 1.05 (95% CI: 0.93–1.17).
Limitations
Effect estimates from this exploratory analysis with age cutpoint chosen after trial completion should be viewed in the context of the overall trial results.
Conclusion
In apparently healthy older people without hyperlipidemia but with elevated high-sensitivity C-reactive protein, rosuvastatin reduces the incidence of major cardiovascular events.
Primary Funding Source
AstraZeneca
Statins are underutilized in high risk older individuals with clear indications (1–3). Moreover, use of statins for primary prevention in older individuals without diabetes remains controversial (4), both because of limited randomized evidence and because the relative hazard associated with elevated cholesterol is markedly attenuated by older age (5). The Framingham coronary heart disease risk score reflects this attenuation in its assignment of 6 points to a man and 8 points to a woman aged 40 years with a total cholesterol of 250 mg/dL, but only 1 and 2 points for men and women, respectively, with this level at age 75 years (6). However, absolute risk is critical for treatment decisions and older age is the dominant risk factor for a first cardiovascular event in individuals without diabetes. Thus, at age 75 years, the Framingham score assigns 13 points to a man and 16 points to a woman, relative to 0 points for either sex at age 40.
Substantial randomized evidence on the efficacy of statins in older people with diabetes or prevalent vascular disease supports guidelines to treat high risk individuals regardless of age (7–10). However, randomized evidence on statin use in primary prevention among older people without diabetes is limited. Among the 5,804 individuals aged 70–82 years randomized in the PROSPER trial (11), pravastatin (40 mg/day) reduced the incidence of important vascular events by 15% (hazard ratio 0.85; 95% CI: 0.74–0.97; P=0.014), but the observed benefit was somewhat weaker in the 3,239 individuals without prior vascular disease (hazard ratio 0.94; 95% CI: 0.77–1.15). Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER, [12]) randomized an older population (mean age 66 years) than prior large primary prevention trials of statins (13–15, mean ages 55–58 years). This report focuses on the treatment effects and safety profile among participants in the JUPITER trial aged 70 years and older, and compares these findings with results in younger participants.
METHODS
Trial Design
JUPITER was a randomized, double-blind, placebo-controlled trial conducted at 1315 sites in 26 countries. Institutional review boards approved the protocol at each site.
Study Population
The main eligibility criteria were age 50 years or older in men and 60 years or older in women, with no history of cardiovascular disease or diabetes, no lipid-lowering therapy within 6 weeks before screening, LDL cholesterol level less than 130 mg per deciliter and high-sensitivity C-reactive protein level of 2.0 mg per liter or more at screening. Additional eligibility and exclusion criteria, including a willingness to participate for the duration of the trial and provision of written informed consent, are described elsewhere (12). Willing and potentially eligible subjects entered a 4-week, placebo run-in phase to test their adherence.
Randomization and Follow-up
Subjects who remained willing and eligible after the run-in phase were randomly assigned in a 1:1 ratio to receive either 20 mg rosuvastatin daily, or matching placebo. Site investigators randomized their subjects via an interactive voice response system that assigned treatment on the basis of a computer-generated list developed by AstraZeneca that was stratified by center. From March 2003 until December 2006, 17,802 participants were randomized.
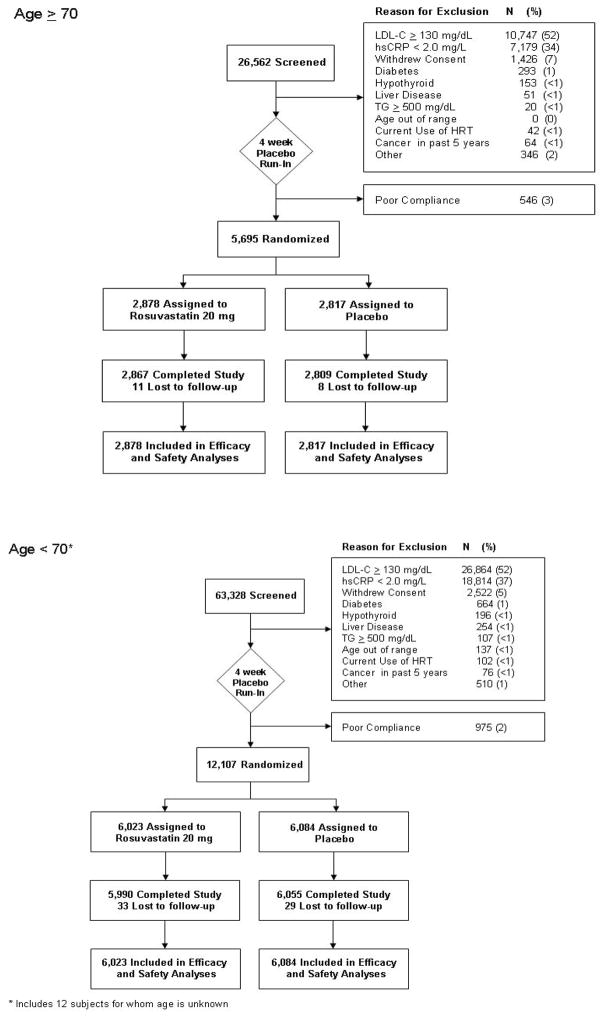
Participants had scheduled follow-up visits at 13 weeks and at 6, 12, 18, 24, 30, 36, 42, 48, 54, and 60 months after randomization. Figure 1 illustrates subject accrual and follow-up by age group.
Figure 1.
Progress of randomization and participation in the JUPITER trial by age group. Randomization was not stratified by age.
JUPITER was designed to continue until accrual of 520 confirmed primary end points, with interim efficacy analyses scheduled to occur upon accrual of 195 and 390 confirmed primary end points. The pre-specified stopping boundary (based on O’Brien-Fleming boundaries determined by the Lan-DeMets approach) was exceeded at the first interim analysis. The independent data and safety monitoring board voted to continue the trial for an additional 6 months, at which time the board determined that the criteria specified in their charter for early stopping were exceeded. Their recommendation on March 29, 2008 to stop the trial was accepted the next day by the steering committee. A closeout visit was scheduled after this date, at which time participants learned their group assignment.
End Points
The primary end point was the occurrence of a first major cardiovascular event, defined as nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from cardiovascular causes. Other pre-specified end points included the components of the primary end point, death from any cause, venous thromboembolism and incident diabetes.
An independent end point committee, masked to randomized treatment assignment, adjudicated all reported primary end points that occurred through March 30, 2008. Follow-up for efficacy end points ended on that date. For safety end points including incident diabetes, blinded treatment and follow-up continued until a participant had a closeout visit and discontinued study therapy. The last closeout visit occurred on August 20, 2008.
Statistical Analysis
The cutpoint at age 70 years for this report was chosen after trial completion but before conducting these analyses because prior primary prevention trials included few individuals in this age group and use of statins for primary prevention in older people is controversial. Analyses of the primary, pre-specified secondary and safety outcomes followed the intention to treat principle and used Cox proportional hazards models to estimate treatment effects, separately by age group. Possible heterogeneity in the treatment effect by age was evaluated by a likelihood ratio test of an age group by treatment interaction in a model fitted to the entire population. Because the competing risk of death is an important consideration in the treatment of older people, we considered composite end points including either death or other end points. Evaluation of the proportional hazards assumption was based on a likelihood ratio test of the interaction between treatment and study time. Alternative analyses stratified on geographic region yielded similar effect estimates.
We also evaluated absolute treatment effects as the difference between the incidence rate of an outcome in the placebo group minus the incidence rate in the rosuvastatin group, separately by age group. We based estimates of the number of patients needed to treat to prevent one event on the difference between Kaplan-Meier estimates of cumulative risk at 4 years (18).
Role of the funding source
The study chair designed and wrote the trial protocol. AstraZeneca financially supported the trial, collected the data, and monitored the sites, but remained blinded to treatment status throughout the trial, and played no role in the analyses or drafting of the primary study results (12, 16–17) or this manuscript.
RESULTS
Participants in JUPITER who were age 70 years or older at randomization had a somewhat different profile of other cardiovascular risk factors, relative to participants age 50–69 years at randomization (Table 1). Higher percentages of older participants were women or had hypertension, and lower percentages were obese or were cigarette smokers, relative to younger participants.
Table 1.
Baseline characteristics of trial participants, according to age and treatment assignment
| Age 70–97 years | Age 50–69 years | |||
|---|---|---|---|---|
| Rosuvastatin (N=2878) | Placebo (N=2817) | Rosuvastatin (N=6023) | Placebo (N=6084) | |
| Age, years, median (interquartile range) | 74 (72–77) | 74 (72–78) | 63 (58–66) | 63 (58–66) |
| Female sex, N (%) | 1485 (51.6) | 1446 (51.3) | 1941 (32.2) | 1929 (31.7) |
| Race or ethnic group, N (%) | ||||
| White | 2030 (70.6) | 1953 (69.3) | 4328 (71.9) | 4372 (71.9) |
| Black | 382 (13.3) | 372 (13.2) | 718 (11.9) | 752 (12.4) |
| Hispanic | 383 (13.3) | 420 (14.9) | 738 (12.3) | 720 (11.8) |
| Other or unknown | 82 (2.8) | 72 (2.6) | 239 (4.0) | 240 (3.9) |
| Geographic region, N (%) | ||||
| United States or Canada | 1009 (35.1) | 1040 (36.9) | 1998 (33.2) | 1994 (32.8) |
| Central or South America | 465 (16.2) | 468 (16.6)) | 842 (14.0) | 831 (13.7) |
| Europe | 970 (33.7) | 889 (31.6) | 2291 (38.0) | 2365 (38.9) |
| South Africa | 416 (14.5) | 398 (14.1) | 839 (13.9) | 844 (13.9) |
| Israel | 18 (0.6) | 22 (0.8) | 53 (0.9) | 50 (0.8) |
| Body mass index, N (%) | ||||
| <25 kg/m2 | 748 (26.1) | 752 (26.8) | 1292 (21.5) | 1281 (21.1) |
| 25 to <30 kg/m2 | 1184 (41.3) | 1175 (41.8) | 2311 (38.5) | 2339 (38.5) |
| ≥ 30 kg/m2 | 938 (32.7) | 884 (31.5) | 2400 (40.0) | 2452 (40.4) |
| Hypertension, N (%) | 1883 (65.5) | 1849 (65.7) | 3196 (53.1) | 3280 (53.9) |
| Current smoker, N (%) | 232 (8.1) | 245 (8.7) | 1168 (19.4) | 1175 (19.3) |
| Metabolic syndrome*, N (%) | 1152 (40.4) | 1105 (39.5) | 2500 (41.8) | 2618 (43.4) |
| Framingham risk score>10, N (%) | 1984 (69.1) | 1948 (69.3) | 2458 (40.9) | 2505 (41.2) |
| hsCRP ≥ 5.0 mg/L, N(%) | 1204 (41.8) | 1211 (43.0) | 2414 (40.1) | 2515 (41.3) |
| LDL cholesterol > 100 mg/dL, N (%) | 1894 (65.8) | 1831 (65.0) | 3887 (64.6) | 3916 (64.4) |
| HDL < 40 mg/dL (men), < 50 mg/dL (women), N (%) | 864 (30.0) | 845 (30.0) | 1969 (32.7) | 2011 (33.1) |
| Triglycerides ≥ 150 mg/dL, N(%) | 811 (28.2) | 809 (28.7) | 2089 (34.7) | 2127 (35.0) |
| Fasting glucose ≥ 100 mg/dL, N(%) | 881 (30.6) | 842 (29.9) | 1874 (31.1) | 1972 (32.4) |
Abbreviations: hsCRP, high-sensitivity C-reactive protein; HDL, high density lipoprotein cholesterol
The metabolic syndrome was defined according to consensus criteria of the American Heart Association and the National Heart, Lung, and Blood Institute (19).
Achieved levels of lipids and high-sensitivity C-reactive protein during follow-up were comparable in older (age ≥ 70 years) and younger subjects. Specifically, among the 89% of subjects with blood samples at 12 months, the median LDL cholesterol in the rosuvastatin group (54 and 55 mg/dL in older and younger subjects, respectively) was half that in the placebo group in each age group. The median high-sensitivity C-reactive protein level (2.3 and 2.2 mg/L in older and younger subjects, respectively) was 36–37% lower in the rosuvastatin group versus placebo, separately in each age group.
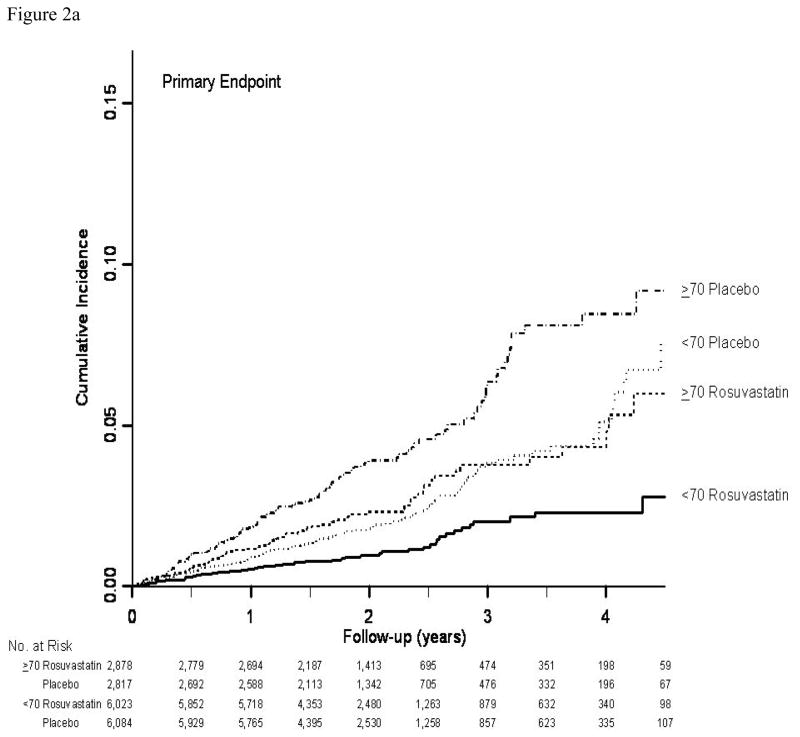
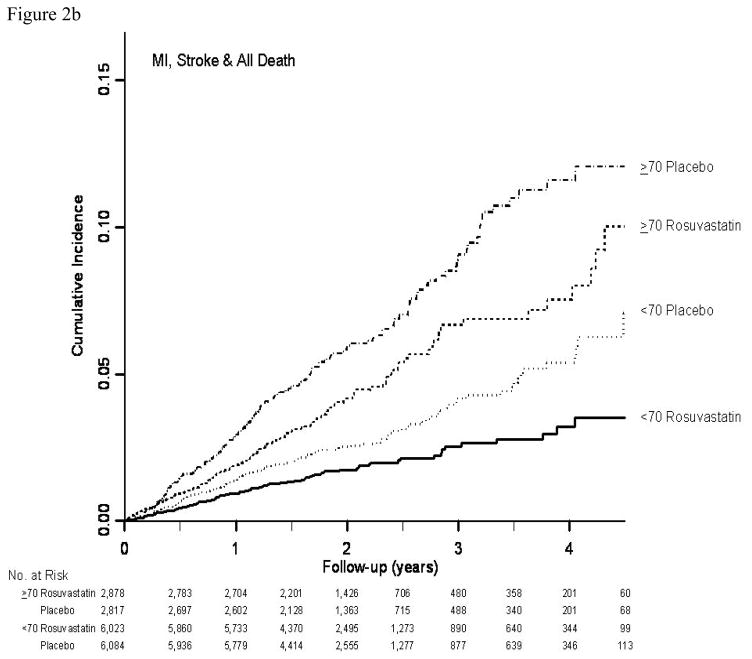
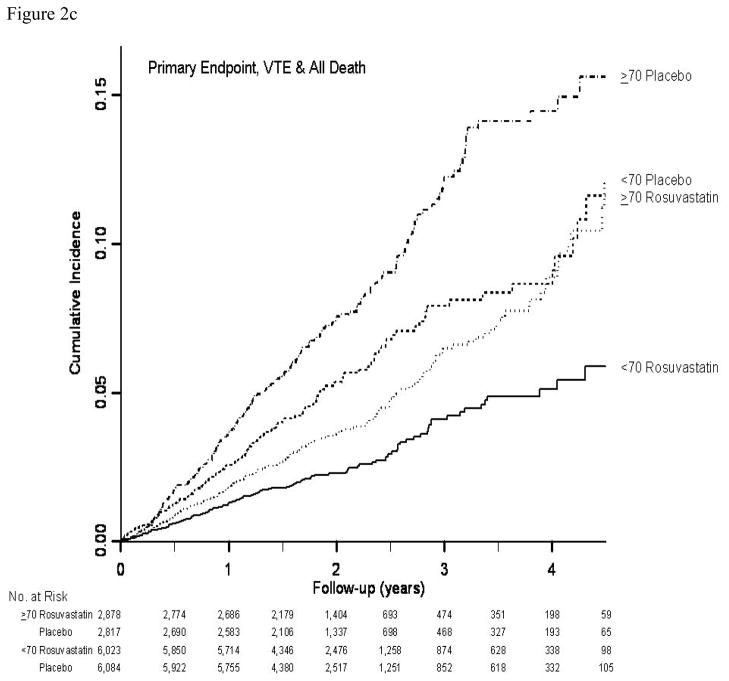
In both age groups combined (12), rosuvastatin was associated with a 44% reduction in the hazard of the primary end point (hazard ratio 0.56; 95% CI: 0.46–0.69; P<0.001). The 32% of participants age 70 years or older accrued 49% (N=194) of the 393 confirmed primary cardiovascular end points in JUPITER (Table 2). For the primary composite cardiovascular end point, as well as for most of its components, relative treatment effects were slightly attenuated in older subjects, but substantial treatment benefits were still seen and there were no significant interactions between age and treatment effect for any outcome (each P>0.10). For the primary end point, as well as for composites including total mortality, a treatment benefit emerged shortly after treatment initiation in both older and younger participants, and no violation of the proportional hazards assumption was seen in either age group (Figure 2a–2c).
Table 2.
Relative and absolute treatment effects according to age
| Rosuvastatin | Placebo | Hazard ratio† (95% CI), P-value | Rate difference† (95% CI) | |||
|---|---|---|---|---|---|---|
| N | Rate* | N | Rate* | |||
| Age 70–97 years (median [maximum] follow-up: 2.0 [5.0] years) | ||||||
| Primary end point | 75 | 1.22 | 119 | 1.99 | 0.61 (0.46–0.82), <0.001 | 0.77 (0.32 to 1.22) |
| Myocardial infarction | 17 | 0.27 | 30 | 0.50 | 0.55 (0.31–1.00), 0.046 | 0.22 (0.00 to 0.44) |
| Stroke | 22 | 0.35 | 39 | 0.64 | 0.55 (0.33–0.93), 0.023 | 0.29 (0.04 to 0.54) |
| Revascularization or hospitalization for unstable angina | 30 | 0.48 | 57 | 0.95 | 0.51 (0.33–0.80), 0.003 | 0.46 (0.16 to 0.76) |
| Cardiovascular death | 21 | 0.34 | 25 | 0.41 | 0.83 (0.47–1.48), 0.53 | 0.07 (−0.14 to 0.29) |
| Any death | 108 | 1.63 | 133 | 2.04 | 0.80 (0.62–1.04), 0.090 | 0.40 (−0.06 to 0.87) |
| Venous thromboembolism | 15 | 0.24 | 25 | 0.41 | 0.59 (0.31–1.11), 0.096 | 0.17 (−.003 to 0.37) |
| MI, stroke or any death | 131 | 2.11 | 183 | 3.04 | 0.70 (0.56–0.87), 0.001 | 0.93 (0.35 to 1.50) |
| Primary endpoint or death or VTE | 165 | 2.69 | 233 | 3.91 | 0.69 (0.56–0.84), <0.001 | 1.23 (0.58 to 1.88) |
| Age 50–69 years (median [maximum] follow-up: 1.9 [5.0] years) | ||||||
| Primary end point | 67 | 0.54 | 132 | 1.06 | 0.51 (0.38–0.69), <0.001 | 0.52 (0.29 to 0.74) |
| Myocardial infarction | 14 | 0.11 | 38 | 0.30 | 0.37 (0.20–0.69), <0.001 | 0.19 (0.08 to 0.30) |
| Stroke | 11 | 0.09 | 25 | 0.20 | 0.45 (0.22–0.91), 0.020 | 0.11 (0.02 to 0.20) |
| Revascularization or hospitalization for unstable angina | 46 | 0.37 | 86 | 0.69 | 0.54 (0.38–0.77), <0.001 | 0.32 (0.14 to 0.50) |
| Cardiovascular death | 14 | 0.11 | 18 | 0.14 | 0.79 (0.39–1.58), 0.50 | 0.03 (−0.06 to 0.12) |
| Any death | 90 | 0.68 | 114 | 0.86 | 0.80 (0.60–1.04), 0.10 | 0.18 (−0.04 to 0.39) |
| Venous thromboembolism | 19 | 0.15 | 35 | 0.28 | 0.55 (0.31–0.96), 0.031 | 0.13 (0.01 to 0.24) |
| MI, stroke or any death | 108 | 0.87 | 170 | 1.35 | 0.64 (0.50–0.81), <0.001 | 0.49 (0.22–0.75) |
| Primary endpoint or death or VTE | 155 | 1.25 | 250 | 2.00 | 0.62 (0.51–0.76), <0.001 | 0.75 (0.44–1.07) |
MI denotes myocardial infarction and VTE venous thromboembolism
Rates are per 100 person-years
Hazard ratios compare hazards in the rosuvastatin group to placebo; rate differences are rates in the placebo group minus those in the rosuvastatin group with 95% confidence intervals based on the normal approximation to the Poisson distribution
Figure 2.
Figure 2a. Cumulative incidence of the primary end point (myocardial infarction, stroke, arterial revascularization, hospitalization for unstable angina, or death from cardiovascular causes) by time, treatment, and age group
Figure 2b. Cumulative incidence of the composite end point including myocardial infarction, stroke or any death by time, treatment, and age group
Figure 2c. Cumulative incidence of the composite end point including the primary end point (myocardial infarction, stroke, arterial revascularization, hospitalization for unstable angina, or death from cardiovascular causes), venous thromboembolism or any death by time, treatment, and age group
The absolute reduction in the incidence of the primary end point associated with rosuvastatin was 48% larger (0.77 versus 0.52 events per 100 person-years) among participants age 70 and older relative to those under 70 years of age. For composite end points that included any death, larger differences in absolute treatment effects were seen between age groups. The estimated number of individuals age 70 or older who need to be treated for 4 years to prevent one primary end point was 24 (95% CI: 15–57) compared with the need to treat 36 (95% CI: 23–77) younger individuals; for the composite including the primary end point, any death or venous thromboembolism, comparable estimates were 17 (95% CI: 12–33) older versus 27 (95% CI: 17–57) younger individuals.
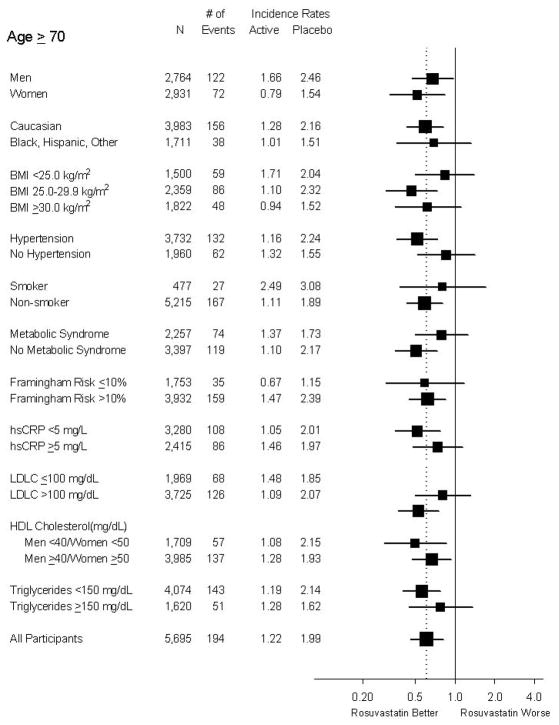
Similar hazard reductions were seen in both older men and women, and no significant heterogeneity was observed across subgroups among older participants (Figure 3). A clear treatment benefit was observed among higher risk older subgroups including those with Framingham risk score above 10%, and those with hypertension.
Figure 3.
Forest plot of the effect of rosuvastatin on the primary end point within subgroups of trial participants aged 70 years of age or older. Hazard ratios with 95% confidence intervals are plotted.
Older participants assigned to placebo had higher rates of any serious adverse event, as well as of most specific adverse events, compared to younger participants (Table 3). Among older participants, rates of muscle weakness, stiffness or pain, renal disorder, bleeding, gastrointestinal disorder, hepatic disorder, and incident diabetes were higher in the rosuvastatin group, but none of these associations was statistically significant (each P>0.10).
Table 3.
Monitored adverse events and other events of interest by age and treatment group
| Age 70–97 years | Age 50–69 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Monitored adverse event | Rosuvastatin | Placebo | Hazard ratio† (95% CI) | Rosuvastatin | Placebo | Hazard ratio† (95% CI) | ||||
| N | Rate* | N | Rate* | N | Rate* | N | Rate* | |||
| Any serious adverse event | 622 | 10.93 | 584 | 10.45 | 1.05 (0.93–1.17) | 730 | 6.07 | 793 | 6.51 | 0.93 (0.84–1.03) |
| Muscle weakness, stiffness or pain | 494 | 8.92 | 467 | 8.50 | 1.04 (0.92–1.19) | 927 | 8.14 | 908 | 7.85 | 1.04 (0.94–1.13) |
| Myopathy | 4 | 0.06 | 3 | 0.05 | 1.31 (0.29–5.84) | 6 | 0.05 | 6 | 0.05 | 1.01 (0.33–3.14) |
| Rhabdomyolosis | 1 | 0 | 0 | 0 | ||||||
| Newly diagnosed cancer | 144 | 2.30 | 155 | 2.54 | 0.91 (0.73–1.14) | 154 | 1.21 | 159 | 1.23 | 0.98 (0.79–1.22) |
| Death from cancer | 18 | 0.27 | 31 | 0.48 | 0.58 (0.32–1.03) | 17 | 0.13 | 27 | 0.20 | 0.63 (0.35–1.16) |
| Gastrointestinal disorder | 665 | 12.41 | 621 | 11.71 | 1.06 (0.95–1.18) | 1088 | 9.72 | 1090 | 9.54 | 1.02 (0.94–1.11) |
| Renal disorder | 222 | 3.63 | 191 | 3.17 | 1.14 (0.94–1.39) | 313 | 2.51 | 289 | 2.28 | 1.10 (0.94–1.29) |
| Bleeding | 127 | 2.04 | 106 | 1.73 | 1.18 (0.91–1.53) | 131 | 1.03 | 169 | 1.32 | 0.78 (0.62–0.98) |
| Hepatic disorder | 61 | 0.96 | 59 | 0.95 | 1.01 (0.71–1.45) | 155 | 1.22 | 127 | 0.99 | 1.24 (0.98–1.57) |
| Newly diagnosed diabetes | 82 | 1.30 | 64 | 1.03 | 1.25 (0.90–1.74) | 188 | 1.48 | 152 | 1.18 | 1.26 (1.02–1.56) |
Rates are per 100 person-years
Hazard ratios compare hazards in the rosuvastatin group to placebo
DISCUSSION
Among the 5,695 randomized participants in the JUPITER trial who were age 70 years or older, rosuvastatin substantially reduced the incidence of major cardiovascular events. In these older people, clear benefits over time emerged shortly after treatment initiation and appeared in analyses of broader end points including total mortality, in separate analyses of myocardial infarction and stroke, among older men and women separately, and in high risk subgroups. The observed relative treatment effects in older people were consistent with those seen in younger trial participants, but absolute event rates and treatment benefits were greater in older people.
The JUPITER trial differed from prior primary prevention trials of statins in its enrollment of an older population, its inclusion of stroke in the primary end point, and its enrollment of individuals with normal LDL cholesterol but elevated high-sensitivity C-reactive protein. Because age is the dominant risk factor for a first cardiovascular event in non-diabetic individuals, the lower age limit and absence of an upper age limit contributed to enrollment of a population that incurred many cardiovascular events. Prevention of stroke is an important treatment target for statin therapy (20), and stroke contributes an increasing proportion of total cardiovascular events with increasing age. Enrollment of individuals with elevated high-sensitivity C-reactive protein contributed further to the identification of a population at increased risk of both coronary heart disease and stroke (21, 22), who were found to benefit from statin therapy in spite of their normal LDL levels.
Meta-analysis of observational studies found that a 1 mmol/L lower total cholesterol level was associated with a 56% reduction (95% CI: 52%–58%) in the hazard of death from ischemic heart disease at ages 40–49, but a 17% reduction (95% CI: 15%–19%) at ages 70–89 years (5). For stroke, a slightly reduced risk associated with lower total cholesterol at ages 40–69 did not persist upon control for blood pressure, and there was no reduction in the hazard of stroke associated with lower cholesterol at ages 70–89. The apparently attenuated associations of total cholesterol levels with cardiovascular events in older people may be partly due to confounding by age-related comorbid conditions (23). Nonetheless, the weakened association of total cholesterol with cardiovascular risk in older people and the limited randomized evidence on statin therapy for primary prevention directed by lipid levels in older people, raise questions about how lipid levels should be used to inform treatment decisions in older people. Relative treatment benefits appear to be independent of baseline lipid levels (24), many older people are at substantial risk in spite of apparently normal lipid levels, and the JUPITER findings indicate that these individuals receive a benefit regardless of their lipid levels if high sensitivity C-reactive protein is elevated.
The JUPITER trial provides new information on the effects of statins in older people that can inform evaluations of the impact of alternative prescribing strategies. Several authors have noted the limitations arising from the need to project prior treatment recommendations for healthy older people from the available randomized evidence in younger people, or in older people with diabetes or prevalent cardiovascular disease, or from the observational data on cholesterol levels and risk of cardiovascular disease (4, 25, 26). For example, in their comparison of alternative treatment strategies, Pletcher et al (26) assumed, in their base-case scenario, that the relative treatment benefit associated with a given LDL cholesterol reduction is much less in an older compared to a younger person. Their sensitivity analyses, under the assumption supported by the JUPITER data that relative treatment benefits are unchanged across age groups, found a preference for strategies more focused on treating older people. The JUPITER trial also points to the need to include stroke prevention in evaluations of cost-effectiveness, both because of its high personal and financial impact as well as its increased percentage of total cardiovascular events with age. Thus, evaluations restricted to coronary events can under-value treatment in older people.
Comorbidity and proximity to death are barriers to preventive care in older people. Physicians reasonably hesitate to initiate a therapy if they expect a patient will not live long enough to benefit. Data from JUPITER indicate that a treatment benefit emerges shortly after initiation, that absolute risk is high, and that the absolute risk reduction is greater in older versus younger individuals. Consideration of composite end points including total mortality provides a treatment evaluation that accounts for the competing risks of death. Adding total mortality to the end point indicates that fewer patients need to be treated to prevent one event.
These exploratory analyses need to be interpreted in light of the overall trial results, but they confirm that the overall treatment effect was reliably seen in older participants. Early stopping of the trial limited the information on the long-term effects of treatment, although cumulative risks between treatment groups continued to diverge up to 4 years of follow-up, and reliable estimates of effects were seen even in subgroups of older participants. Stopping early on the basis of a principled and conservative monitoring plan yields a valid estimate of treatment effects (27) and meets ethical requirements to inform participants when equipoise no longer holds and society when better treatments are available (28,29).
Overall, among individuals age 70 years and older in this randomized trial, rosuvastatin was associated with a significant reduction in the rate of a first major cardiovascular event. Because older participants had much higher event rates, absolute treatment benefits were greater in this age group.
Acknowledgments
by AstraZeneca. Dr. Glynn received additional support from the National Institute on Aging (R01 AG18833).
Supported by a grant from AstraZeneca. Dr. Glynn received additional support from grant AG18833 from the US National Institute on Aging
Footnotes
Reproducible Research Statement:
Protocol: not available
Statistical Code: Available to interested readers by contacting Dr. Glynn at rglynn@rics.bwh.harvard.edu
Data: not available
References
- 1.DeWilde S, Carey IM, Bremner SA, Richards N, Hilton SR, Cook DG. Evolution of statin prescribing 1994–2001: a case of agism but not of sexism? Heart. 2003;89:417–421. doi: 10.1136/heart.89.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients: the treatment-risk paradox. JAMA. 2004;291:1864–1870. doi: 10.1001/jama.291.15.1864. [DOI] [PubMed] [Google Scholar]
- 3.Setoguchi S, Glynn RJ, Avorn J, Levin R, Winkelmayer WC. Ten-year trends of cardiovascular drug use after myocardial infarction among community-dwelling persons > or =65 years of age. Am J Cardiol. 2007;100:1061–7. doi: 10.1016/j.amjcard.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 4.Abramson J, Wright JM. Are lipid-lowering guidelines evidence based? Lancet. 2007;369:168–169. doi: 10.1016/S0140-6736(07)60084-1. [DOI] [PubMed] [Google Scholar]
- 5.Prospective Studies Collaboration. Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. Erratum in: Lancet. 2008; 372:292. [DOI] [PubMed] [Google Scholar]
- 6.National Cholesterol Education Program. NIH Publication No. 01-3305. US Department of Public Health, Public Health Service, National Institute of Health, National Heart, Lung, and Blood Institute; [Accessed July 11, 2009]. ATP III Guidelines At-A-Glance Quick Desk Reference. www.nhlbi.nih.gov/guidelines/cholesterol/atglance.pdf. [Google Scholar]
- 7.Lewis SJ, Moye LA, Sacks FM, et al. Effect of pravastatin on cardiovascular events in older patients with myocardial infarction and cholesterol levels in the average range: results of the Cholesterol and Recurrent Events (CARE) trial. Ann Intern Med. 1998;129:681–689. doi: 10.7326/0003-4819-129-9-199811010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Hunt D, Young P, Simes J, et al. Benefits of pravastatin on cardiovascular events and mortality in older patients with coronary heart disease are equal to or exceed those seen in younger patients: results from the LIPID trial. Ann Intern Med. 2001;134:931–940. doi: 10.7326/0003-4819-134-10-200105150-00007. [DOI] [PubMed] [Google Scholar]
- 9.Wenger NK, Lewis SJ, Herrington DM, et al. Outcomes of using high- or low-dose atorvastatin in patients 65 years of age or older with stable coronary heart disease. Ann Intern Med. 2007;147:1–9. doi: 10.7326/0003-4819-147-1-200707030-00002. [DOI] [PubMed] [Google Scholar]
- 10.Cholesterol Treatment Trialists’ (CTT) Collaborators. Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 14.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS: Air Force/Texas Coronary Artherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 17.Glynn RJ, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Ridker PM. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman DG, Andersen P-K. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–1495. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: executive summary. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 20.Amarenco P, Labreuche J, Lavallée P, Touboul P-J. Statins in stroke prevention and carotid atherosclerosis: systematic review and meta-analysis. Stroke. 2004;35:2902–2909. doi: 10.1161/01.STR.0000147965.52712.fa. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 22.Everett BM, Kurth T, Buring JE, Ridker PM. The relative strength of C-reactive protein and lipid levels as determinants of ischemic stroke compared with coronary heart disease in women. J Am Coll Cardiol. 2006;48:2235–2242. doi: 10.1016/j.jacc.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corti MC, Guralnik JM, Salive ME, Harris T, Ferrucci L, Glynn RJ, Havlik RJ. Clarifying the direct relation between total cholesterol levels and death from coronary heart disease in older persons. Ann Intern Med. 1997;126:753–760. doi: 10.7326/0003-4819-126-10-199705150-00001. [DOI] [PubMed] [Google Scholar]
- 24.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 25.McAlister FA, van Diepen S, Padwal RS, Johnson JA, Majumdar SR. How evidence-based are the recommendations in evidence-based guidelines? PLOS Med. 2007;4:e250. doi: 10.1371/journal.pmed.0040250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pletcher MJ, Lazar L, Bibbins-Domingo K, Moran A, Rodondi N, Coxson P, Lightwood J, Williams L, Goldman L. Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Ann Intern Med. 2009;150:243–254. doi: 10.7326/0003-4819-150-4-200902170-00005. [DOI] [PubMed] [Google Scholar]
- 27.Friedlin B, Korn E. Stopping clinical trials early for benefit: impact on estimation. Clin Trials. 2009;6:119–125. doi: 10.1177/1740774509102310. [DOI] [PubMed] [Google Scholar]
- 28.Korn EL, Friedlin B, Mooney M. Stopping or reporting early for positive results in randomized clinical trials: the National Cancer Institute Cooperative Experience from 1990 to 2005. J Clin Oncol. 2009;27:1712–1721. doi: 10.1200/JCO.2008.19.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sargent D. Early stopping for benefit in National Cancer Institute-sponsored randomized phase III trials: the system is working. J Clin Oncol. 2009:1543–1544. doi: 10.1200/JCO.2008.20.8611. [DOI] [PubMed] [Google Scholar]