Abstract
Complement cascade (CC) becomes activated and its cleavage fragments play a crucial role in the mobilization of hematopoietic stem/progenitor cells (HSPCs). Here, we sought to determine which major chemottractant present in peripheral blood (PB) is responsible for the egress of HSPCs from the BM. We noticed that normal and mobilized plasma strongly chemoattracts HSPCs in a stromal derived factor-1 (SDF-1)-independent manner because i) plasma SDF-1 level does not correlate with mobilization efficiency, ii) the chemotactic plasma gradient is not affected in the presence of AMD3100, and iii) it is resistant to denaturation by heat. Surprisingly, the observed loss of plasma chemotactic activity after charcoal stripping suggested involvement of bioactive lipids and we focused on sphingosine-1 phosphate (S1P), a known chemoattracant of HSPCs. We found that S1P i) creates in plasma a continuously present gradient for BM-residing HSPCs, ii) is at physiologically relevant concentrations a chemoattractant several magnitudes stronger than SDF-1, and iii) its plasma level increases during mobilization due to CC activation and the interaction of membrane attack complex (MAC) with erythrocytes that are a major reservoir of S1P. We conclude and propose a new paradigm that S1P is a crucial chemoattractant for BM-residing HSPCs and that CC via MAC induces release of S1P from erythrocytes for optimal egress/mobilization of HSPCs.
Keywords: S1P, SDF-1, CXCR4, stem cells, homing, MAC
Introduction
Hematopoietic stem/progenitor cells (HSPCs) circulate in peripheral blood (PB) under steady state conditions at a very low level to keep a pool of stem cells in balance in the bone marrow (BM) microenvironment located in distant bones. Therefore, PB could be envisioned as a highway by which HSPCs relocate in the body between hematopoietic BM endosteal and endothelial niches. HSPCs are mobilized from BM into PB during infection,1,2 and tissue injury,3,4 and after administration of some pharmacological agents [e.g., granulocyte colony stimulating factor (G-CSF)5 or some polysaccharides (e.g., Zymosan)6]. However, the molecular mechanisms controlling mobilization of HSPCs are still not well understood. Evidence is accumulating that the crucial role in this process involves attenuation of the stromal-derived growth factor-1 (SDF-1)-CXCR4 interaction between BM-secreted SDF-1 and HSPC-expressed CXCR47 and the adhesive interaction between Very Late Antigen-4 (VLA-4; α4β1 integrin) expressed on HSPCs and its ligand Vascular Adhesion Molecule-1 (VCAM-1; CD106), which is expressed in the BM microenvironment.8–11
Increasing evidence demonstrates also that HSPC mobilization is regulated/orchestrated by elements of innate immunity, in particular by complement cascade (CC) protein cleavage fragments12,13 and neutrophils,14–16 which all play a pivotal and, until recently, underappreciated role in this process. Recently we reported that CC is activated in BM during mobilization of HSPCs and that C5 cleavage fragments direct egress of HSPCs from BM into PB mainly by promoting proteolytic activity of the BM environment and inducing BM egress of granulocytes. Granulocytes are the first cells to egress and thus pave the way for HSPCs to follow in their footsteps.16
In this study, we sought to determine which major chemottractant is present in PB that is responsible for egress of HSPCs and whether activation of CC plays some role in its level/expression. We observed that plasma derived from normal and mobilized PB strongly chemoattracts murine BM HSPCs and that this chemotactic effect was not dependent on plasma SDF-1 levels because: i) it occurs in the presence of the CXCR4 antagonist AMD3100; ii) it is robust to heat-inactivated plasma; and iii) ELISA studies revealed negligible concentrations of SDF-1 in plasma, which did not correlate with good or poor mobilizer status. However, the chemotactic activity of plasma was abolished after charcoal stripping what suggested involvement of bioactive lipids. Based on this we focused on sphingosine-1 phosphate (S1P), a known chemoattractant for HSPCs, whose major reservoir in PB is erythrocytes.17,18 To support this notion plasma isolated from mobilized mice contains traces of free hemoglobin and the level of hemolysis correlated with CC activation and generation of membrane attack complex (MAC).
Based on this we postulate that HSPCs are actively retained/anchored in BM niches in for example, a SDF-1-CXCR4 and VCAM-1-VLA-4 dependent manner that counteracts the continuous influence of the S1P-mediated chemotactic gradient of plasma. During mobilization CC becomes activated and MAC interaction with circulating erythrocytes leads to an additional increase in plasma S1P. Thus, S1P is a crucial plasma component that chemoattracts HSPCs and directs their release from the BM niches into PB.
Materials and Methods
Animals
Pathogen-free, 4- to 6-week-old C5−/−, C5+/+ mice were purchased from the Jackson Laboratory (Bar Harbor, ME; http://www.jax.org). C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD; http://www.cancer.gov). All mice were allowed to adapt for at least 2 weeks and used for experiments at age 6 to 8 weeks. Animal studies were approved by the Animal Care and Use Committee of the University of Louisville (Louisville, KY).
Plasma
PB samples were obtained from 16 patients. Clinical characteristics of these patients are presented in Table 1 (see supplement). There were 11 male and 5 female patients, 8 diagnosed with non-Hodgkin lymphoma (NHL), 5 with Hodgkin's lymphoma (HL), 2 with germ cell tumor, and one with active myoblastic leukemia (AML), who had been mobilized with chemotherapy followed by G-CSF (Filgrastim 5μL/kg BID; Amgen, Thousand Oaks, CA) in accordance with guidelines approved by the Human Ethics Research Board of the Faculty of Medicine and Dentistry at the University of Alberta, Edmonton, Canada. During mobilization CD34+ cell and white blood cell (WBC) counts were monitored. Patients were classified as good mobilizers when their CD34+ cell count reached > 50 cells/μL, and as poor mobilizers when it was below 50 cells/μL, on the day of leukapheresis.
Murine plasma was obtained from the vena cava (1 ml syringe containing 50 μl of 100 mM EDTA) from normal and AMD3100-, or zymosan-mobilized mice. Plasma samples were prepared by taking the top fraction after centrifugation at 1,000×g for 10 min at 4°C. Samples were stored at −80°C until use. In some experiments we employed heat-inactivated (56°C, 30 min), boiled (10 min), or charcoal-stripped plasma prepared as described elsewhere.19
Plasma SDF-1, S1P and C5 cleavage fragment and free hemoglobin measurements
Plasma SDF-1 levels were evaluated by employing the sandwich enzyme-linked immunosorbent assay (ELISA) using the commercially available ELISA system (R & D Systems, Minneapolis, MN, USA). S1P levels were analyzed using a commercially available plasma S1P detection kit (Echelon Biosciences Inc., UT, USA).
Plasma CC activation levels were analyzed by a sandwich ELISA as described elsewhere.20 Briefly, plasma samples were incubated in triplicate wells of microtiter plates, which had been pre-coated with antigen-specific capture Abs (C5a). Repeated washing and aspiration were conducted before each incubation step to remove unbound materials from the assay plates. This step was followed by incubation with specific biotin-labeled capture Abs and an enzyme reagent. Substrate solution was added to the wells of the microtiter plates and color developed in proportion to the amount of antigen bound in the initial step of the individual assay. The color development was stopped with the addition of an acid solution and the intensity of color was read using a microtiter plate spectrophotometer. Hemolysis levels were analyzed by measuring absorbance of plasma at 540 nm as described elsewhere21 and calculated as percentage of hemolysis expressed as fold increase of hemoglobin in mobilized plasma as compared to normal plasma.
Mobilization
Mobilization in mice was induced by G-CSF, zymosan, or AMD3100. For G-CSF mobilization mice were injected subcutaneously (s.c.) with 250 μg/kg of human G-CSF (Amgen, Thousand Oaks, CA; http://www.amgen.com) daily for 6 days. For zymosan mobilization, mice were injected intravenously (i.v.) with 0.5 mg/mouse and for AMD3100 mobilization with a dose of 5 mg/kg s.c. At 6 h after the last G-CSF administration or 1 h after zymosan or AMD3100 injection, mice were bled into microvette EDTA-coated tubes (Sarstedt Inc., Newton, NC; http://www.sarstedt.com) from the retro-orbital plexus to obtain a complete blood count (CBC) and 450 μl of PB was obtained from the vena cava with a 25-gauge needle and 1 ml syringe containing 50 μl of 100 mM ethylenediaminetetraacetic acid (EDTA). Some mice were treated with deoxypyridoxin (DOP, 30 mg/L) with glucose (10 g/L) in drinking water, as described elsewhere,22 for 14 hrs before BM harvest or AMD3100 administration.
Evaluation of HSPC migration and mobilization in mice
To count the number of HSPCs, we employed colony forming assay and fluorescence-activated cell sorting (FACS) analysis for Sca-1+/c-Kit+/Lin−/CD34− (SKL) cells. The following formulae were used for evaluation of circulating CFU-GM and SKL cells, respectively: number of white blood cells (WBCs) × number of CFU-GM colonies/number of WBCs plated = number of CFU-GM per microliter of PB) and number of WBCs × number of SKL cells)/number of gated WBCs = number of SKL cells per microliter of PB..
Colony-forming assay
To evaluate the number of circulating progenitor cells after mobilization, a colony-forming cell assay was employed. Red blood cells (RBCs) were lysed with BD Pharm Lyse buffer (BD Biosciences, San Jose, CA; http://www.bdbiosciences.com). Nucleated cells were subsequently washed twice and used for colony-formation assays. Briefly, cells were resuspended in methylcellulose base media provided by the manufacturer (R&D Systems, Inc., Minneapolis, MN; http://www.rndsystems.com) supplemented with granulocyte macrophage colony-stimulating factor (GM-CSF, 25 ng/ml) plus interleukin-3 (IL-3, 10 ng/ml) for colony forming units (CFU) granulocyte/macrophage (GM), granulocyte colony stimulating factor (G-CSF, 20 ng/ml) for CFU-granulocyte (G), macrophage colony stimulating factor (M-CSF, 10 ng/ml) for CFU macrophage (M), erythropoietin (EPO, 5 unit/ml, Stem cell Tech, http://www.stemcell.) plus stem cell factor (SCF, 5 ng/ml) for burst-forming units (BFU-E), and thrombopoietin (TPO, 100 ng/ml) for CFU-megakaryocytes (Megs). All growth factors were purchased from the same company unless otherwise mentioned. Cultures were incubated for 7 days, at which time they were scored under an inverted microscope for the number of each colonies.
FACS analysis
Cell staining was performed in medium containing 2% fetal bovine serum (FBS). All monoclonal antibodies (mAbs) were added at saturating concentrations and the cells were incubated for 30 min on ice, washed twice, resuspended in staining buffer at a concentration of 5×106 cells/ml, and subjected to analysis using an LSR II (BD, Mountainview, CA). The following anti-mouse mAbs were used to detect fluorescein isothiocyanate (FITC)-anti-CD117 (c-Kit) (clone 2B8; BioLegend, San Diego, CA) and phycoerythrin (PE)-Cy5 anti-mouse Ly-6A/E (Sca-1) (clone D7; eBioscience™, San Diego, CA). All anti-mouse lineage markers (Lin) were conjugated by PE and purchased from BD Biosciences: anti-CD45R/B220 (clone RA3-6B2); anti-Gr-1 (clone RB6-8C5); anti-T-cell receptor β (TCRβ; clone H57-597); anti-TCRγδ (clone GL3); anti-CD11b (clone M1/70); anti-Ter-119 (clone TER-119); and anti-CD34 (clone RAM34).
Bone marrow nucleated cells (BMNCs)
BMNCs were prepared by flushing femurs and tibias of pathogen-free, 6- to 8-week-old mice without enzymatic digestion. BMNCs were lysed with BD Pharm Lyse buffer to remove RBCs, washed, and resuspended in RPMI containing 0.5% bovine serum albumin (BSA). In some experiments, BMNCs were exposed to AMD3100 (5 μM), heat inactivated normal plasma from the same donor, or S1P for 1 h at 37°C before use in the trans-well migration assay.
Trans-well migration assay
The trans-well migration assay was performed as described elsewhere.16 Briefly, unless otherwise indicated, RBC-lysed BMNCs from C57BL/6 mice were resuspended in assay media (RPMI containing 0.5% BSA) and equilibrated for 10 min at 37°C. Assay medium (650 μl) containing test reagents was added to the lower chambers of a Costar Trans-well plate (Costar Corning, Cambridge, MA, USA). Aliquots of cell suspension (1×106 cells/100 μl) were loaded onto the upper chambers with 5 μm-pore filters, then incubated for 3 h (37°C, 5% CO2). Cells from the lower chambers were scored using FACS analysis for migration of BMNCs to test regents. The results are express as fold increase, i.e., the ratio of the number of cells that migrated toward the test reagents to the number of cells that migrated toward the medium alone. In some experiments, migrated cells harvested from the lower chambers were plated in colony forming assays as mentioned earlier.
MAC deposition
Immunolocalization of each MAC in femur was achieved using the immunoperoxidase system. Briefly, 4 μm paraffin sections, after deparaffinization and rehydration, were treated with 0.3% hydrogen peroxide in absolute methanol for 30 min to quench endogenous peroxidase activity and incubated in 10% normal goat serum for 60 min to block non-specific binding. Following overnight incubation with rabbit anti-murine C5b-9 Ab (1:100, Abcam # ab55811), sections were exposed to HRP-conjugated goat-anti rabbit IgG (1:1000, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 60 min. Immunoreactivity was visualized using the liquid DAB Substrate Chromogen System (Sigma-Aldrich Co, St Louis MO, USA). Sections were rinsed thoroughly in tap water, lightly counterstained with hematoxylin, dehydrated through graded ethanol, cleared in xylene and mounted using mounting media.
Statistical analysis
Arithmetic means and standard deviations were calculated using Instat 1.14 software (Graphpad, San Diego, CA). Statistical significance was defined as P<0.05. Data were analyzed using Student's t-test for unpaired samples.
Results
Plasma SDF-1 level does not correlate with mobilization efficiency of HSPCs
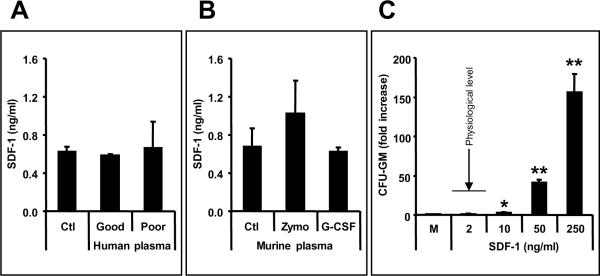
Our first aim was to determine whether SDF-1 plasma level correlates with efficiency of mobilization of CD34+ cells; hence we employed ELISA to measure SDF-1 level in plasma obtained from 16 patients (classified as good and poor mobilizers). We found that plasma SDF-1 concentration is about 600 pg/ml and does not increase during G-CSF-induced mobilization. Moreover, both good (n=9) and poor (n=7) mobilizers had similarly low levels of SDF-1 (Figure 1, panel A and Supplementary Table I).
Figure 1. Plasma SDF-1 level does not correlate with mobilization efficiency of HSPCs.
Panel A - SDF-1 concentrations are low (less than 1 ng/ml) and do not differ significantly in the plasma of patients who are poor (n=7 or good (n=9) G-CSF mobilizers Panel B - As in patients, SDF-1 concentration is murine plasma is also low (less than 2 ng/ml) and does not increase significantly during Zymosan- or G-CSF-induced mobilization. Panel C – SDF-1 at a physiological concentration does not show chemotactic activity against murine BM HSPCs. The data shown in Panels B and C represent the combined results from three independent experiments carried out in triplicate per group (n=9). * p<0.05, ** p<0.01
As observed in patients' plasma, the SDF-1 level was also very low in murine plasma and did not increase significantly after G-CSF- or Zymosan-induced mobilization (Figure 1, panel B). Of note, but as expected and shown in Figure 1 panel C,, this low physiological concentration of SDF-1 (~ 2 ng/ml) does not have significant chemotactic activity against murine CFU-GM (Figure 3 panel C).
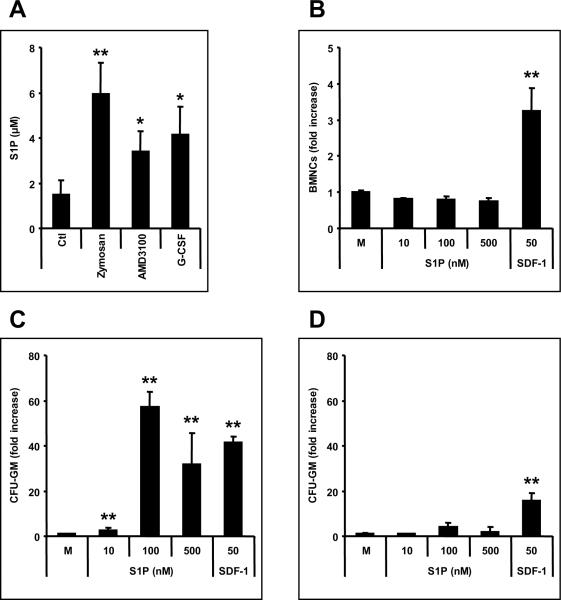
Figure 3. S1P is a major chemotactic component of plasma.
Panel A - Murine plasma S1P levels increase during mobilization (10 min after zymosan-, 1 h after AMD3100-, and 6 h at day 6 after the last G-CSF-treatment). Panel B and C - However, S1P does not chemoattract differentiated/mature BMNCs; (B), it strongly chemoattracts BM-residing naïve HSPCs (C). Panel D – S1P has much lower chemotactic activity against G-CSF-mobilized PB circulating HSPCs. Ctl, normal mouse plasma; M, media only control; SDF-1 (ng/ml). * p<0.01, ** p<0.001. The data shown represent the combined results from three independent experiments carried out in triplicate per group (n=9).
HSPCs egress from BM into PB in SDF-1-independent manner
In order to identify factor/s responsible for chemoattraction/egress of HSPCs from BM into PB, we evaluated the chemotactic activity of normal murine and human plasma (Figure 2 and Supplementary Figure 1). Figure 2 panel A shows that murine CFU-GM are chemoattracted by murine plasma much stronger than by plasma physiological SDF-1 level (1–2ng/ml).
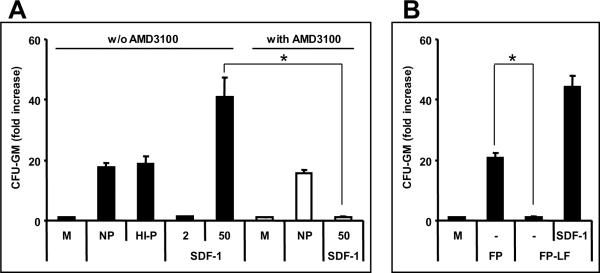
Figure 2. HSPCs egress from BM into PB in an SDF-1-independent manner.
Panel A – Murine CFU-GM are much more efficiently chemoattracted by murine plasma than by a physiological concentration of SDF-1. Potential involvement of SDF-1 can be excluded because normal CFU-GM progenitor cells (black bar) responded to heat-inactivated plasma (HI-P) as well as the presence of 5 μM AMD3100 (white bar - NP). However, exposure to AMD3100-inhibited chemotaxis to SDF-1. Panel B - CFU-GM cells do not migrate to charcoal-stripped, lipid-free plasma; however they respond normally to the SDF-1 added (50 ng/ml) charcoal-stripped plasma. M, media-alone control; NP, normal murine plasma (2.5%); HI-P, heat-inactivated murine plasma (2.5%); SDF-1 (ng/ml); FP, filtered normal plasma filtered with 0.22 μm membrane; FP-LF, charcoal-stripped and then filtered plasma. * p<0.01. The data shown are the combined results from three independent experiments carried out in triplicate per group (n=9).
Potential involvement of SDF-1 in plasma chemotaxis was excluded by showing that AMD3100 treated murine CFU-GM responded to a plasma gradient and that this chemotactic response was not affected by heat inactivation (Figure 2, panel A). Interestingly, as is shown in Figure 2, panel B, removal of lipids from plasma by charcoal stripping abrogated plasma chemotactic activity but did not affect chemotactic responsiveness to SDF-1 (50 ng/ml) that was added to the plasma before charcoal stripping. This suggests an involvement of lipid components in observed plasma chemotactic activity. Overall both murine and human 2–5% plasma (Figure 2 and Supplementary Figure 1, panels A and B) chemoattract BM-derived CFU-GM; however, this chemotactic effect decreased with higher plasma concentration.
S1P level in PB increases during mobilization
S1P is a bioactive lipid that was reported to chemottract HSPCs.23 Thus, we measured S1P level in murine plasma during mobilization and found that it increases 2–4 times depending on the mobilization agent employed (Figure 3, panel A). We noticed that although S1P did not chemoattract BMNCs (Figure 3, panel B) it strongly chemoattracted BM-residing CFU-GM (Figure 3, panel C) as well as other clonogeneic progenitors (Supplementary Figure 2). This effect seems to be specific for BM-residing naïve CFU-GM, but was significantly weaker for CFU-GM circulating in PB (Figure 3, panel D). In control experiments, blood-isolated CFU-GM also displayed weaker chemotactic response to SDF-1 as compared to BM naïve counterparts. We also noticed in our chemotactic assays that the responsiveness of BM-derived CFU-GM is S1P dose-dependent and shows a bell-shaped curve, being most optimal at dose ~ 100 nM and decreasing with higher S1P concentrations (Supplementary Figure 1, panel C). This bell-shaped curve for S1P chemotactic responsiveness corresponds to chemotactic activity in murine and human plasma (Supplementary Figure 1, panels A and B), which also show a bell- shaped curve.
To explore further the involvement of S1P, we evaluated the chemotactic responsiveness of BM-isolated CFU-GM that were exposed to plasma (Figure 4, panel A) or preincubated with S1P before the chemotaxis assay (Figure 4, panel B). Both pre-incubation of BM-derived CFU-GM with plasma and S1P inhibited their chemotactic responsivenss to S1P but not to SDF-1 (Figure 4, A and B). This indicates that the chemotactic responsivenss to S1P may be affected/desensitized by prior exposure to this bioactive lipid.
Figure 4. S1P exposed- or plasma pre-incubated BM-HSPCs do not respond to S1P.
Pre-incubation of BM-HSPCs with plasma from the same donor (Panel A, striped bar) or with S1P (Panel B), gray bar-100 nM, white bar-500 nM) for 1 h abrogated migration of BM-naïve CFU-GM progenitors to an S1P gradient. M, media alone control. * p<0.001. The data shown represent the combined results from three independent experiments carried out in triplicate per group (n=9).
S1P is released during mobilization form erythrocytes in a MAC-dependent manner
Erythrocytes are a major source/reservoir of S1P in PB.17,18 Based on our data that the later steps of CC activation are crucial for egress of HSPCs from BM into PB16,20 we tested the possibility that the final product of CC activation, MAC, could be involved in the release of S1P from erythrocytes. Figure 5, panel A shows that activation of the later steps of CC activation as evaluated by C5a ELISA correlates with the plasma free hemoglobin level. The generation of MAC deposits in BM of mobilized wild type mice and lack of these deposits in C5−/− mice that do not activate the later steps of CC and do not generate MAC was confirmed by immunohistochemical staining (Figure 5, panel B).Figure 5, panel C shows that murine CFU-GM show the bell-shaped curve in chemotactic responsiveness to erythrocyte lysates.
Figure 5. The role of MAC in mobilization.
Panel A – Free hemoglobin level in plasma correlates with strength of CC activation. Panel B – MAC deposits are detectable in BM of G-CSF-mobilized wt but not C5−/− mice. Diffuse MAC deposition is visible in endothelial cells (arrow), osteoblasts (arrow head), and interstitium. Original magnification ×200. Panel C – Chemotactic response of BM CFU-GM to erythrocyte lysates prepared from plasma-washed erythrocytes followed by repeated freezing and thawing. Ctl, normal mouse plasma; M, media only control. * p<0.01, ** p<0.001. The data shown represent the combined results from three independent experiments carried out in triplicate per group (n=9).
DOP-treated mice are poor mobilizers
It has been reported that activity of enzyme that degrades S1P (S1P lyase) depends on vitamin B6 that serves as a co-enzyme, and DOP, a vitamin B6 antagonist, decreases its activity in tissues.22 Therefore, to better address the role of S1P in the release of HSPCs from BM we treated mice with DOP and subsequently evaluated the chemotactic response of BM CFU-GM from control and DOP-treated mice to S1P. Figure 6, panel A shows that CFU-GM cells from DOP-treated BM, which were exposed in the BM environment to a high level of S1P (due to lack of S1P lyase activity) do not respond to a S1P gradient. Furthermore, we also noticed that DOP-treated mice became poor mobilizers (Figure 6, panels B–D). Again this could be explained by desensitization of their responsiveness to a S1P gradient after exposure to the high concentration of S1P that accumulates in BM due to lack of S1P lyase activity.
Figure 6. Impaired responsiveness of HSPCs from DOP- treated mice to S1P.
Panel A - BM HSPCs harvested from DOP- treated mice (white bar) do not migrate to a S1P gradient. Panel B - D - DOP treated mice are poor AMD3100 mobilizers *p<0.0001. The data shown represent the combined results from three independent experiments carried out in triplicate per group (n=9) and the combined results of three independent mobilization experiments carried out with 5 mice per group (n=15).
Discussion
The process of HSPC mobilization is still not fully understood and a significant number of patients, particularly those with previous history of chemotherapy, are poor mobilizers.24,25 Several BM microenvironment-related factors have been identified that regulate mobilization, including: i) release of proteases by activated myeloid cells, which perturb the interactions of SDF-CXCR4, VLA-4-VCAM-1,9,10 and KL-c-KitR axes26; ii) release of neurotransmitters by neural fibers that innervate BM tissue27; iii) downregulation of SDF-1 mRNA expression in the osteoblastic stem cell niche28,29; iv) activation of osteoclasts30; and v) upregulation of the serpin family protease inhibitors.31,32
We reported CC is activated in BM during mobilization of HSPCs and that CC cleavage fragments direct egress of HSPCs into PB by modulating the activity of granulocytes.16 Supporting this notion was our finding that mice that do not activate efficiently the later steps of CC are poor mobilizers13,16,20 and, as reported by several groups, mobilization of HSPCs is severely reduced in neutropenic mice.33,34 It has been also demonstrated that granulocytes are a major source of proteolytic enzymes in the BM that perturb HSPC retention signals and are the first cells to egress from BM.16
To clarify the role of later steps of CC activation and granulocytes we demonstrated that C5 cleavage fragments affect several important neutrophil-mediated steps of HSPC mobilization. First, they may increase secretion of proteases by BM-residing granulocytes and their precursors, which leads to enzymatic disintegration of HSPC anchorage signals in BM niches. Second, C5 cleavage fragments along with IL-8, NAP-2, Gro-β create in plasma a chemotactic gradient for C5aR+ granulocytes and promote their egress into PB. Granulocytes, being highly enriched in proteolytic enzymes, are crucial for “permeabilization” of the BM-blood barrier and thus these cells pave the way for HSPCs expressing proteolytic enzymes at a low level to follow in their footsteps.
G-CSF is currently the most frequently used mobilizing agent that efficiently mobilizes HSPCs after a few consecutive daily injections. Mobilization can also be induced within hours after administration of certain chemokines [e.g., IL-8,32 growth-related oncogene protein-beta (Gro-β),34 macrophage inhibitory protein-1 alpha (MIP-1α),35 or small molecular CXCR4 receptor, e.g., AMD3100, and VLA4, e.g., BIO5192, antagonists36). Additionally, in experimental animals, mobilization also occurs within 1 h after a single injection of some polysaccharides (e.g., zymosan, fucoidans).37
It has been envisioned that HSPCs released from their hematopoietic niches egress from the BM into PB in response to increasing plasma SDF-1 levels.5,6,38,39 However, the role of plasma SDF-1 level in the egress of HSPCs is not clear.40 We report here that plasma derived from normal and mobilized PB strongly chemoattracts murine HSPCs. More important, this chemotactic effect is not dependent on SDF-1 concentration because: i) it occurs in the presence of the CXCR4 antagonist AMD3100; ii) it is robust to heat-inactivated plasma; and iii) ELISA studies reveal negligible concentrations of SDF-1 in plasma, which did not correlate with good or poor mobilizer status. This last observation is in agreement with a recent report indicating that plasma SDF-1 levels do not correlate with G-CSF-induced mobilization efficiency in patients.41 However, as reported, some priming factors released during mobilization such as complement cascade protein C3 cleavage fragments (C3a and desArgC3a), as well as cathelicidin released from activated granulocytes, could enhance responsiveness of HSPCs to SDF-12, 16; nevertheless, this priming phenomenon does not explain the remarkable chemotactic potential of plasma. Based on this, we sought to determine which major chemoattractant is present in PB that is responsible for the egress of HSPCs and whether the activation of later steps of CC plays some role in its level.
We found that the chemotactic activity of plasma was almost completely abolished after charcoal stripping, suggesting involvement of a bioactive lipid. Since we detected free hemoglobin in the plasma of mobilized animals we explored the possibility that MAC generated during the activation of the last step of CC could affect erythrocytes which release some chemotactic factor/s. In support of this we found that erythrocyte lysates indeed exert a strong chemotactic effect on HSPCs, which is in agreement with observations that HSPCs are robustly mobilized during intravascular lysis of erythrocytes, e.g., in sickle cell crisis.42
It is well known that erythrocytes store in blood and release S1P,17,18, a bioactive lipid that has been reported to posses chemotactic activity against HSPCs as well as regulate the egress of lymphocytes into blood and lymph.43,44 Of note, S1P is the only known chemoattractant for HSPCs besides SDF-1 and has been postulated to play a role in CD34+ cell homing to BM. The pro-homing/chemotactic effects of S1P are mediated by five G-protein coupled seven-transmembrane span receptors (S1P1 – S1P5).45,46 While binding of S1P to S1P1 receptor promotes the chemotaxis of CD34+ cells,23 activation of the S1P2 receptor has an opposite effect.47 In addition, binding of S1P to S1P3 increases CXCR4 receptor signaling and enhances the responsiveness of HSPCs to an SDF-1 gradient.48
In this work we provide evidence that S1P is a major chemotactic component of normal plasma that directs egress of HSPCs into PB. We demonstrate that S1P is already present in plasma under normal steady-state conditions and creates a gradient continuously chemoattracting BM-residing HSPCs. To counteract this S1P egress gradient HSPCs are actively retained in BM via SDF-1-CXCR4 and VLA4-V-CAM1 interactions. Therefore, perturbation of these interactions by i) creating a proteolytic microenvironment that affects retention signals, or ii) direct blocking of CXCR4 of VLA-4 by small molecule antagonists (eg., AMD 3100 or BIO5192, respectively) is sufficient to shift a BM-retention balance towards a plasma S1P gradient that directs egress of HSPCs into PB. Furthermore, as we demonstrated, since during mobilization CC is activated and MAC generated, this leads to additional increases in plasma S1P released from erythrocytes. It is known that erythrocytes are highly protected from MAC by CD59 and decay-accelerating factor (DAF) receptors (which are lacking on erythrocytes in paroxysmal nocturnal hemoglobinuria (PNH) patients, a disorder characterized by erythrocyte lysis).49. However, expression of these receptors on erythrocytes does not give complete protection from activated MAC. Our data support the notion that erythrocytes form a buffer system that controls S1P levels in PB and release of S1P from erythrocytes as seen during hemolysis,42, 49 or, as shown here, after mobilization induced MAC exposure increase the free levels of S1P that regulate BM-egress of HSPCs.
Our data also indicate that the chemotactic responsiveness of HSPCs to S1P depends on S1P level and source of cells. Accordingly, S1P that strongly chemoattracts HSPCs at doses of 100–500 nM, inhibits their chemoattraction in higher concentrations. It is possible that higher doses of S1P promote signaling through the S1P2 receptor, which in contrast to S1P provides chemotactic inhibitory signals. This explains why we observed optimal chemotaxis of HSPCs to diluted murine and human plasma and its inhibition in the presence of higher plasma concentrations. Moreover, while naïve BM-derived CFU-GM showed robust chemotaxis to S1P, this effect was not evident for CFU-GM already mobilized and circulating in PB or for BM-derived CFU-GM exposed to S1P or pre-incubated with plasma. We observed a similar effect for HSPCs from DOP-treated animals that express high levels of S1P in the BM microenvironment. These data could be explained by desensitization of cell responsiveness due to S1P1 downregulation after exposure to S1P.47,50
Interestingly, while S1P strongly chemoattracts HSPCs it does not affect the migration of granulocytes. Therefore, optimal HSPCs mobilization depends on the activation of the later steps of CC and the generation of C5 cleavage fragments that induce egress of granulocytes. Based on this, C5 cleavage fragments and S1P together orchestrate egress of HSPCs by inducing BM-egress of granulocytes that pave the way for HSPCs (C5 cleavage fragments), and by the direct chemoattraction by S1P of HSPCs that follow the granulocytes.
Based on these data, we propose the following scenario (Figure 7). In the steady-state condition, HSPCs are retained in BM due to active SDF-1-CXCR4/VLA-4-VCAM1 interactions that counteract the continuous chemotactic S1P gradient present in PB. To induce mobilization, a mobilizing agent (e.g., G-CSF) activates CC in BM which subsequently increases the release of proteolytic enzymes from granulocytes which in turn perturb SDF-1-CXCR4/VLA-4-VCAM1 interactions or simply block these axes (e.g., AMD3100 or BIO5192, respectively) (Step I). The mobilization process subsequently activates CC and the later steps of this cascade are required for optimal egress of HSPCs. Accordingly, C5 cleavage fragments promote egress of granulocytes that pave the way for the egress of HSPCs (Step II). These observations made in mice16 wererecently confirmed in patients.51 Simultaneously, concentration of S1P in plasma increases due to the release of S1P from erythrocytes in a MAC-dependent manner, providing a more optimal gradient for HSPCs. Thus S1P accumulation in BM sinusoids along with C5 cleavage fragments, but not changes in plasma SDF-1 levels, are crucial executors of HSPC egress from BM into PB (Step III).
Figure 7. Novel mechanistic insight into BM HSPC mobilization.
HSPCs are actively retained in BM and retention signals in the BM niches counteract a S1P-mediated chemotactic plasma gradient. Mobilizing agents disrupt major HSPC-anchoring signals (i.e., CXCR4-SDF-1 and VLA4-VCAM-1 interactions) and release HSPCs from their niches (Step I). First, as reported previously16,20,51 activated granulocytes egress from BM into sinusoids in response to C5 cleavage fragments and thus pave the way for HSPC (Step II). Next, MAC generated in the final step of CC activation enhances release of S1P from erythrocytes into the plasma, and the plasma S1P level directs egress of HSPCs into PB (Step III).
Supplementary Material
Acknowledgments
Supported by NIH grants R01 CA106281 and R01 DK074720 and Stella and Henry Endowment and European Union structural funds, Innovative Economy Operational Program POIG.01.01.02-00-109/09-00 to MZR and NIH 1RC1 HL099447 to MJL.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
References
- 1.Welner RS, Kincade PW. Stem cells on patrol. Cell. 2007;131(5):842–844. doi: 10.1016/j.cell.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee H, Ratajczak MZ. Innate immunity: a key player in the mobilization of hematopoietic stem/progenitor cells. Arch Immunol Ther Exp (Warsz) 2009;57(4):269–278. doi: 10.1007/s00005-009-0037-6. [DOI] [PubMed] [Google Scholar]
- 3.Kassirer M, Zeltser D, Gluzman B, Leibovitz E, Goldberg Y, Roth A, et al. The appearance of L-selectin(low) polymorphonuclear leukocytes in the circulating pool of peripheral blood during myocardial infarction correlates with neutrophilia and with the size of the infarct. Clin Cardiol. 1999;22(11):721–726. doi: 10.1002/clc.4960221109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyne L, Hausdorff JM, Knight E, Dukas L, Azhar G, Wei JY. Neutrophilia and congestive heart failure after acute myocardial infarction. Am Heart J. 2000;139(1Pt1):94–100. doi: 10.1016/s0002-8703(00)90314-4. [DOI] [PubMed] [Google Scholar]
- 5.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3(7):687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney EA, Lortat-Jacob H, Priestley GV, Nakamoto B, Papayannopoulou T. Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice: involvement in mobilization of stem/progenitor cells. Blood. 2002;99(1):44–51. doi: 10.1182/blood.v99.1.44. [DOI] [PubMed] [Google Scholar]
- 7.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 8.Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. J Clin Invest. 1999;104(9):1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lévesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98(5):1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 10.Lévesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111(2):187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lévesque JP, Hendy J, Winkler IG, Takamatsu Y, Simmons PJ. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol. 2003;31(2):109–117. doi: 10.1016/s0301-472x(02)01028-7. [DOI] [PubMed] [Google Scholar]
- 12.Molendijk WJ, van Oudenaren A, van Dijk H, Daha MR, Benner R. Complement split product C5a mediates the lipopolysaccharide-induced mobilization of CFU-s and haemopoietic progenitor cells, but not the mobilization induced by proteolytic enzymes. Cell Tissue Kinet. 1986;19(4):407–417. doi: 10.1111/j.1365-2184.1986.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 13.Reca R, Cramer D, Yan J, Laughlin MJ, Janowska-Wieczorek A, Ratajczak J, et al. A novel role of complement in mobilization: immunodeficient mice are poor granulocyte-colony stimulating factor mobilizers because they lack complement-activating immunoglobulins. Stem Cells. 2007;25(12):3093–3100. doi: 10.1634/stemcells.2007-0525. [DOI] [PubMed] [Google Scholar]
- 14.Liu F, Poursine-Laurent J, Link DC. Expression of the G-CSF receptor on hematopoietic progenitor cells is not required for their mobilization by G-CSF. Blood. 2000;95(10):3025–3031. [PubMed] [Google Scholar]
- 15.Pruijt JF, Verzaal P, van Os R, de Kruijf EJ, van Schie ML, Mantovani A, et al. Neutrophils are indispensable for hematopoietic stem cell mobilization induced by interleukin-8 in mice. Proc Natl Acad Sci U S A. 2002;99(9):6228–6233. doi: 10.1073/pnas.092112999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HM, Wu W, Wysoczynski M, Liu R, Zuba-Surma EK, Kucia M, et al. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23(11):2052–2062. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hänel P, Andréani P, Gräler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21(4):1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 18.Ohkawa R, Nakamura K, Okubo S, Hosogaya S, Ozaki Y, Tozuka M, et al. Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. Ann Clin Biochem. 2008;45(Pt 4):356–63. doi: 10.1258/acb.2007.007189. [DOI] [PubMed] [Google Scholar]
- 19.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279(5356):1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 20.Lee HM, Wysoczynski M, Liu R, Shin DM, Kucia M, Botto M, et al. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia. 2009 doi: 10.1038/leu.2009.271. doi: 10.1038/leu.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ringstad L, Andersson Nordahl E, Schmidtchen A, Malmsten M. Composition effect on peptide interaction with lipids and bacteria: variants of C3a peptide CNY21. Biophys J. 2007;92(1):87–98. doi: 10.1529/biophysj.106.088161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309(5741):1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 23.Seitz G, Boehmler AM, Kanz L, Möhle R. The role of sphingosine 1-phosphate receptors in the trafficking of hematopoietic progenitor cells. Ann N Y Acad Sci. 2005;1044:84–89. doi: 10.1196/annals.1349.011. [DOI] [PubMed] [Google Scholar]
- 24.Glaspy JA, Shpall EJ, LeMaistre CF, Briddell RA, Menchaca DM, Turner SA, et al. Peripheral blood progenitor cell mobilization using stem cell factor in combination with filgrastim in breast cancer patients. Blood. 1997;90(8):2939–2951. [PubMed] [Google Scholar]
- 25.Chabannon C, Le Corroller AG, Viret F, Eillen C, Faucher C, Moatti JP, et al. Cost-effectiveness of repeated aphereses in poor mobilizers undergoing high-dose chemotherapy and autologous hematopoietic cell transplantation. Leukemia. 2003;17(4):811–813. doi: 10.1038/sj.leu.2402867. [DOI] [PubMed] [Google Scholar]
- 26.Bellucci R, De Propris MS, Buccisano F, Lisci A, Leone G, Tabilio A, et al. Modulation of VLA-4 and L-selectin expression on normal CD34+ cells during mobilization with G-CSF. Bone Marrow Transplant. 1999;23(1):1–8. doi: 10.1038/sj.bmt.1701522. [DOI] [PubMed] [Google Scholar]
- 27.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 28.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276(47):43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 29.Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106(9):3020–3027. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kollet O, Dar A, Lapidot T. The multiple roles of osteoclasts in host defense: bone remodeling and hematopoietic stem cell mobilization. Annu Rev Immunol. 2007;25:51–69. doi: 10.1146/annurev.immunol.25.022106.141631. [DOI] [PubMed] [Google Scholar]
- 31.Winkler IG, Levesque JP. Mechanisms of hematopoietic stem cell mobilization: When innate immunity assails the cells that make blood and bone. Exp Hematol. 2006;34(8):996–1009. doi: 10.1016/j.exphem.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 32.van Pel M, van Os R, Velders GA, Hagoort H, Heegaard PM, Lindley IJ, et al. Serpina1 is a potent inhibitor of IL-8-induced hematopoietic stem cell mobilization. Proc Natl Acad Sci USA. 2006;103(5):1469–1474. doi: 10.1073/pnas.0510192103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruijt JF, Fibbe WE, Laterveer L, Pieters RA, Lindley IJ, Paemen L, et al. Prevention of interleukin-8-induced mobilization of hematopoietic progenitor cells in rhesus monkeys by inhibitory antibodies against the metalloproteinase gelatinase B (MMP-9) Proc Natl Acad Sci USA. 1999;96(19):10863–10868. doi: 10.1073/pnas.96.19.10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King AG, Horowitz D, Dillon SB, Levin R, Farese AM, MacVittie TJ, et al. Rapid mobilization of murine hematopoietic stem cells with enhanced engraftment properties and evaluation of hematopoietic progenitor cell mobilization in rhesus monkeys by a single injection of SB-251353, a specific truncated form of the human CXC chemokine GRO-beta. Blood. 2001;97(6):1534–1542. doi: 10.1182/blood.v97.6.1534. [DOI] [PubMed] [Google Scholar]
- 35.Liles WC, Broxmeyer HE, Rodger E, Wood B, Hübel K, Cooper S, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102(8):2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 36.Ramirez P, Rettig MP, Uy GL, Deych E, Holt MS, Ritchey JK, et al. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood. 2009;114(7):1340–3. doi: 10.1182/blood-2008-10-184721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cramer DE, Wagner S, Li B, Liu J, Hansen R, Reca R, et al. Mobilization of hematopoietic progenitor cells by yeast-derived beta-glucan requires activation of matrix metalloproteinase-9. Stem Cells. 2008;26(5):1231–1240. doi: 10.1634/stemcells.2007-0712. [DOI] [PubMed] [Google Scholar]
- 38.Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97(11):3354–3360. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 39.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195(9):1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozuka T, Ishimaru F, Fujii K, Masuda K, Kaneda K, Imai T, et al. Plasma stromal cell-derived factor-1 during granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization. Bone Marrow Transplant. 2003;31(8):651–654. doi: 10.1038/sj.bmt.1703901. [DOI] [PubMed] [Google Scholar]
- 41.Cecyn KZ, Schimieguel DM, Kimura EY, Yamamoto M, Oliveira JS. Plasma levels of FL and SDF-1 and expression of FLT-3 and CXCR4 on CD34+ cells assessed pre and post hematopoietic stem cell mobilization in patients with hematologic malignancies and in healthy donors. Transfus Apher Sci. 2009;40(3):159–167. doi: 10.1016/j.transci.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Lamming CE, Augustin L, Blackstad M, Lund TC, Hebbel RP, Verfaillie CM. Spontaneous circulation of myeloid-lymphoid-initiating cells and SCID-repopulating cells in sickle cell crisis. J Clin Invest. 2003;111(6):811–819. doi: 10.1172/JCI15956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei SH, Rosen H, Matheu MP, Sanna MG, Wang SK, Jo E, et al. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol. 2005;6(12):1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- 44.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316(5822):295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 45.Lynch KR. Lysophospholipid receptor nomenclature. Biochim Biophys Acta. 2002;1582(1–3):70–71. doi: 10.1016/s1388-1981(02)00138-5. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92(5):913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 47.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8(10):753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sainz J, Sata M. CXCR4, a key modulator of vascular progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27(2):263–265. doi: 10.1161/01.ATV.0000256727.34148.e2. [DOI] [PubMed] [Google Scholar]
- 49.Bessler M, Hiken J. The pathophysiology of disease in patients with paroxysmal nocturnal hemoglobinuria. Hematology Am Soc Hematol Educ Program. 2008:104–110. doi: 10.1182/asheducation-2008.1.104. [DOI] [PubMed] [Google Scholar]
- 50.Sensken SC, Bode C, Gräler MH. Accumulation of fingolimod (FTY720) in lymphoid tissues contributes to prolonged efficacy. J Pharmacol Exp Ther. 2009;328(3):963–969. doi: 10.1124/jpet.108.148163. [DOI] [PubMed] [Google Scholar]
- 51.Jalili A, Shirvaikar N, Marquez-Curtis N, Qiu Y, Korol Ch, Lee H, et al. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: Further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells. Exp Hematol. 2010 doi: 10.1016/j.exphem.2010.02.002. doi:10.1016/j.exphem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.