Abstract
Objective
Sleep disturbances are common in major depressive disorder (MDD), although polysomnographic (PSG) abnormalities are more prevalent in adults than in children and adolescents with MDD. Sleep spindle activity (SPA) is associated with neuroplasticity mechanisms during brain maturation and is more abundant in childhood and adolescence than in adulthood, and as such, may be a more sensitive measure of sleep alteration than PSG in early-onset depression. This study investigated SPA changes related to early-onset MDD, comparing those already ill and those at high-risk for MDD to healthy non-depressed controls.
Method
The study included 63 participants (8-15 year-olds): 21 currently depressed, 21 at high-risk for MDD based on positive family history of MDD, and 21 healthy controls with no personal or family history of psychiatric illness. All participants maintained a regular sleep/wake schedule for 5 days, followed by 2 nights in the lab. SPA was analyzed in Stage 2 of non-Rapid Eye Movement (NREM) sleep.
Results
SPA differed significantly between groups, particularly in the late part of the night (F2,62=7.3 p=0.001). Although, the difference was greatest between MDD and healthy controls, both the MDD (p=0.0004) and at high-risk groups (p=0.02) had significantly lower SPA compared to healthy controls. SPA deficit was more prominent in females than males (F5,62=5.19 p=0.005).
Conclusions
Low SPA characterizes youths with MDD and those at high-risk, particularly among girls, suggesting that early-onset depression and risk for the illness are associated with decreased neuroplasticity.
Keywords: childhood depression, spindles, sleep, risk for MDD, neuroplasticity
Introduction
Major depressive disorder (MDD) is highly prevalent in children and adolescents at a rate of 4-11% and can be more severe and chronic at this age than in adult onset 1. Higher rates of recurrence 2, 3, suicide 4, 5, and substance abuse disorder 6 are evident in early-onset depression compared to late-onset depression7. It is also during these stages of human developmental that sex-differences in MDD are apparent, with a 2:1 female/male ratio of incidence 8, 9. It is also well documented that MDD aggregates in families 10, and the strong correlation between age of onset of MDD and family loading has led many researchers to consider early-onset MDD the most genetic form of the illness 11, 12. Accordingly, research with a focus on children and adolescents at high-risk for MDD is important to understand the contribution of the genetic programming to depression as well as to identify abnormal neurophysiological substrates that confer susceptibility for this disorder.
In the past few decades, researchers have focused on changes in sleep architecture as potential neurophysiological substrates mediating alterations in neuroplasticity related to MDD. The most reliable sleep electroencephalogram (EEG) changes associated with MDD include lower sleep efficiency, shorter latency to rapid eye movement (REM) sleep, increased REM sleep, and altered slow-wave activity compared to healthy controls 13. Significant differences may be stronger when age and sex are taken into consideration 14. However, despite sound evidence showing that MDD onset and course of illness is sex-dependent, divergent brain pathophysiologies remain largely understudied in boys and girls with MDD 15,16. Perhaps as a contributing factor, sleep EEG measures have shown consistency among adult studies, whereas findings in children and adolescents with MDD have been more equivocal 14, 17. Nevertheless, sleep EEG measures have been successfully utilized to identify youths at high-risk for MDD and to predict both onset of MDD and course of illness 16, 18-20.
It has been proposed that change in incidence of several sleep rhythms changes throughout human development has implications in brain development 21-24. Therefore, researchers have focused on age-related sleep changes to better identify biological risk factors for early-onset MDD. Among those rhythms are sleep spindles, bursts of activity with frequency of 12-16Hz and duration of 0.5-3 sec that constitutes the hallmark of Stage 2 of non-REM (NREM) sleep 25. Sleep spindle activity (SPA) is generated and regulated by the thalamocortical network 26, and reaches maximum level in the late part of the night 27. Several findings support an active role for SPA in neuroplasticity mechanisms. Animal and human studies have shown that SPA synchronizes the flow of information from limbic structures to cortex 28, 29. This mechanism could lead to the strengthening of cortical synaptic connections through a long-term potentiation (LTP)-like mechanism 30 during brain maturation and memory consolidation. Regarding the role of SPA in brain maturation, it has been shown that SPA peak at 12-14 years and a decline thereafter 23. Furthermore, SPA is affected in children with mental retardation 31, supporting the notion that SPA plays a significant role in neuroplasticity and proper neural circuit formation. SPA generation is also modified by sex with higher levels in young adult females than males 32. However, no studies have addressed the impact of sex on SPA in childhood and adolescence. Altogether, these findings suggest that SPA changes during development illustrate neuroplasticity mechanisms underlying brain maturation that could be different for females and males.
There have been few studies of SPA in MDD with contradictory results. De Maertelaer et al's study showed a decrease in SPA in depressed patients compared to healthy individuals 33 whereas two other studies found no difference in SPA between depressed and healthy individuals 34, 35. Unlike De Maertelaer and collaborators, the last two studies did not include children and adolescents in their samples, which considering the changes in SPA during development 23 is a limitation. One additional study failed to show differences in SPA between depressed and healthy individuals, despite the inclusion of youths in their sample 36. However, authors restricted their analysis of SPA to the first NREM period only, when SPA is minimal 37.
The present study was undertaken to evaluate SPA in youths with MDD and in those at high risk, based on positive family history, compared to healthy non-depressed controls. It was hypothesized that SPA would be lower in both the MDD and high-risk youths, particularly among girls.
Method
Participants
Twenty-one depressed (MDD: 11 males and 10 females), twenty-one at high-risk for depression (HR: 11 males and 10 females), and twenty-one healthy non-depressed controls (HC: 11 males and 10 females) ranging from 8-15 years were recruited for this study. Participants were recruited through published advertisements and posted flyers at community centers, hospitals, outpatient psychiatric clinics, and pediatric clinics. Self-referral or referral from a community clinician was also permitted. Inclusion criteria for all participants consisted of ability to provide informed written consent (parent) and assent (child), and no medication (for at least 4 weeks) or counseling at the time of the clinical interview. Additional inclusion criteria for healthy controls were no personal or family history of psychopathology in first-degree relatives. Those at high-risk for depression had no personal history of psychopathology, but had at least one parent with a history of treated MDD. For the MDD group, inclusion required a current diagnosis of nonpsychotic MDD, single or recurrent, according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), and symptomatic at the time of enrollment. Exclusion criteria for all participants included significant head injury or unconsciousness for > 5 minutes (lifetime); independent/intrinsic sleep disorder based on history or polysomnogram; significant previous or concurrent medical illness by history, physical exam, or clinical lab tests. Additional exclusion criteria included lifetime diagnosis of anorexia or bulimia or substance abuse in the last 6 months.
The high-risk group was ascertained first then the HC and MDD groups were recruited as an exact sex-match and within the given age range. An age match within 6 months was obtained in almost all cases.
Clinical Assessment
A brief telephone screen was administered to determine potential eligibility. All participants were then scheduled for a full clinical interview. Prior to the initial interview, the study was explained and written informed consent was obtained from the parent(s) and assent from the participant. All study participants underwent the same initial psychiatric evaluations. At the initial visit, each participant and parent(s) was interviewed separately using the Schedules for Affective Disorders and Schizophrenia for School-Aged Children: Present and Lifetime 38. Additionally, depressive-symptom severity was assessed using the Children's Depression Rating Scale-Revised (CDRS-R) 39. A minimum score of > 40 on the CDRS-R was required for entry into the study, indexing moderate depressive-symptom severity and matching the criterion of our previous work 16, 40, 41. While the child was being interviewed, a separate interviewer obtained family history from parents using the Family History Diagnostic Interview. The Children's Global Assessment Scale (CGAS) 42 and the Family Global Assessment Scale (FGAS) 43 assessed overall functioning of the child and the family, respectively. Tanner maturation (1-5 scores) was self-assessed by participants using the “Typical Progression of Pubertal Development Chart” adapted from Tanner 44. Breast and pubic hair development were assessed for girls, and genital and pubic hair development was assessed for boys.
Procedures
Sleep recording and scoring
All participants agreed to follow their usual school-week's bed- and rise-time schedule throughout the study, established by sleep history at enrollment. Actigraphs (Actiwatch-L™, Mini-Mitter) were worn throughout the week and sleep/wake diaries were collected daily during the home recording period. Data from the actigraphs were downloaded prior to their first night in the laboratory to ensure that the participants adhered to their regularized rise- and bed-times.
Each participant spent 2 consecutive nights in Sleep Laboratory. Night 1 served as laboratory adaptation and as an additional screen for the presence of independent sleep disorders, and night 2 as the study night. Night 1 recordings also included chest and abdomen respiration bands, nasal-oral thermistors, and leg electrodes. Electrode placement on the study night included F3, F4, C3, C4, O1, O2, P3, P4, left and right EOG, recorded from the upper and lower canthi, and a bipolar, chin-cheek EMG. EEG electrodes were referenced to the ear lobes linked through a 10 KΩ resistor to minimize non-homogeneous current flow and potential artifactual hemispheric asymmetries, as is standard in our laboratory. EEG was transduced by Grass™ P511 A/C amplifiers set at sensitivity of 5 (50μV, 0.5 s calibration), corresponding to a gain of 50,000. The half-amp low- and high-bandpass filters were set at 0.3 and 30 Hz, respectively. A 60-Hz notch filter attenuated electrical noise.
Visual stage scoring was conducted in 30 s epochs, according to standard sleep staging criteria, described in Rechtschaffen, and Kales 45, by research personnel trained at better than 90 % agreement on an epoch-by-epoch basis. Sleep latency was defined as the first consecutive 10 minute block of any sleep stage (except REM) with no more than 2 minutes of waking time, reflecting persistent sleep onset. Total sleep period (TSP) was defined as the time from lights out to lights on. The REM latency was defined as the minutes from sleep onset to the first epoch of REM sleep with no minimum duration criterion. Sleep efficiency is calculated as the total amount of sleep time divided by the total sleep period. The number of arousals was defined as the total number of waking episodes of at least 30 sec duration. Percentages of each of the sleep stages within TSP were also computed. The personnel who scored the records were blind to the diagnostic group, age or gender.
Spindle detection and analysis
Sleep spindles were analyzed in the left-frontal (F3) electrode on stage 2 segments of the first four NREM sleep periods, following convention. Spindles were automatically detected utilizing the software HypnoLab 1.2 (SWS Soft, Italy). The detection criterion in the HypnoLab program was set to identify spindles with frequency of 11–16Hz, amplitude of 14μV or higher, and duration of 0.5–3sec. Following automatic detection of spindles by the software, a researcher, blind to the clinical condition and diagnosis, visually confirmed each of the spindles selected by the program. Spindle density (SD) was defined as the number of 0.5 sec spindles per number of stage 2 epochs in a particular NREM period and used as the dependent measures of SPA.
Statistical Analysis
The statistical analysis software (SAS) version 9.1.3 for Windows was utilized for all statistical analyses. SD was compared between groups across the first four NREM periods using a repeated-measures analysis of variance (ANOVA), after statistical significant difference was established with a multivariate ANOVA (MANOVA). Least-squares multiple comparisons tested differences between individual means at an experiment-wise p<0.05, only if a significant overall ANOVA effect was obtained. The experiment-wise probability is equivalent to a Bonferroni adjustment and provides protection against Type 1 errors. Separate ANOVAs were computed for SD in each of the first fourth NREM periods. There are usually 4-5 NREM periods in youths, but since we used repeated measures analysis to investigate SPA changes across the night, we include the same number of NREM periods for each subject in each group. Pearson correlation coefficients were also calculated to assess the relationship between SD and comorbid symptomatology in the MDD group, and between SD and average Tanner scores in all three groups (HC, HR, and MDD). Data are provided as mean ± standard deviation, unless specified otherwise.
Results
Demographic and clinical characteristics of the sample
Demographic and clinical information are shown in Table 1. The age of males and females in each of the groups is as follows: HC (males=13.0±2.1, females=11.4±2.4); HR (males=12.9±3.3, females=12.6±2.2); MDD (males=12.2±2.7, females=11.3±2.5). There were no significant between-group differences in age (F2,62=0.7 p>0.05) or Tanner developmental maturation scores (F2,62=0.5 p>0.05).
Table 1.
Demographic and clinical features of the sample by diagnostic group. Data are shown as means ± standard deviation.
| HC1 n=21 |
HR n=21 |
MDD n=21 |
|
|---|---|---|---|
| Age | 12.2±2.4 | 12.8±2.8 | 11.8±2.6 |
| Tanner scores | 3.2±1.4 | 2.9±1.3 | 2.8±1.4 |
| FGAS | 90.4±5.3 | 75.2±7.7 | 62.6±10 |
| CGAS | 89.2±4.9 | 78.1±5.3 | 51.1±6.5 |
| CDRS-R | 18.6±1.8 | 20.8±1.7 | 58.1±5.8 |
| Age of onset | — | — | 10.8±3.0 |
| Length of current depressive episode2 | — | — | 12.6±14.8 |
| Suicide attempts, (n) | — | — | 1 |
| Ideation, 1-5 scale | — | — | 2.2±0.8 |
| Family history of MDD (n) | — | 21 | 15 |
| Comorbid psychiatric diagnosis (n) | — | — | 15 |
| ADHD | — | — | 6 |
| SAD | — | — | 1 |
| Generalized Anxiety | — | — | 2 |
| Dysthymic Disorder | — | — | 4 |
| Phobia | — | — | 1 |
| OCD | — | — | 1 |
Note: Bold font indicates significant between-group differences. ADHD = Attention Deficit Hyperactivity Disorder; HC = Healthy Control; HR = High Risk; MDD = Major Depressive Disorder; OCD = Obsessive compulsive disorder; SAD = Seasonal Affective Disorder
n=20 for Family Global Assessment Scale (FGAS), Clinical Global Assessment Scale (CGAS), and Children's Depression Rating Scale – Revised (CDRS-R);
in months
There were between-groups differences for the Family Global Assessment Scale (FGAS: F2,61=62.7 p<0.0001), the Children's Global Assessment Scale (CGAS: F2,61=250 p<0.0001), and the Children's Depression Rating Scale (CDRS: F2,61=753.3 p<0.0001). Multiple comparisons revealed that all three groups differed from each other (p<0.0001) for FGAS and CGAS, with significantly lower scores in the MDD group than in both the HR and HC groups, but the HR group was also significantly lower than HCs. With regard to CDRS, individuals in the MDD group were different from those in the HC (p<0.0001) and HR (p<0.0001) groups, with no difference between HC and HR groups. Note that between group comparisons on FGAS, CGAS and CDR-S were based on n=20 for the HC, as data were missing.
Sleep macroarchitecture
The means and standard deviations for select sleep macroarchitectural variables are presented in Table 2. We found significant between-group changes in the time spent in stage 1 of NREM sleep (F5,62=8.64 p<0.0001), amount of awake and movement time (F5,62=6.21 p<0.0001), sleep efficiency (F5,62=3.44 p=0.009), REM density (F5,62=10.13 p<0.0001), and number of arousals (F5,62=12.9 p<0.0001). Multiple comparisons revealed that these changes were driven by the high-risk group, who showed less time in stage 1, less arousal, less awake and movement, better sleep efficiency, and higher REM density compared to MDD and HC groups. No differences were observed between HCs and MDDs. Groups did not differ in time spent in stage 2 of NREM sleep (F5,62=0.8 p=0.55). It is notable that sleep macroarchitectural variables typically associated with depression such as total sleep time, sleep latency, and REM sleep latency did not differentiate between groups, while sleep spindle activity clearly delineated groups by condition and by sex (see below).
Table 2.
Means and standard deviations of sleep macroarchitecture by group (collapsed by gender).
| Sleep variable | HC n=21 |
HR n=21 |
MDD n=21 |
|---|---|---|---|
| Total sleep period, min. | 503.1 ± 49.9 | 499.4 ± 55.2 | 489.1 ± 54 |
| Sleep latency, min. | 14.7 ± 10.7 | 15.8 ± 16.5 | 18.2 ± 12.2 |
| REM latency, min | 101.2 ± 37.8 | 106.7 ± 43.3 | 97.4 ± 41.7 |
| % Stage 11 | 8.2 ± 5.1 | 2.1 ± 2.9 | 9.2 ± 3.4 |
| % Stage 21 | 49.3 ± 6.8 | 48.5 ± 12.3 | 48.5 ± 6.9 |
| % SW (Stage 3 + 4)1 | 19.4 ± 5.6 | 25.7 ± 11.4 | 18.6 ± 6.9 |
| % REM1 | 18.9 ± 2.4 | 21.5 ± 4.4 | 19.7 ± 4.2 |
| % Awake and movement1 | 4.3 ± 1.9 | 2.0 ± 1.8 | 4.0 ± 1.5 |
| Sleep efficiency | 92.8 ± 2.8 | 95.0 ± 3.1 | 92.4 ± 3.0 |
| REM density | 2.1 ± 0.6 | 3.9 ± 1.5 | 2.0 ± 0.5 |
| Slow-wave min in 1st NREM2 | 58.6 ± 22.6 | 65.7 ± 29.1 | 49.0 ± 24.9 |
| Number of arousals | 27.0 ± 6.7 | 12.6 ± 6.8 | 23.2 ± 6.5 |
Note: Bold font indicates significant between-group differences. HC = Healthy Control; HR = High Risk; MDD = Major Depressive Disorder; REM = Rapid Eye Movement sleep; NREM = non-Rapid Eye Movement sleep; SW = Slow Wave sleep
relative to total sleep period
minutes of Stage 3 +4
Sleep spindle characteristics
MANOVA yielded overall multivariate significance for the SD. There was a NREM period main effect (F3,55=9.27, p<0.0001) and a NREM period by group by sex interaction (F15,171=1.90, p=0.02). Univariate ANOVA was conducted for SD across the night and in each of the first four NREM periods.
Spindle density across the night
One-way ANOVA for SD across the night showed between-groups differences (F2,60=5.25 p=0.008). Multiple comparisons indicated that youths in the MDD group had significantly lower SD (p=0.002) than HCs. The HR group (0.9±0.8) showed an intermediate level of SD between HC (1.5±1.2) and MDD (0.6±0.6) groups, with a trend towards statistical significance in the comparison with HC (p=0.056). These results are illustrated in Figure 1.
Figure 1.
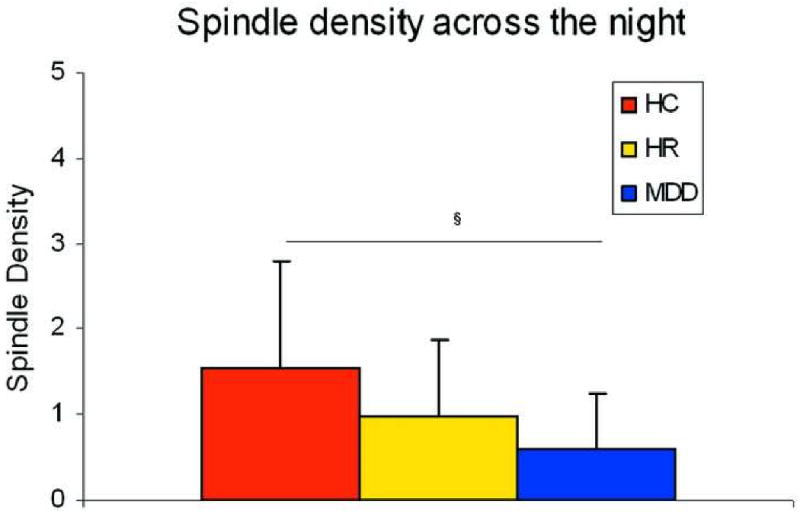
Spindle density across the night. Note: HC = Healthy Control; HR = High Risk; MDD = Major Depressive Disorder
§ p<0.005
Spindle density in each NREM period
Since sleep spindles have been shown to be unequally distributed across the night, with a higher level of activity in the late part of the night 37, between-group differences were evaluated across the first four NREM periods.
The repeated-measures ANOVA for SD revealed a significant NREM period main effect (F3,171=12.7 p<0.0001) and a NREM period by group by sex interaction (F15,171=1.86, p<0.03). Univariate ANOVAs were used to determine whether SD showed between-group difference across NREM periods. No significant group differences were found in the first (F2,62=2.4 p<0.05) or second (F2,62=2.65 p<0.05) NREM periods. However, group main effects were evident in the third NREM period (F2,62=3.51 p=0.03). Least square, multiple comparisons indicated that both the HR (p=0.03) and MDD (p=0.02) groups had lower SD (HC=1.78±1.32; HR=0.97±0.89; MDD=0.78±0.86) than HCs.
ANOVA in the fourth NREM period echoed the findings in the third NREM period, with significant group effects (F2,62=7.3 p=0.001). SD (HC=1.97±1.45; HR=1.12±1.13; MDD=0.67±0.61) was significantly lower in HR (p=0.02) and MDD (p=0.0004) groups compared to controls. See Figure 2 for a representation of these effects.
Figure 2.
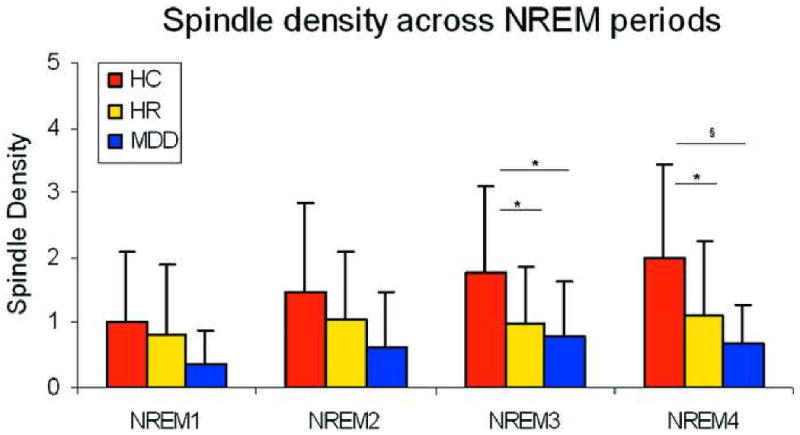
Spindle density in each of the non-Rapid Eye Movement (NREM) periods. Note: HC = Healthy Control; HR = High Risk; MDD = Major Depressive Disorder
* p<0.05, § p<0.005
Sex-differences in spindle density in the third and fourth NREM periods
Based on the significant NREM period by group by sex interaction (F15,171=1.90, p=0.02) obtained in the MANOVA, we investigated the influence of sex on SD changes. No significant group differences were detected in the first (F5,62=2.1 p>0.05) or second (F5,62=1.7 p>0.05) NREM periods. ANOVA in the third NREM period produced significant results (F5,62=3.04 p=0.02). HC-females (HC-F) had significantly higher SD (HC-F=1.6±1.5; HR-F=1.3±0.9; MDD-F=1.0±1.0; HC-M=1.5±1.5; HR-M=0.6±0.6; MDD-M=0.5±0.6) than MDD-males (MDD-M) (p=0.003), MDD-F (p=0.04), and HR-M (p=0.004). In addition, SD was higher in HC-M compared to MDD-M (p=0.03) and HR-M (p=0.04). We observed more robust results in the fourth NREM period (F5,62=5.19 p=0.005). The between-group interaction was primarily driven by girls in the HC group (HC-F=2.6±1.4; HR-F=1.5±1.3; MDD-F=0.6±0.5; HC-M=1.4±1.2; HR-M=0.7±0.7; MDD-M=0.6±0.7). This group expressed significantly higher SD than any other group (p range 0.01-0.0001). These results are depicted in Figure 3.
Figure 3.
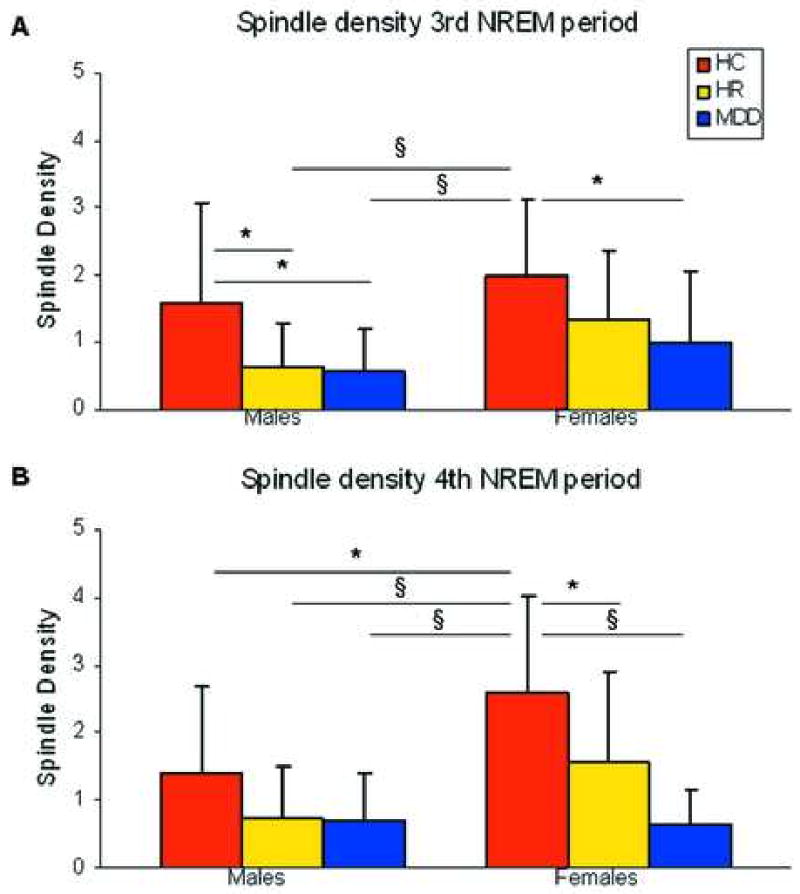
Spindle density in the 3rd (A) and 4th (B) non-Rapid Eye Movement (NREM) periods across sex. Note: HC = Healthy Control; HR = High Risk; MDD = Major Depressive Disorder
* p<0.05, § p<0.005
Correlation analyses
As a final set of analyses, we computed correlations between SD and several of the clinical and demographic variables. The goal was two-fold, to determine potential moderator variables of SD within and between groups, and to aid with interpretation of our results.
Pearson's correlation coefficients were computed between SD and Tanner score within each group to ensure that differences in sexual maturity between groups did not contribute to lower SD in the MDD and HR groups. Tanner score was not significantly correlated with SD in HC [r21=.02], HR [r21=.09] or MDD [r21=.11] groups p>.05.
We were also interested in the influence of symptom severity and psychiatric co-morbidity on SD since there the high number of depressed participants with co-morbid illness. The number of co-morbid illnesses within the MDD group accounted for less than 2 percent of the variance in SD [r21= -0.13 p>0.05]. Further, symptom severity did not correlate significantly with SD in the MDD [r21=-0.07], HR [r21=0.34] or HC group [r20=-0.07], p>.05. Although the HR group showed a positive correlation, whereas both MDD and HCs showed negative correlations between CDRS score and SD, the correlations were not significant in any group and accounted for less than 15 % of the variance within the HR group. Thus, it was not those with the most severe symptoms that showed the lowest SD.
Interestingly, FGAS and CGAS did correlate with SD, but only in the HC group [r20 = 0.47; 0.46 p<.04; respectively]. However, neither correlation was significant by Bonferroni criteria. The FGAS and CGAS accounted for only marginal variance in SD in the MDD group [r21=-0.14; -0.09; p>.05; respectively] and in the HR group [r21=-.014; -0.09; p>.05; respectively].
No other analyses were conducted.
Discussion
The major finding of the present study is that decreased SPA during sleep characterizes both youths with MDD and those at high-risk for the illness, based on family history, compared to healthy non-depressed controls. SPA was lowest in currently depressed children and adolescents, and intermediate in the high-risk group suggesting that reduced neuroplasticity at this age might constitute a vulnerability factor for MDD.
Based on our findings in high-risk children and adolescents, we speculate that the existence of a predisposing genetic programming for MDD may trigger the development of a thalamocortical circuit with limited SPA generation. It has been shown that neuronal circuit in the brain are formed through information encoded in the genetic programming 46. This reduced SPA might produce abnormalities in brain maturation that could result in reduced neuroplasticity and susceptibility for the onset of MDD. If our hypothesis is correct, decreased SPA can be regarded as a biological risk marker for early-onset depression. However, we recognize that we cannot assert that a decline in SPA is antecedent to MDD or predict onset without longitudinal follow-up or without extending our studies to other mental disorders.
Depressed children and adolescents showed a larger decrease in SPA than high-risk in the comparison with healthy controls, suggesting that onset of depressive symptoms is associated with lower neuroplasticity levels than mere genetic predisposition. Considering the proposed function of sleep spindles 28, 29, it is possible that the dramatic reduction observed in depressed youth is a compensatory brain mechanism to limit the flow of information (e.g. emotionally harmful intrusive and persistent thoughts) from limbic structures to cortex.
However, the group differences in SPA were greater in females than in males. In comparison with healthy controls, depressed and at high-risk males exhibited similar reduction in SPA in the latter part of the night. Unlike males, SPA did differ between girls at risk versus those already ill with MDD. SPA was lowest in depressed females and intermediate in at high-risk females but still lower in comparison to healthy control females. This disparity between depressed and at high-risk for depression females implies that a further reduction in SPA may accompany full onset of clinical symptoms of depression. However, it is not just an increase in symptoms that appears to moderate SPA, as CDRS scores were not significantly correlated with SPA in either the HR or MDD groups. Thus, it appears that SPA reflects vulnerability to disease of depression, not to the symptoms of the illness. It also appears that the expression of this vulnerability is stronger in girls than in boys.
This finding parallels previous clinical studies reporting that females are twice as likely to suffer from MDD than males 47, 48, a risk that increases with the beginning of the reproductive years. Gonadal hormones have been extensively researched as significant risk factors in the precipitation and course of MDD in females 49, 50, and could also interfere with spindle activity generation. It has been shown that estrogen and progesterone alter neuronal firing 51, the sleep cycle, and EEG frequencies 52, 53 through their effect on the serotonergic, noradrenergic, cholinergic, and GABAergic systems 54. Noteworthy is the influence of estrogen on the GABAergic system, which may alter spindle activity. Spindles are generated by the hyperpolarizing action of thalamic-reticular neurons on thalamocortical cells via GABAergic receptors 55, 56. If MDD and those at high-risk have impaired neurotransmitter regulation, particularly GABAergic, gonadal hormones may have aberrant effects on neuroplasticity leading to impaired neuronal synchronization in the thalamocortical circuit and eventually to a decrease in SPA.
The finding of decreased SPA in depressed children and adolescents is not consistent across all previous studies 33-36. We suggest that part of the disparity between studies is attributable to the age differences among studies. SPA follows a well-defined developmental course with a peak around age 12-14 and a decline thereafter 23. It is expected that changes in neuroplasticity and homeostatic mechanisms affecting SPA will be more evident during youth and young adulthood than in older adults—as observed in our results. Additional evidence for a role of SPA in brain plasticity during youth originates from studies of the function of spindles in memory formation. This research has reported changes in SPA after learning in young, but not older adults 57. Altogether, these findings support our hypothesis that changes in SPA would be more evident in childhood and adolescence, an age when neuroplasticity is at its peak, than in adulthood. Of course, longitudinal studies are necessary to determine the true developmental time course across sex or diagnostic group. The cross-sectional approach severely limits our ability to address developmental or age-related differences. Nevertheless, its is important to underscore that in our study SPA, but not sleep macroarchitectural variables, discriminate between diagnostic groups, suggesting that SPA might be a more sensitive measure of sleep alteration in adolescent depression than standard polysomnographic measures.
The finding of “better” sleep in the high risk group than either HC or MDD groups was unexpected and difficult to interpret, since all participants maintained regularized sleep schedules prior to and throughout the study, verified by actigraphy and sleep diaries. Participants were also age-matched across groups and therefore minimizing the contribution of age-related differences in sleep measures between groups. We have no good explanation as to why the high-risk group had less sleep fragmentation and awake time. Nevertheless, this bolsters our SPA findings. Lower SPA in the HR and MDD groups was not due to less Stage 2 sleep or more sleep fragmentation.
There are limitations in the present study that need to be considered. Our sample size included only 10 females and 11 males in each of the three groups for a total of 21 participants per group. Although this is a reasonable sample size for this type of sleep study, it limited the statistical power of some of our comparisons, particularly interaction effects. Separate analyses between SPA and clinical characteristics are necessary to fully understand the differences between boys and girls in this study. Unfortunately, too few subjects were included within each group and gender to permit such a comparison here. Also, the small sample size limited further stratification of the sample into age groups to examine group differences before and after the developmental peak of SPA. Further, a longitudinal design is necessary to fully address maturational differences in SPA within individuals in each group. This is a very important question that is currently the focus of research in our laboratory. Of course, the most important limitation of this study is that we can only define risk for MDD within an individual. The validity of our interpretation of the results from this study rests on demonstrating that those in the HR group with the lowest SPA are most likely to develop later depression.
Nevertheless, the present study is a first step in assessing the utility of SPA in differentiating children and adolescents at high-risk for MDD from healthy controls. We will continue to follow this sample longitudinally to fully assess the predictive value of decreased SPA and initiate studies examining the direct role of SPA changes in neuroplasticity in early-onset depression.
Acknowledgments
This research was supported under R01-MH077744 (RA); R01-MH056953 (RA), UL1RR024986 (JL) and the Cohen Family Fund (RA).
The authors would like to thank Raffaele Ferri, M.D. and Francesco Rundo, B.E. from the Sleep Research Centre, Department of Neurology I.C., OASI Institute, Troina, Italy for technical support, the research team of the Sleep and Chronophysiology Laboratory at the University of Michigan for running participants, and Holli Bertram, M.S.W. from the University of Michigan for conducting clinical interviews. The authors extend their deepest thanks to the children and adolescents and families who participated in this study.
Footnotes
Disclosure: Drs. Lopez, Hoffman, and Armitage report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Zisook S, Rush AJ, Albala A, et al. Factors that differentiate early vs. later onset of major depression disorder. Psychiatry Res. 2004;129(2):127–40. doi: 10.1016/j.psychres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs M, Feinberg TL, Crouse-Novak MA, Paulauskas SL, Finkelstein R. Depressive disorders in childhood. I. A longitudinal prospective study of characteristics and recovery. Arch Gen Psychiatry. 1984;41(3):229–37. doi: 10.1001/archpsyc.1984.01790140019002. [DOI] [PubMed] [Google Scholar]
- 3.Rao U, Ryan ND, Birmaher B, et al. Unipolar depression in adolescents: clinical outcome in adulthood. J Am Acad Child Adolesc Psychiatry. 1995;34(5):566–78. doi: 10.1097/00004583-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Rao U, Weissman MM, Martin JA, Hammond RW. Childhood depression and risk of suicide: a preliminary report of a longitudinal study. J Am Acad Child Adolesc Psychiatry. 1993;32(1):21–7. doi: 10.1097/00004583-199301000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Weissman MM, Bland RC, Canino GJ, et al. Prevalence of suicide ideation and suicide attempts in nine countries. Psychol Med. 1999;29(1):9–17. doi: 10.1017/s0033291798007867. [DOI] [PubMed] [Google Scholar]
- 6.Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J. Childhood and adolescent depression: a review of the past 10 years. Part II. J Am Acad Child Adolesc Psychiatry. 1996;35(12):1575–83. doi: 10.1097/00004583-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman J, Martin A, King RA, Charney D. Are child-, adolescent-, and adult-onset depression one and the same disorder? Biol Psychiatry. 2001;49(12):980–1001. doi: 10.1016/s0006-3223(01)01127-1. [DOI] [PubMed] [Google Scholar]
- 8.Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107(1):128–40. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- 9.Hankin BL, Abramson LY. Development of gender differences in depression: description and possible explanations. Ann Med. 1999;31(6):372–9. doi: 10.3109/07853899908998794. [DOI] [PubMed] [Google Scholar]
- 10.Williamson DE, Birmaher B, Axelson DA, Ryan ND, Dahl RE. First episode of depression in children at low and high familial risk for depression. J Am Acad Child Adolesc Psychiatry. 2004;43(3):291–7. doi: 10.1097/00004583-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs M, Devlin B, Pollock M, Richards C, Mukerji P. A controlled family history study of childhood-onset depressive disorder. Arch Gen Psychiatry. 1997;54(7):613–23. doi: 10.1001/archpsyc.1997.01830190033004. [DOI] [PubMed] [Google Scholar]
- 12.Todd RD, Neuman R, Geller B, Fox LW, Hickok J. Genetic studies of affective disorders: should we be starting with childhood onset probands? J Am Acad Child Adolesc Psychiatry. 1993;32(6):1164–71. doi: 10.1097/00004583-199311000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand Suppl. 2007;433:104–15. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 14.Dahl RE, Ryan ND, Birmaher B, et al. Electroencephalographic sleep measures in prepubertal depression. Psychiatry Res. 1991;38(2):201–14. doi: 10.1016/0165-1781(91)90045-q. [DOI] [PubMed] [Google Scholar]
- 15.Armitage R, Hoffmann RF. Sleep EEG, depression and gender. Sleep Med Rev. 2001;5(3):237–46. doi: 10.1053/smrv.2000.0144. [DOI] [PubMed] [Google Scholar]
- 16.Robert JJ, Hoffmann RF, Emslie GJ, et al. Sex and age differences in sleep macroarchitecture in childhood and adolescent depression. J Electrocardiol. 2006;29(3):351–8. doi: 10.1093/sleep/29.3.351. [DOI] [PubMed] [Google Scholar]
- 17.Ivanenko A, Crabtree VM, Gozal D. Sleep and depression in children and adolescents. Sleep Med Rev. 2005;9(2):115–29. doi: 10.1016/j.smrv.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Rao U, Hammen CL, Poland RE. Risk Markers for Depression in Adolescents: Sleep and HPA Measures. Neuropsychopharmacology. 2009;34(8):1936–45. doi: 10.1038/npp.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulton MK, Armitage R, Rush AJ. Sleep electroencephalographic coherence abnormalities in individuals at high risk for depression: a pilot study. Biol Psychiatry. 2000;47(7):618–25. doi: 10.1016/s0006-3223(99)00163-8. [DOI] [PubMed] [Google Scholar]
- 20.Armitage R. Microarchitectural findings in sleep EEG in depression: diagnostic implications. Biol Psychiatry. 1995;37(2):72–84. doi: 10.1016/0006-3223(94)00082-E. [DOI] [PubMed] [Google Scholar]
- 21.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106(13):5177–80. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feinberg I, Campbell IG. Sleep EEG changes during adolescence: An index of a fundamental brain reorganization. Brain Cogn. 2009;72(1):56–65. doi: 10.1016/j.bandc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Scholle S, Zwacka G, Scholle HC. Sleep spindle evolution from infancy to adolescence. Clin Neurophysiol. 2007;118(7):1525–31. doi: 10.1016/j.clinph.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–19. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 25.Loomis AL, Harvey EN, Hobart G. Potential rhythms of the cerebral cortex during sleep. Science. 1935;81(2111):597–8. doi: 10.1126/science.81.2111.597. [DOI] [PubMed] [Google Scholar]
- 26.Steriade M. Impact of network activities on neuronal properties in corticothalamic systems. J Neurophysiol. 2001;86(1):1–39. doi: 10.1152/jn.2001.86.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch JC, Fourment A, Marc ME. Sleep-related variations of membrane potential in the lateral geniculate body relay neurons of the cat. Brain Res. 1983;259(2):308–12. doi: 10.1016/0006-8993(83)91264-7. [DOI] [PubMed] [Google Scholar]
- 28.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21(5):1123–8. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 29.Clemens Z, Molle M, Eross L, Barsi P, Halasz P, Born J. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain. 2007;130(Pt 11):2868–78. doi: 10.1093/brain/awm146. [DOI] [PubMed] [Google Scholar]
- 30.Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25(41):9398–405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shibagaki M, Kiyono S, Watanabe K. Spindle evolution in normal and mentally retarded children: a review. J Electrocardiol. 1982;5(1):47–57. doi: 10.1093/sleep/5.1.47. [DOI] [PubMed] [Google Scholar]
- 32.Huupponen E, Himanen SL, Varri A, Hasan J, Lehtokangas M, Saarinen J. A study on gender and age differences in sleep spindles. Neuropsychobiology. 2002;45(2):99–105. doi: 10.1159/000048684. [DOI] [PubMed] [Google Scholar]
- 33.de Maertelaer V, Hoffman G, Lemaire M, Mendlewicz J. Sleep spindle activity changes in patients with affective disorders. J Electrocardiol. 1987;10(5):443–51. doi: 10.1093/sleep/10.5.443. [DOI] [PubMed] [Google Scholar]
- 34.Goetz RR, Goetz DM, Hanlon C, Davies M, Weitzman ED, Puig-Antich J. Spindle characteristics in prepubertal major depressives during an episode and after sustained recovery: a controlled study. J Electrocardiol. 1983;6(4):369–75. doi: 10.1093/sleep/6.4.369. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds CF, III, Kupfer DJ, Taska LS, et al. EEG sleep in elderly depressed, demented, and healthy subjects. Biol Psychiatry. 1985;20(4):431–42. doi: 10.1016/0006-3223(85)90045-9. [DOI] [PubMed] [Google Scholar]
- 36.Ferrarelli F, Huber R, Peterson MJ, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483–92. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 37.Himanen SL, Virkkala J, Huupponen E, Niemi J, Hasan J. Occurrence of periodic sleep spindles within and across non-REM sleep episodes. Neuropsychobiology. 2003;48(4):209–16. doi: 10.1159/000074639. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Poznanski EO, Freeman LN, Mokros HB. Children's Depression Rating Scale - Revised (September 1984) Psychopharmacol Bull. 1985;21:979–89. [Google Scholar]
- 40.Armitage R, Emslie GJ, Hoffmann RF, et al. Ultradian rhythms and temporal coherence in sleep EEG in depressed children and adolescents. Biol Psychiatry. 2000;47(4):338–50. doi: 10.1016/s0006-3223(99)00129-8. [DOI] [PubMed] [Google Scholar]
- 41.Emslie GJ, Armitage R, Weinberg WA, Rush AJ, Mayes TL, Hoffmann RF. Sleep polysomnography as a predictor of recurrence in children and adolescents with major depressive disorder. Int J Neuropsychopharmacol. 2001;4(2):159–68. doi: 10.1017/S1461145701002383. [DOI] [PubMed] [Google Scholar]
- 42.Shaffer D, Gould MS, Brasic J, et al. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40(11):1228–31. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 43.Mrazek DA, Mrazek P, Klinnert M. Clinical assessment of parenting. J Am Acad Child Adolesc Psychiatry. 1995;34(3):272–82. doi: 10.1097/00004583-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Tanner JM. Growth at Adolescence. 2nd. Springfield: Charles C.Thomas; 1962. [Google Scholar]
- 45.Rechtschaffen A, Kales A. National Institute of Healthy Publication No 204. US Goverment Printing Office; Washington, DC: 1968. A Manual of Standarized Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. [Google Scholar]
- 46.Cohen-Cory S. The developing synapse: construction and modulation of synaptic structures and circuits. Science. 2002;298(5594):770–6. doi: 10.1126/science.1075510. [DOI] [PubMed] [Google Scholar]
- 47.Earls F. Sex differences in psychiatric disorders: origins and developmental influences. Psychiatr Dev. 1987;5(1):1–23. [PubMed] [Google Scholar]
- 48.Breslau N, Chilcoat H, Schultz LR. Anxiety disorders and the emergence of sex differences in major depression. J Gend Specif Med. 1998;1(3):33–9. [PubMed] [Google Scholar]
- 49.Halbreich U, Lumley LA. The multiple interactional biological processes that might lead to depression and gender differences in its appearance. J Affect Disord. 1993;29(2-3):159–73. doi: 10.1016/0165-0327(93)90030-n. [DOI] [PubMed] [Google Scholar]
- 50.Parry BL, Haynes P. Mood disorders and the reproductive cycle. J Gend Specif Med. 2000;3(5):53–8. [PubMed] [Google Scholar]
- 51.Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38(4):379–95. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- 52.Driver HS, Dijk DJ, Werth E, Biedermann K, Borbely AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81(2):728–35. doi: 10.1210/jcem.81.2.8636295. [DOI] [PubMed] [Google Scholar]
- 53.Manber R, Armitage R. Sex, steroids, and sleep: a review. J Electrocardiol. 1999;22(5):540–55. [PubMed] [Google Scholar]
- 54.Halbreich U, Kahn LS. Role of estrogen in the aetiology and treatment of mood disorders. CNS Drugs. 2001;15(10):797–817. doi: 10.2165/00023210-200115100-00005. [DOI] [PubMed] [Google Scholar]
- 55.Bazhenov M, Timofeev I, Steriade M, Sejnowski T. Spiking-bursting activity in the thalamic reticular nucleus initiates sequences of spindle oscillations in thalamic networks. J Neurophysiol. 2000;84(2):1076–87. doi: 10.1152/jn.2000.84.2.1076. [DOI] [PubMed] [Google Scholar]
- 56.Contreras D, Steriade M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J Physiol. 1996;490(Pt 1):159–79. doi: 10.1113/jphysiol.1996.sp021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peters KR, Ray L, Smith V, Smith C. Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. J Sleep Res. 2008;17(1):23–33. doi: 10.1111/j.1365-2869.2008.00634.x. [DOI] [PubMed] [Google Scholar]


