Abstract
Background
Cryptococcal meningitis (CM) remains a common AIDS-defining illness in Africa and Asia. Sub-clinical cryptococcal antigenemia is frequently unmasked with antiretroviral therapy (ART). We sought to define the cost-effectiveness of serum cryptococcal antigen (CRAG) screening to identify persons with sub-clinical cryptococcosis and the efficacy of preemptive fluconazole.
Methods
609 ART-naïve adults with AIDS initiating ART in Kampala, Uganda had a serum CRAG prospectively measured during 2004–2006. The number needed to test/treat (NNT) with a positive CRAG was assessed for ≥30-month outcomes.
Results
In the overall cohort, 50 persons (8.2%) were serum CRAG positive when starting ART. Of 295 people with CD4+ ≤ 100cells/μL and without prior CM, 26 (8.8%: 95% CI: 5.8–12.6%) were CRAG positive of whom 21 were promptly treated with fluconazole (200–400mg) for 2–4 weeks. Clinical CM developed in 3 fluconazole-treated persons, and 30-month survival was 71% (95% CI: 48%–89%). In the 5 CRAG positive persons with CD4+≤ 100 cell/μL treated with ART but did not fluconazole, all died within 2 months of ART initiation.
The NNT with CRAG screening and fluconazole to prevent one CM case is 11.3 (95%CI: 7.9–17.1) at costs of $190 (95%CI: $132–$287). The NNT to save one life is 15.9 (95%CI: 11.1–24.0) at costs of $266 (95% CI: $185–$402). The cost per disability-adjusted life year (DALY) saved is $21 (95%CI: $15 to $32).
Conclusions
Integrating CRAG screening into HIV care, specifically targeting people with severe immunosuppression (CD4+≤ 100 cells/μL) should be implemented in treatment programs in resource-limited settings. ART alone is insufficient treatment for CRAG-positive persons.
Keywords: HIV, Cryptococcal Meningitis, Screening, Cost analysis, antiretroviral therapy
Introduction
The global annual burden of cryptococcal meningitis (CM) is estimated at 957,900 cases resulting in an estimated 624,700 deaths within 3 months of cryptococcal infection.[1] Sub-Saharan Africa has the highest burden with a median incidence of 3.2% among all HIV-infected people resulting in 720,000 CM cases annually with high mortality of 20–50%.[2–5] Even with antiretroviral therapy (ART) availability, CM-related mortality remains high.[5, 6] Most CM occurs in AIDS patients with advanced immunosuppression. Among patients with CM in Tanzania and Uganda, 80–90% had CD4+ T-cell counts ≤ 100 cells/μL.[4, 5, 7] Most CM occurs in ART naïve persons;[8] however, the unmasking of ART-associated CM within the first weeks is also common. We have previously reported a high incidence of ART-associated CM in patients who are cryptococcal antigen (CRAG) positive who are not treated with fluconazole.[9]
Early diagnosis and treatment is paramount to reducing CM-related mortality. CM is a sub-acute meningitis in which the polysaccharide CRAG is detectable in serum a median of 3 weeks prior to onset of clinical symptoms.[7, 10] The subacute nature allows for effective interventions. Methods to prevent this CM-related mortality would include: 1) earlier HIV diagnosis and ART initiation prior to AIDS, 2) primary prophylaxis with fluconazole in persons with AIDS, and 3) screening and treatment for occult cryptococcosis. Both earlier ART and primary fluconazole prophylaxis are effective interventions,[11–13] yet widespread implementation requires improvements in HIV testing and treatment infrastructure. For those people presenting with already advanced HIV, preventative options are limited.
Despite published data on increased mortality in patients with cryptococcal antigenemia, the utility of serum CRAG testing to identify asymptomatic cryptococcosis and the clinical impact of preemptive fluconazole treatment have not been clearly defined in the ART era.[14, 15] We analyzed the cost-effectiveness of performing routine testing of asymptomatic persons for serum CRAG at time of ART initiation in Uganda, specifically in persons with CD4+≤100 cells/μL.
Methods
Data were collected from a prospective cohort of 609 ART-naïve people who initiated ART between 2004–2006 at the Infectious Disease Institute (IDI) in Kampala, Uganda, of whom 559 have been previously clinically described.[9] In brief, ART eligible adults (≥ 18 years) were enrolled if they had: (1) confirmed HIV-1, (2) regular clinic attendance, based on ≥2 clinic visits in the prior 6 months, (3) stable residence within ≤20 km, (4) willingness to exclusively receive HIV care at the clinic for ≥2 years, (5) ART eligible according to WHO 2003 and National Ministry of Health guidelines: CD4+ count ≤200 cell/μL or WHO stage IV, (6) informed consent.
The first-line ART regimen prescribed was stavudine or zidovudine, plus lamivudine, and either nevirapine or efavirenz. Daily co-trimoxazole prophylaxis was provided regardless of CD4+ count. CD4+ was measured every 3 months (Becton Dickinson, Mountain View, CA). At enrollment, study physicians conducted clinical evaluations, including full medical history and physical examination. A qualitative, undiluted serum CRAG was measured at ART initiation regardless of symptoms (Wampole Laboratories, Princeton, NJ). During 2004–2006, no clinic-wide protocol existed for clinical intervention with a positive CRAG in an asymptomatic person, thus intervention depended on physician discretion. Patients were followed for a median of 3.9 years (minimum 2.5 years). No patients were lost to follow up. This research was approved by the ethics committees of Makerere University and Uganda National Council for Science and Technology.
We subsequently assessed interventions and patient-outcomes within this prospective cohort using chart review. We determined the mean incidence with 95% confidence interval (95% CI) of symptomatic CM disease, and the number needed to test and treat (NNT) prior to ART initiation to prevent one case of CM or one death as well as the costs associated with screening.
The cost of CRAG testing was calculated based on current actual cost in Kampala, Uganda. In 2010, the CRAG cost was US$16.75 at the Makerere University-Johns Hopkins University (MU-JHU) laboratory, a College of American Pathologists (CAP)-certified laboratory in Kampala, Uganda which adheres to CAP and Good Clinical Laboratory Practice (GCLP) standards for all testing. This CRAG cost is a total cost encompassing reagents ($5.43/test; 32% of CRAG cost), daily positive/negative quality controls ($2.72; 16%), lab disposable supplies ($0.95; 6%), labor ($3.04; 18%), external quality assurance testing ($0.62; 4%), laboratory overhead ($3.19; 19%), margin ($0.80; 5%). The MU-JHU Laboratory is a financially self-sustainable laboratory which is not subsidized from external sources. The lab survives solely on testing income to provide world-class lab testing in a resource-limited setting. Thus, the cost estimate used was a real world, actual cost. This real world cost, never-the-less, may be higher than laboratory costs in other countries. The 2010 CRAG cost in South Africa via the National Health Laboratory System (NHLS) is $5.61 (33%).
The cost of fluconazole was not included in the model because the average anti-fungal medicine use was less in the screened and preemptively treated group (2–4 weeks), than in the untreated group who developed clinical CM requiring longer and more expensive anti-fungal treatment courses (i.e. 2 weeks of amphotericin induction, 8 weeks of fluconazole 400mg consolidation, and then secondary fluconazole 200mg prophylaxis). Amphotericin cost ($17.50/day) is based on 2010 actual Ugandan costs, which are two-fold higher than 2009 negotiated costs in South Africa.
Results
Patients and Cryptococcal Antigen (CRAG) screening
Of 609 HIV-infected adults with CD4+<200 cells/μL, 50 (8.2%) were CRAG+ when starting HIV therapy (Figure 1). Of the cohort, 418 (69%) were female, and the mean cohort CD4+ was 79 cells/μL with 311 (51%) patients having CD4+ counts ≤100 cells/μL. The median CD4+ of CRAG+ persons was 15 (IQR: 4 to 59) cells/μL and lower than the overall cohort (P<.001). Of 50 CRAG+ persons, 17 had pre-ART diagnosed and treated CM, and were excluded from analysis of screening, thus 33 (5.6%) had incident cryptococcemia. Of 295 people with CD4+ ≤100 cells/μL and without prior CM, 26 persons were CRAG+ and were classified as having incident cryptococcal antigenemia (8.8%; 95% CI: 5.8–12.6%). All were relatively asymptomatic, and none had clinical symptoms concerning for meningitis. Fluconazole therapy was given to 21 with doses varying between 200–400mg for 2–4 weeks duration. Only three treated with fluconazole developed CM at a median time of 4 weeks during a median follow up time of 47 months (minimum: 30 months).
Figure 1. Study Profile.
Of 295 people with CD4+≤ 100 cells/μL without CM, 26 (8.8%; 95% CI: 5.8–12.6%) were serum CRAG positive prior to initiating ART.
Mortality
There were 77 HIV-related deaths in the overall cohort with 19 (25%) attributed to central nervous system infections.[9] Eleven people died of known CM, and all were CRAG+ at baseline (7 incident CM, 4 prior CM). Among people without prior CM with a positive CRAG and CD4+≤ 100 cells/μL, the mortality rate was 42% (11/26; 95% CI: 23% to 63%). Of 21 who received fluconazole therapy, 6 (29%) died during follow-up (Figure 1). Causes of death included: CM (n=3); d4T-related lactic acidosis (n=1); putative toxoplasmosis (n=1), and unknown (n=1). Of deaths, two received fluconazole 400mg while four received fluconazole 200mg for 2 weeks.
In five persons with CD4+≤ 100 cells/μL treated with ART only and without fluconazole, putative causes of death included: CM (n=2); lymphoma (n=1); toxoplasmosis (n=1) and unknown (n=1), all occurring within 2 months of starting ART. Retrospectively, in the absence of imaging studies and biopsy diagnoses, the toxoplasmosis and lymphoma may have been inflammatory intra-parenchymal masses due to cryptococcal immune reconstitution inflammatory syndrome (IRIS); however, consent for post-mortem examinations were not given.
In those with higher CD4+ >100 cells/μL, the CRAG+ incidence was 2.3% (7/298), excluding one person with known prior CM. Of the 7 CRAG+, 86% (6/7) survived. Of the six survivors, four received fluconazole and two remained asymptomatic without fluconazole. The one person who died did not receive fluconazole. In all people with incident cryptococcal antigenemia, fluconazole use was associated with survival (OR=9.5; 95% CI: 1.5 to 60, P=.017). The 2-year survival without fluconazole among all incident CRAG+ subjects was 25% and was 0% in those with CD4+≤100 cells/μL (Figure 2). In a multivariate logistic regression model, fluconazole remained independently associated with survival (OR=34.6; 95% CI: 1.7 to 703: P=.021) among all 33 subjects with incident CRAG+. Baseline CD4+ was loosely associated with >30-month survival with increasing odds foreach pre-ART 25 CD4+/μL increase above zero (OR=1.9; 95% CI: .96 to 3.8; P=.064). Baseline viral load was associated with mortality in a univariate model (P=.013); however in multivariate regression models, viral load, CD4%, weight, height, BMI, age, sex, or hemoglobin were not predictive of 30-month survival whenever fluconazole use was included in the model.
Figure 2. Survival of People with Asymptomatic Cryptococcal Antigenemia starting HIV Therapy.
Kaplan-Meier curve displays the 2 year survival with and without preemptive fluconazole use in 33 asymptomatic people starting HIV antiretroviral therapy (ART) who have a positive serum cryptococcal antigen (CRAG) test. Survival curves are adjusted for pre-ART CD4 as a covariate by Cox-regression. Without CD4 adjustment, the survival in ‘No Fluconazole’ is 25% in all people and 0% in persons with CD4+<100 cells/μL. No mortality occurred after 2 years through a median follow up of 3.9 years on ART. Follow up was complete, and no persons were right-hand censored.
Cost-Effectiveness
Using these outcomes, a cost-effectiveness analysis was performed excluding the 17 persons with prior known history of cryptococcosis. The analysis determined the number needed to CRAG test and treat with fluconazole to prevent CM and CM-related death. In order to detect one person who was CRAG+, the NNT for screening was 11.3 (95% CI: 7.9–17.1) in those with CD4+≤100 cells/μL. To prevent one death, 15.9 (95% CI: 11.1–24.0) people would need to be screened and treated to increase survival for one person for >30 months on ART.
Based on the CRAG cost at a CAP-certified clinical laboratory ($16.75/test), this translates into $190 (95% CI: $132–$286) to detect one asymptomatic person. To save one person’s life by preemptive fluconazole therapy, the cost is $266 (95% CI: $185 to $402) to prevent one fatal CM case occurring within 30 months of ART initiation. Assuming an average increase in life expectancy of 12.5 years with ART,[16] this equates to $21 (95% CI: $15 to $32) per disability-adjusted life year (DALY) saved. The functional quality of life was normal in survivors with a median Karnofsky performance status of 100 at last follow up (mean 96, minimum 90).
In the absence of CD4+ testing, using WHO clinical stage criteria of screening all people with stage III/IV disease or CD4+<200 cells/μL, the cost to benefit ratio was less favorable. For the total cohort, the asymptomatic CRAG positivity was 5.6% (95% CI: 3.9% to 7.7%). The NNT for CRAG screening to prevent one death was 31.5 (95% CI: 22.7 to 45.4) with a cost of $527 (95% CI: $380 to $760) assuming mean fluconazole efficacy (76%) and mean survival without preemptive therapy (25%) among the total cohort. In using WHO clinical stage criteria, the cost per DALY saved is $42 (95% CI: $30 to $61). Specifically, the incremental cost of screening persons with CD4+ counts between 101–200 cells/μL is a NNT of 148 (95% CI: 69 to 401) with estimated costs of $2,479 (95% CI: $1,153 to $6,723) to prevent one death.
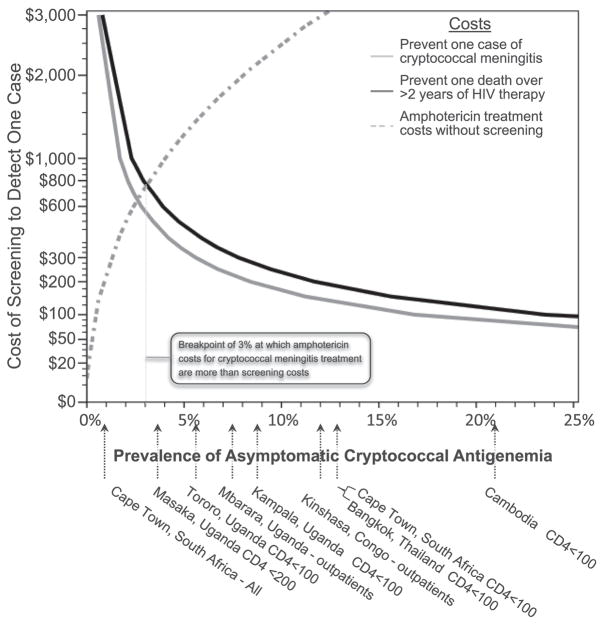
These estimates do not include the avoidable costs of hospitalization for clinical CM and amphotericin costs (~$245 for 14 days of amphotericin deoxycholate). Above a CRAG+ prevalence of approximately 3%, the amphotericin deoxycholate cost for CM treatment becomes exponentially greater than the CRAG screening cost. This does not include the costs of hospitalization and supportive care which are highly variable by region.
Using mean estimates for survival and effectiveness of fluconazole therapy, we present cost-threshold estimates for screening in populations with different CRAG+ prevalence (Figure 3). For example, in populations with a CRAG prevalence of ≥4.7%, the cost of CRAG screening when starting ART is ≤$500 to prevent one death and is $40 per DALY. Conversely in this scenario, if health systems chose not to implement CRAG screening, approximately $1150 in amphotericin costs would accrue with worse survival outcomes.
Figure 3. Cost of Serum Cryptococcal Antigen Screening based on Asymptomatic Prevalence.
The figure displays the relative cost-effectiveness of cryptococcal antigen (CRAG) screening and preemptive fluconazole therapy based on the prevalence of antigenemia within a given population and outcomes from Kampala, Uganda. The cost to prevent one case of clinical cryptococcal meningitis (black line) and to prevent one death from CM (gray line) are presented with survivors living for >2.5 years on ART. Below the x-axis, displays the reported cross-sectional prevalence rates of asymptomatic antigenemia in various outpatient clinic populations in HIV-infected people without a prior history of cryptococcal meningitis. [10, 12, 14, 15, 17–19] Above a prevalence of approximately 3%, the cost of amphotericin deoxycholate for treatment of people unmasking ART-associated cryptococcal meningitis ($245 per person) is greater than the costs of screening and treating with preemptive fluconazole, not including the additional hospitalization costs. CRAG screening costs are based on $16.75 per test, and different implementation costs would affect the cost-benefit curves proportionally.
Economies of Scale for CRAG Testing
Our 2010 CRAG testing cost was $16.75; however this is based the actual volume of 3–5 CRAG tests per day at the MU-JHU laboratory in Uganda. A previous published CDC-Uganda cost estimate was $3.97 per sample for CRAG testing with bulk purchase of reagents only.[15] The CRAG testing cost is highly dependent on the testing volume, as there are fixed costs, such as: laboratory overhead, external quality assurance (EQA), and daily quality control (QC) which do not increase with additional testing. The QC cost per test is highly dependent on volume, whereby for each batch of samples tested – one positive and one negative control are used.
There are additional efficiencies in batch testing. By incorporating a simple model based on increased testing volume, the price per test decreases dramatically. For example, if CRAG testing increased from 3–5 tests to 16–20 tests per day, the CRAG cost would decline by 45% ($9.29 per test), due to a drop in reagents costs by bulk purchasing (3.97/test),[15] distribution of the daily QC ($0.15/test), and annual EQA costs ($0.15/test) which all are fixed costs. As well, there would be a moderate reduction in the cost of disposable supplies ($0.72/test) and labor costs ($2.10/test) because the lab preparation time and effort are applied for the whole run. Finally, the overhead ($1.77/test) and margin ($.044/test) are affected accordingly, based on distributing these costs on increased volume.
Using these estimates for increased CRAG testing, the overall cost-effectiveness is further beneficial. Incorporating these economies of scale, to save one person’s life by preemptive fluconazole therapy, the cost is $148 (95% CI: $103 to $223) to prevent one fatal CM case, and the cost is $12 (95% CI: $8 to $18) per DALY saved. With the list cost in South African NHLS (i.e. one-third the estimate used here), the cost of screening is $7 (95% CI: $5 to $11) per DALY saved, under the same conditions. We believe that pre-ART screening in persons with CD4+≤100 cells/μL is highly beneficial.
Discussion
We report a prevalence of new cryptococcal antigenemia of 8.8% among patients with CD4+ T-cell counts ≤100 cells/μL at this urban health facility in Kampala, Uganda, with an overall prevalence of 13.5% including those with prior CM. In this representative prospective Sub-Saharan African cohort, we have shown that initial CRAG screening prior to starting ART in patients with CD4+T cell counts ≤ 100 cells/μL can prevent disease and death in 8% of patients started on ART. These prevalence rates are comparable with 21% in Cambodia, 13% in South Africa, 12.9% in Bangkok, and 5.8% in Tororo, Uganda among patients with CD4+≤100 cells/μL.[10, 14, 15, 17] CRAG+ prevalence in outpatients has also been reported from Masaka, Uganda (3.8%), Mbarara, Uganda (7.5%), and Kinshasa, Congo (12.2%).[12, 18, 19] In the absence of ART, occult cryptococcal antigenemia precedes clinical CM symptoms by a median of 22 days.[7] Unfortunately, the attributable mortality risk being CRAG+ is 17–18% in rural community cohorts from Uganda,[7, 15] and asymptomatic, untreated cryptococcal antigenemia independently predicts death during the first 12 weeks of ART.[1, 20, 21] The unmasking of clinical CM after initiating ART is relatively common accounting for 30% of CM diagnosed in two 2006 African cohorts and the proportion is likely increasing [7, 15] These data reiterate the importance of screening for opportunistic infections prior to initiating ART.[20, 21]
The 2009 U.S. D.H.H.S. guidelines for managing opportunistic infections unequivocally state, “because the incidence of cryptococcal disease is relatively low, routine testing of asymptomatic people for serum cryptococcal antigen is not recommended (DIII),” nor is primary prophylaxis recommended in the U.S.[22] Yet, we strongly advocate that a different standard is necessary in resource-limited settings where the prevalence of asymptomatic antigenemia is high and the numbers starting ART with AIDS is also still alarmingly commonplace. In Africa, fluconazole primary prophylaxis is effective and safe in improving survival in people with CD4+<200 cells/μL.[12] We would extend this finding further in that those who are not receiving fluconazole prophylaxis with CD4+<100 cells/μL should receive CRAG screening prior to ART initiation.
One necessity for a good screening test is an effective intervention. In our experience, untreated patients had a 75% mortality rate overall and 100% of those with CD4+≤ 100 died. With use of fluconazole, 71% survived for >2.5 years. The 75% mortality is unfortunately similar to published CM-related 12 week mortality in Africa.[1, 5, 8, 23] The untreated CRAG+ survival (25%) in Uganda was lower than that seen in a prior cohort which reported a 56% survival in Cape Town, South Africa.[14] While Uganda is a resource-limited region, the differences in survival are likely partially related to the degree of immunosuppression of the Uganda cohort. In CRAG+ persons, the median CD4s were 15 cells/μL in Uganda vs. 46 cells/μL in South Africa.[14] We previously have reported that Ugandans with CD4+<25 cells/μL have 2.5-fold higher odds of mortality after starting ART.[9]
No previous studies have investigated the cost-benefit of CRAG screening in HIV-infected persons to identify those with cryptococcosis in sub-Saharan Africa, nor determined the optimal treatment strategy. Our prospective observational experience strongly favors fluconazole therapy for asymptomatic cryptococcal antigenemia, and that ART alone is insufficient. This differs from other opportunistic infections such as Kaposi Sarcoma where ART alone can be curative in localized disease.[24, 25] The optimal dose and duration of fluconazole is unknown. The majority of people in this study received between 2–4 weeks of fluconazole at doses of 200–400mg. Higher doses of fluconazole are likely to be more effective as 800mg of fluconazole is fungicidal whereas dosing at ≤400mg is fungistatic.[23] Based on the available data, we would recommend 800mg fluconazole for at least a 4-week course in combination with ART for CRAG+ asymptomatic persons without clinical meningitis. Prospective trials of the optimal preemptive therapy in these patients are warranted. Limitations of the study include its observational nature whereas a randomized trial would have been more ideal. Our cost-effectiveness analysis was conservative and did not include further savings from reduced antifungal use and savings from avoidance of hospitalizations. All of these would enhance the cost-effectiveness of the CRAG screening as would economies of scale. The moderate size of the cohort allows for an approximate but non-precise estimate. The magnitude of the benefit of CRAG screening is overwhelming, such that the degree of precision should not cast doubt on the efficacy of CRAG screening and treatment. The local prevalence of CRAG antigenemia and the degree of immunosuppression will affect the absolute benefit.
Conclusion
We demonstrate the cost-effectiveness of CRAG testing in resource-limited settings in persons with CD4+≤ 100 cells/μL initiating ART and that ART alone is insufficient. We believe serum cryptococcal antigen screening should be integrated in national ART treatment programs in Sub-Saharan Africa, specifically targeting patients with severe immunosuppression (CD4+≤100 cells/μL), as CRAG screening is both cost-effective and affordable to reduce early mortality on ART.
Acknowledgments
DBM, DRB, YCM were involved in concept design and data analysis. BC, BAC, were involved in data collection. MRK, PRB, AK, DBM, YCM and DRB were involved in critical revision of the manuscript for important intellectual content. We thank all the Medical Officers and Nursing staff at the Infectious Disease Institute (IDI) who managed the patients and Ms. Agnes Kiragga for assisting with data extraction.
FUNDING
The Infectious Disease Institute is supported in part by a philanthropic grant from Pfizer pharmaceuticals (BC, AK, and MK), and this research by the University of Minnesota Academic Health Center (PRB and DBM), and NIH (K23AI073192-01A2 DRB; R34 AI081554 DRB, DBM, PRB).
Footnotes
FINANCIAL DISCLOSURES
All the authors listed have no financial disclosure to declare.
ACCESS TO DATA
(DBM) and (DRB) have full access to all study data and take responsibility for the integrity and accuracy of the data analysis.
CONFLICTS OF INTEREST
Pfizer manufactures and donates fluconazole for use in Sub-Saharan Africa via the Pfizer Diflucan Partnership program. Pfizer had no role in any aspect of this project.
The authors have no potential conflicts of interest.
References
- 1.Park BJ, Wannwmuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Mayanja-Kizza H, Oishi K, Mitarai S, et al. Combination therapy with fluconazole and flucytosine for cryptococcal meningitis in Ugandan patients with AIDS. Clin Infect Dis. 1998;26:1362–6. doi: 10.1086/516372. [DOI] [PubMed] [Google Scholar]
- 3.Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS. 2007;21:2119–29. doi: 10.1097/QAD.0b013e3282a4a64d. [DOI] [PubMed] [Google Scholar]
- 4.Kisenge PR, Hawkins AT, Maro VP, et al. Low CD4 count plus coma predicts cryptococcal meningitis in Tanzania. BMC Infect Dis. 2007;7:39. doi: 10.1186/1471-2334-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kambugu A, Meya DB, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. 2008;46:1694–701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bicanic T, Meintjes G, Rebe K, et al. Immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis: a prospective study. J Acquir Immune Defic Syndr. 2009;51:130–4. doi: 10.1097/QAI.0b013e3181a56f2e. [DOI] [PubMed] [Google Scholar]
- 7.French N, Gray K, Watera C, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16:1031–8. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- 8.Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45:76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 9.Castelnuovo B, Manabe YC, Kiragga A, Kamya M, Easterbrook P, Kambugu A. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first 3 years after antiretroviral therapy initiation in an urban African cohort. Clin Infect Dis. 2009;49:965–72. doi: 10.1086/605500. [DOI] [PubMed] [Google Scholar]
- 10.Micol R, Lortholary O, Sar B, et al. Prevalence, determinants of positivity, and clinical utility of cryptococcal antigenemia in Cambodian HIV-infected patients. J Acquir Immune Defic Syndr. 2007;45:555–9. doi: 10.1097/QAI.0b013e31811ed32c. [DOI] [PubMed] [Google Scholar]
- 11.Singh N, Barnish MJ, Berman S, et al. Low-dose fluconazole as primary prophylaxis for cryptococcal infection in AIDS patients with CD4 cell counts of < or = 100/mm3: demonstration of efficacy in a positive, multicenter trial. Clin Infect Dis. 1996;23:1282–6. doi: 10.1093/clinids/23.6.1282. [DOI] [PubMed] [Google Scholar]
- 12.Parkes-Ratansh R, Kamali A, Wakeham K, et al. Successful primary prevention of cryptococcal disease using fluconazole prophylaxis in HIV-infected Ugandan adults, Abstract 32. CROI, 16th Annual Conference; Montreal, Canada. 2009. [Google Scholar]
- 13.Wright P, Inverarity D. Human immunodeficiency virus (HIV) related cryptococcal meningitis in rural central Thailand--treatment difficulties and prevention strategies. Southeast Asian J Trop Med Public Health. 2007;38:58–61. [PubMed] [Google Scholar]
- 14.Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48:856–62. doi: 10.1086/597262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liechty CA, Solberg P, Were W, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007;12:929–35. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 16.Walensky RP, Wolf LL, Wood R, et al. When to start antiretroviral therapy in resource-limited settings. Ann Intern Med. 2009;151:157–66. doi: 10.7326/0003-4819-151-3-200908040-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pongsai P, Atamasirikul K, Sungkanuparph S. The role of serum cryptococcal antigen screening for the early diagnosis of cryptococcosis in HIV-infected patients with different ranges of CD4 cell counts. J Infect. 2010 doi: 10.1016/j.jinf.2010.03.015. In Press. [DOI] [PubMed] [Google Scholar]
- 18.Tassie JM, Pepper L, Fogg C, et al. Systematic screening of cryptococcal antigenemia in HIV-positive adults in Uganda. J Acquir Immune Defic Syndr. 2003;33:411–2. doi: 10.1097/00126334-200307010-00019. [DOI] [PubMed] [Google Scholar]
- 19.Desmet P, Kayembe KD, De Vroey C. The value of cryptococcal serum antigen screening among HIV-positive/AIDS patients in Kinshasa, Zaire. AIDS. 1989;3:77–8. doi: 10.1097/00002030-198902000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Jenny-Avital ER, Abadi M. Immune reconstitution cryptococcosis after initiation of successful highly active antiretroviral therapy. Clin Infect Dis. 2002;35:e128–33. doi: 10.1086/344467. [DOI] [PubMed] [Google Scholar]
- 21.Lawn SD, Bekker LG, Myer L, Orrell C, Wood R. Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. AIDS. 2005;19:2050–2. doi: 10.1097/01.aids.0000191232.16111.f9. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan J, Benson C, Holmes K, Brooks J, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR. 2009;58:1–207. [PubMed] [Google Scholar]
- 23.Longley N, Muzoora C, Taseera K, et al. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis. 2008;47:1556–61. doi: 10.1086/593194. [DOI] [PubMed] [Google Scholar]
- 24.Aversa SM, Cattelan AM, Salvagno L, et al. Treatments of AIDS-related Kaposi’s sarcoma. Crit Rev Oncol Hematol. 2005;53:253–65. doi: 10.1016/j.critrevonc.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Cattelan AM, Calabro ML, De Rossi A, et al. Long-term clinical outcome of AIDS-related Kaposi’s sarcoma during highly active antiretroviral therapy. Int J Oncol. 2005;27:779–85. [PubMed] [Google Scholar]