Abstract
Trophic deprivation mediated neuronal death is important during development, acute brain or nerve trauma, and neurodegeneration. Serum deprivation (SD) approximates trophic deprivation in vitro, and an in vivo model is neuronal death in the mouse dorsal lateral geniculate nucleus (LGNd) after ablation of the visual cortex (VCA). Oxidant-induced intracellular Zn2+ release, ([Zn2+]i), from metallothionein-3 (MT-III), mitochondria, or “protein Zn2+” was implicated in trophic deprivation neurotoxicity. We previously showed that neurotoxicity of extracellular Zn2+ required entry, elevation in [Zn2+]i, reduction of NAD+ and ATP levels causing inhibition of glycolysis and cellular metabolism. Exogenous NAD+ and sirtuin inhibition attenuated Zn2+ neurotoxicity. Here we show that: 1) Zn2+ is released intracellularly after oxidant and SD injuries, and sensitivity to these injuries is proportional to neuronal Zn2+ content; 2) NAD+ loss is involved; restoration of NAD+ using exogenous NAD+, pyruvate, or nicotinamide attenuated these injuries, and potentiation of NAD+ loss potentiated injury; 3) Neurons from genetically modified mouse strains which reduce intracellular Zn2+ content (MT-III knockout), reduce NAD+ catabolism (PARP-1 knockout), or increase expression of an NAD+ synthetic enzyme (Wlds) each had attenuated SD and oxidant neurotoxicities; 4) Sirtuin inhibitors attenuated, and sirtuin activators potentiated these neurotoxicities; 5) VCA induces Zn2+ staining and death only in ipsilateral LGNd neurons, and a 1ppm Zn2+ diet attenuated injury; 6) Finally, NAD+ synthesis and levels are involved because LGNd neuronal death after VCA was dramatically reduced in Wlds animals, and by intraperitoneal pyruvate or nicotinamide. Zn2+ toxicity is involved in serum and trophic deprivation induced neuronal death.
Keywords: visual cortex ablation, mouse, pyruvate, sirtuin, dorsal lateral geniculate nucleus
Target deprivation mediated neuronal death plays a large role during development, trauma, and neurodegeneration. In the developing nervous system, 20–80% of all neurons produced during embryogenesis die before reaching adulthood as a result of competition between neurons for innervation of their targets. This results in matching of the size of the target cell population with the number of innervating neurons (Oppenheim, 1991; Purves et al., 1988). Target deprivation mediated neuronal death is apoptotic and occurs by programmed cell death (PCD) (Deshmukh & Johnson, 1997; Martin et al., 1998). PCD is required for the elimination of excess cells during the sculpting of organs or tissues, and cells not required for a new developmental stage (Ellis et al., 1991). Target deprivation mediated neuronal death is also prevalent after trauma or neurodegeneration. Zn2+ staining was reported to have a one to one correspondence with those neurons undergoing developmental neuronal death (Lee et al., 2006). Furthermore, in vivo intracellular Zn2+ chelation using TPEN (N,N,N'N'-tetrakis(−)[2-pyridylmethyl]-ethylenediamine), was recently suggested to attenuate this developmental neuronal death (Cho et al.).
Serum Deprivation Models Target Deprivation, and Both Induce an Oxidative Injury
We and others have shown that serum deprivation (SD) induces substantial oxidative stress leading to partial ATP depletion, K+ loss involving inhibition of the Na+/K+ ATPase, and the apoptotic cascade (Wang et al., 2003). We are using oxidative stress and serum deprivation as in vitro models approximating target deprivation to test therapeutics and implicate pathways. Reactive oxygen species (ROS), apoptosis, and target deprivation have also been suggested to play roles in progressive neurodegenerative diseases (reviewed in Martin et al., 1998). The ROS generated during serum or target deprivations was shown to be a required activating signal for immediate early genes causing an apoptotic cascade leading to caspase activation, mitochondrial dysfunction, and programmed cell death (reviewed in Chang et al., 2002; Deshmukh & Johnson, 1997; Martin et al., 1998; Martin et al., 2003). ROS mediated Zn2+ release plays a role in H2O2 mediated injuries perhaps through autophagy (Hwang et al., 2008). We propose that ROS generated by serum or target deprivation mediated injuries also causes ROS mediated Zn2+ release. Therefore, therapeutics that target Zn2+ neurotoxicity are effective against these ROS-mediated injuries (see model in Figure 1).
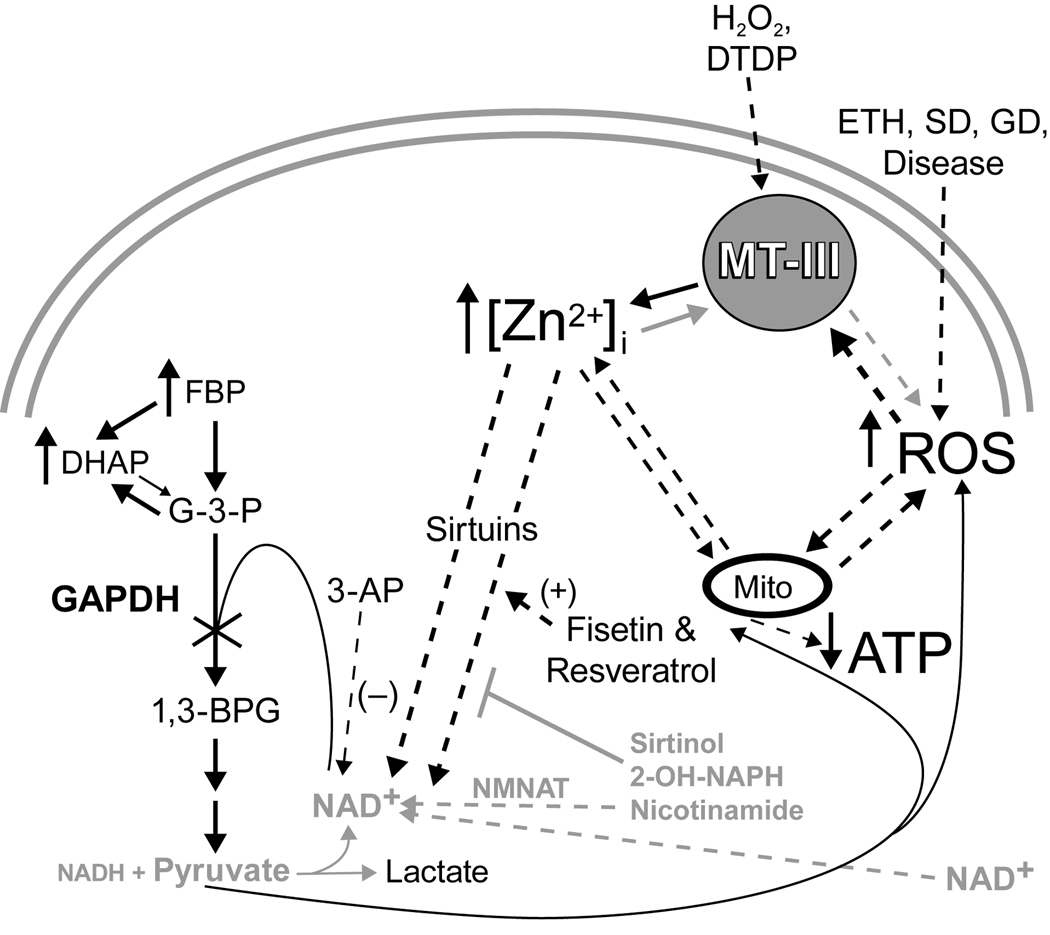
Figure 1. Model of Zinc Neurotoxicity.
The resulting increased [Zn2+]i may cause direct inhibition of mitochondria, or an indirect inhibition of GAPDH and mitochondria by a reduction in NAD+ levels induced by an unknown NAD+ catabolizing enzyme (sirtuins). Pyruvate and nicotinamide prevent NAD+ loss and enzyme inhibition. Black = Toxic, Gray = Therapeutic.
We chose the visual cortex ablation (VCA) induced death of LGNd neurons as our in vivo model of trophic deprivation because of its reproducibility and ease with the intent of defining mechanisms involved and therapeutic doses which would then be tested in more complex models of neurodegeneration. This model was shown to induce a Zn2+ mediated death of LGNd neurons after visual cortex (V1) ablation in adult mice that was associated with Zn2+ staining (Land & Aizenman, 2005). The cortical neurotrophic factors neurotrophin 4/5, nerve growth factor, and brain derived neurotrophic factor (BDNF) have been implicated as the target derived factors required for LGNd neuronal survival (Berardi & Maffei, 1999; Caleo et al., 2003). The hallmarks of trophic-factor deprivation induced apoptosis: ROS, DNA damage, and cytochrome C release have all been demonstrated in LGNd neurons after visual cortex ablation (Agarwala & Kalil, 1998; Al-Abdulla et al., 1998; Martin et al., 1998; Martin et al., 2003). We show here that [Zn2+]i, and [NAD+]i concentrations are key to oxidant, and serum or target deprivation mediated neurotoxicities, and that the NAD+ synthetic and sirtuin pathways are involved in vitro and in vivo (Figure 1).
MATERIALS AND METHODS
Cell culture and toxicity studies
Near-pure cortical neuronal cell cultures were prepared from E15 PARP-1 −/−, +/+, Wlds, MT-III knockout, and Swiss Webster mice as previously described (Gottron et al., 1997; Sheline & Choi, 1998). Dissociated cortical neurons were plated in Eagles’s minimum essential medium (MEM; Earle’s salts, glutamine-free, Gibco/Invitrogen, Carlsbad, CA) containing 21 mM glucose, 5% fetal bovine serum, and 5% horse serum at a density of 5 hemispheres/plate onto poly-D-lysine/laminin coated 24-well plates. An additional 10 µM ZnCl2 was added to the plating medium for Zn2+ loaded cultures. At 2–3 days in vitro (DIV) cytosine arabinoside was added to 10 µM to inhibit glial growth. Slow toxicity was initiated by exposure to oxidants (ETH 20–60 µM, H2O2 40–100 µM, DeNO 200–400 µM) in minimal essential defined media for 24 hours (supplemented with 1 µM MK-801 and 50 ng/ml of BDNF, Cellsciences, Canton, MA) to keep near-pure neuronal cultures from undergoing excitotoxic cell death or spontaneous apoptosis in the absence of astrocytes or serum). Therapeutic compounds were present during the entire exposure period as indicated. Serum deprivation experiments were performed at DIV7 after washing 7 times to remove all serum, cultures were exposed in MEM + 1 µM MK-801 and cell death assayed 30 hrs later. Glucose deprivation experiments were performed after washing 7 times in MEM with amino acids and vitamins, but lacking glucose, cultures were exposed in MEM lacking glucose + 1 µM MK-801 and 50 ng/ml of BDNF for 5 hours followed by 2 times wash into MEM (21 mM glucose + 1 µM MK-801 and 50 ng/ml of BDNF) and cell death was assayed 24 hrs later. Cell death was assessed by lactate dehydrogenase efflux to the bathing medium, and staining with propidium iodide or trypan blue (Sheline & Choi, 1998).
Determination of levels of NAD+, and NADH
Near-pure cortical neuronal cultures were used for these measurements. Nicotinamide adenine dinucleotide (NAD+) measurements were made on cell lysates prepared by lysis in 0.2 N NaOH, 1 mM EDTA at 0C. This lysate was split and part of it directly hydrolyzed at 80C for 20 min, and the other part acidified followed by hydrolysis at 80C for 20 min. These lysates were neutralized and stored at −80C. Alkaline hydrolysis destroys NAD+, whereas acid hydrolysis destroys NADH allowing for determinations of metabolites (FBP, ATP, ADP, NAD+, and NADH) using the malate dehydrogenase/alcohol dehydrogenase cycling pair (Passonneau & Lowry, 1993). The dynamic range of this cycling assay can be varied (10−15 to 10−9 moles) by the amount of cycling enzymes used and the duration of the cycling time. 2 µl of acid extract (∼1250 cells) was added directly to 100 µl of NAD+ cycling reagent (100 mM Tris-HCl, pH 8.1, 2 mM β-mercaptoethanol, 2 mM oxaloacetate, 0.3 M ethanol, 0.02% BSA, and 25 µg/ml of yeast alcohol dehydrogenase and 2.5 µg/ml malic dehydrogenase) and incubated at 25°C for 1h to obtain 2000 cycles of malate amplification. Termination by heating at 100°C for 5 min was followed by addition of 1 ml of malate indicator reagent (50 mM amino-methylpropanol (pH 9.9), 5 mM L-glutamate, 0.2 mM NAD+, 5 µg/ml malic dehydrogenase, and 2 µg/ml glutamate oxaloacetate transaminase). This reaction was incubated for 10 min at 25 °C. The NADH generated from malate was measured fluorimetrically (excitation at 365 nm, emission monitored at 460 nm) (Cai et al., 2006; Lin et al., 2001). The results obtained are compared to a calibration curve, and normalized to protein content; replicate and duplicate reactions are highly reproducible.
Visual cortex ablation
C57/Bl6, C57/Bl6/Wlds, ZnT3, and MT-III knockout adult male mice (3–6 months of age) were used, and visual cortex ablation performed as described (Agarwala & Kalil, 1998; Land & Aizenman, 2005; Muessel et al., 2000). Briefly, anesthesia was induced by 5% isoflurane (Hospira, Inc., Lake Forest, IL), and maintained with 1.5% isoflurane through a mask. Mice were placed in a stereotax, and using aseptic precautions, a bone flap was made above the visual cortex and a unilateral lesion of visual cortex made by subpial suction (between 0.5 and 3.5 mm anterior to bregma, and 0.5 to 3.5 mm lateral to bregma, and the aspiration syringe inserted ∼2 mm ventral to bregma, just touching the corpus callosum). The lesion was covered with a gelfoam sponge moistened in 0.9% saline, and the skin closed with 6-0 suture. The animals were temperature maintained at 37C for 3 hrs, and observed for 24 hrs post-surgery. Intraperitoneal injections of pyruvate, lactate, or nicotinamide were performed at 500 mg/kg 3×/week starting immediately following the surgery. Where indicated a zinc deficient diet (Harlan Teklad, Indianapolis, IN) supplemented with 1 ppm zinc in the water was given to the mice for 4 weeks prior to and following the ablation (50 ppm is in normal rodent chow plus an indeterminate amount of zinc is present in the tap water normally given to mice). Animals were euthanized at 3 or 7 days post injury by an anesthetic overdose and cervical dislocation; the brains removed, fresh frozen, and processed for Zinpyr-1 (ZP1, TefLabs, Galveston, TX), Nissl, and FluoroJadeB stainings (FJB). These mouse strains were maintained at Washington University’s transgenic animal facility. This was a double blind trial, where the animals were age matched, and efforts to minimize animal usage and suffering were taken. Animal usage was minimized by performing both ZP1 and FJB staining on the same animals. If excessive distress would have been noted, animals would have been given appropriate analgesics. Housing and anesthesia concurred with guidelines established by the institutional Animal Studies Committee, and were in accordance with the PHS Guide for the Care and Use of Laboratory Animals, USDA Regulations, and the AVMA Panel on Euthanasia guidelines.
Histology
Cryostat sections of the fresh frozen brain of 16 um thickness were post-fixed in 4% paraformaldehyde in PBS for 15 min, followed by alkaline dehydration and labeling using FluoroJadeB staining (Chemicon/Millipore, Billerica, MA, 0.0004% for 20 min). Sections were mounted and fluorescence microscopy used to detect staining of dead or dying neurons using FluoroJade B (ex. 460 and em. 530). Photomicrographs of the LGNd were taken of 20 sections separated by 5 sections spanning the entire injury. Quantification of neuronal death was determined after counting of these stained neurons by a rater blinded to the genotype, or treatment condition. The average of the total number of stained neurons in 20 sections is presented ± SEM.
Zinc staining
PNC cultures were loaded with 5 µM TSQ to determine basal zinc loading, or were preloaded with 2.5 µM FluoZin3-AM (Invitrogen/Life Technologies, Carlsbad, CA) for 30 minutes prior to oxidant or therapeutic exposures for the indicated times. FluoZin3 staining was quantitated using Metamorph software (Molecular Devices/Danaher, Washington, DC). Briefly, background fluorescence was determined in regions with no cellular structures, and fluorescence signals of greater than 250% of background from structures > 4 um and < 15 um were quantitated. Data is expressed as percent of the fluorescence of control cultures at 1 hour with an n>300.
For in vivo Zn2+ stainings, fresh frozen cryostat sections were air-dried and immersed in 5 µM zinpyr-1 (ZP1) in PBS for 2 min, and rinsed in normal saline before fluorescent microscopy (ex. 460 nm, em. 530nm). FluoroJadeB staining was performed on adjacent cryostat sections to correlate the changes in Zn2+ staining pattern with neuronal death. Quantification of Zn2+ staining was determined after counting of these stained neurons by a rater blinded to the genotype, or treatment condition. The average of the total number of stained neurons in 20 sections spanning the injury site is presented ± SEM.
Genetically manipulated mice
Experiments described utilized genetically manipulated mice. As is true for all knockout animals, there is the possibility of a compensatory change in expression of another family member, or an alternative pathway. To minimize strain differences we have backcrossed the zinc transporter 3, (ZnT3) and MT-III knockout mice (originally prepared in a hybrid 129/Sv background) into C57/Bl6/J for at least 13 generations. We have used the appropriate wildtype littermates, C57/Bl6/J or hybrid line parental controls for the MT-3 or PARP-1 knockouts, and C57/Bl6/Ola for the Wlds lines. The ZnT3 and MT-III knockout mice were kindly supplied by Dr. Palmiter and were then backcrossed. The PARP-1 knockout and the Wlds mice are from Jackson Laboratories (Bar Harbor, ME) and Harlan Labs (Loughborough, UK) respectively.
Reagents
Unless otherwise stated, all reagents were from Sigma Chemical Co (St. Louis, MO).
Data analysis and statistics
The changes in neuronal death, and [NAD+]i were determined in cultures under the conditions and genotypes stated. The mean ± SEM are plotted and the n is given for each experiment in the figure legends. Results were compared to sham wash or saline injection controls, and toxin or injury exposure alone. One-way ANOVA was used to assess variance in each set of experiments, followed by a Bonferroni test. Significance was achieved by a P value of less than 0.05.
RESULTS
Prior Zn2+ loading of neuronal cultures potentiated SD and oxidant neurotoxicity, and Zn2+ chelation attenuated SD and oxidant neurotoxicity
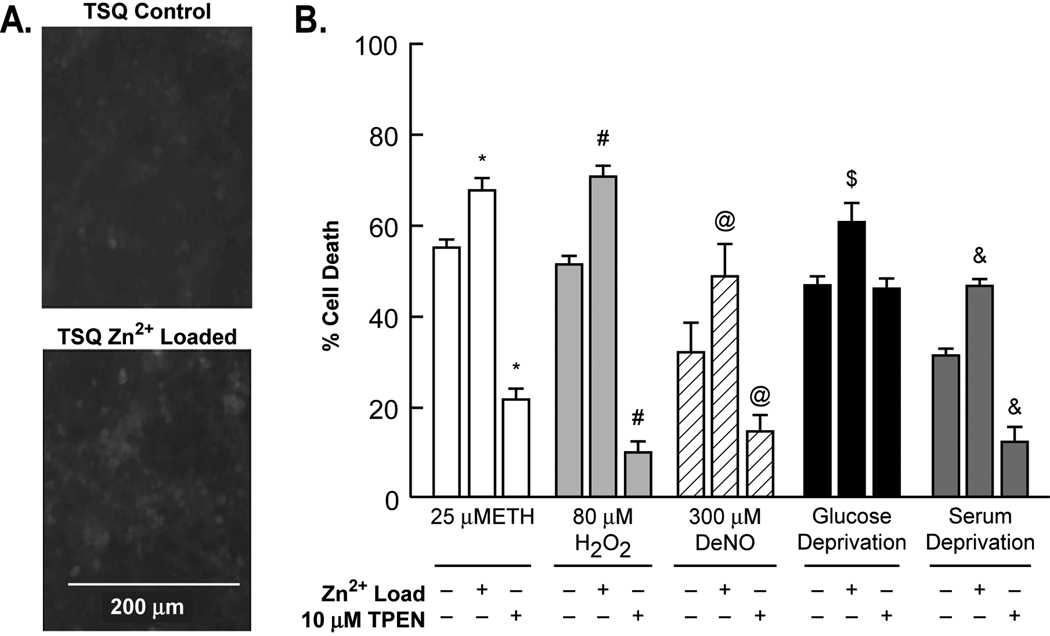
Neuronal cultures derived from embryonic cortex which has not yet been loaded with Zn2+ (typically occurs at postnatal day 14–28) would be expected to have reduced Zn2+. Near-pure cortical neuronal cultures derived from E15 embryos do not stain well with 6-Methoxy-(8-p-toluenesulfonamido)quinoline (TSQ) (DIV 7–9), but cultures grown in the presence of media supplemented with 10 µM Zn2+ in addition to the ∼1–3 µM Zn2+ in the growth medium (MS + 10% FBS) stain better with TSQ, without affecting the number of neurons in the cultures (data not shown). The additional 10 µM Zn2+ makes the Zn2+ concentration in the growth medium approximately equal to that in cerebral spinal fluid or serum of mice or humans (13–20 µM) (Cesur et al., 2005; Davis et al., 1998). These Zn2+ loaded cultures were more susceptible to oxidant-mediated neurotoxicity (ethacrynic acid-ETH, H2O2, serum and glucose deprivation), and the intracellular Zn2+ chelator, TPEN, attenuated these neurotoxicities. In addition, the nitric oxide generator diethyl amine NONOate (DeNO), induced neuronal death that was equally attenuated with TPEN, and was potentiated by Zn2+ loading (Figure 2 and Supplementary Figure 1). H2O2 and serum deprivation were especially responsive to TPEN whereas glucose deprivation was not. Zn2+ loading potentiated NAD+ loss, and Zn2+ chelation attenuated loss (Table 1).
Figure 2. Zn2+ loading potentiated, and Zn2+ chelation attenuated SD, and oxidant mediated neurotoxicity.
A. Near-pure neuronal cultures were cultured for 8 days - or + an additional 10 µM Zn2+ after which the neurons were stained for Zn2+ using TSQ and fluorescence photomicrographs taken. B. Near-pure cortical neuronal cultures were exposed chronically to the indicated conditions. Neuronal death was determined 24–36 hrs later by lactate dehydrogenase release to the medium scaled to the level associated with near complete death produced by exposure to 20 µM A23187 for 24 hrs, = 100 (mean ± SEM, n = 6–18 cultures per condition). * # $ & @ indicate difference from appropriate oxidant exposure in the absence of added Zn2+ at P<0.05
Table 1. NAD+ levels are reduced by SD and oxidant exposures, and are restored by pyruvate, nicotinamide, and TPEN.
Near-pure cortical neuronal cultures were exposed and total cellular NAD+ was isolated at the times indicated which are prior to neuronal death (n >10).
| Condition | NAD+ (nmols/mg) | FBP (nmols/mg) |
|---|---|---|
| Control | 2.9 ± 0.2 | 4.0 ± 0.3 |
| + Zn2+ preload | 2.76 ± 0.2 | N.P. |
| 30 µM Ethacrynic acid (2 hr) | 1.69 ± 0.15 * | 11.3 ± 0.5 * |
| + 10 mM pyruvate | 2.27 ± 0.13 # | 5.1 ± 0.3 # |
| + 10 mM nicotinamide | 2.35 ± 0.13 # | 5.3 ± 0.4 # |
| + Zn2+ preload | 0.29 ± 0.03 # | N.P. |
| 80 µM H2O2 (2 hr) | 0.69 ± 0.03 * | 14.3 ± 0.6 * |
| + 10 mM nicotinamide | 2.64 ± 0.18 # | 6.4 ± 0.8 # |
| + 10 µM TPEN | 1.82 ± 0.07 # | 7.2 ± 0.7 # |
| + Zn2+ preload | 0.29 ± 0.03 # | N.P. |
| Serum deprivation (6 hr) | 2.01 ± 0.12 * | 7.9 ± 0.3 * |
| + 10 mM pyruvate | 2.45 ± 0.04 # | 5.0 ± 0.4 # |
| + 10 mM nicotinamide | 2.66 ± 0.05 # | 5.2 ± 0.5 # |
| + 10 µM TPEN | 2.62 ± 0.2 # | 6.2 ± 0.2 # |
| + Zn2+ preload | 1.39 ± 0.07 # | N.P. |
indicates a significant difference from control.
indicates a significant difference from toxin alone at P < 0.05 by one-way ANOVA followed by a Bonferroni test.
Oxidative injuries induced an increase in intracellular free zinc [Zn2+]i
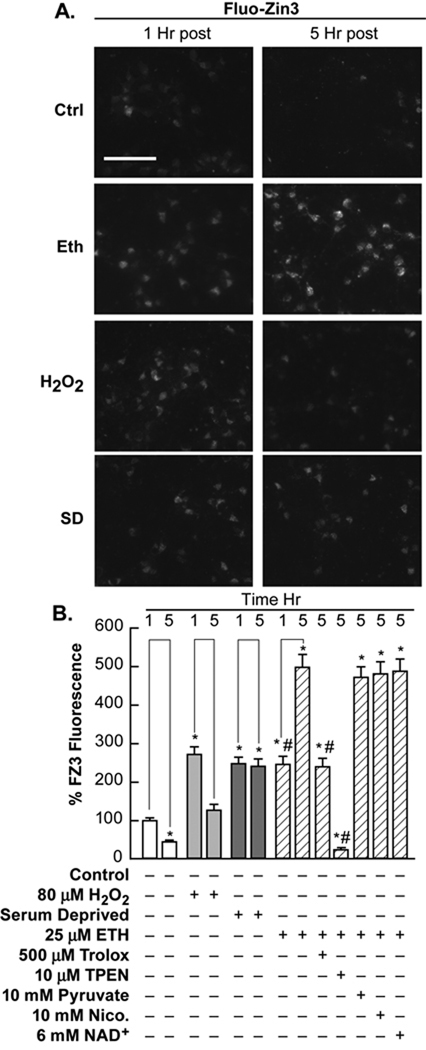
PNC cultures were preloaded with the zinc specific fluorescent dye, FluoZin3-AM at 2.5 µM for 30 min. Excess dye was washed out and cultures were exposed as indicated. All three oxidant exposures caused a significant increase in [Zn2+]i at 1 and 5 hours post exposure. This increase in [Zn2+]i was prevented by addition of the Zn2+ chelator, N,N,N'N'-tetrakis(−)[2-pyridylmethyl]-ethylenediamine (TPEN), and was attenuated by the ROS scavenger, trolox (500 µM), but not by pyruvate, nicotinamide, or NAD+ (Figure 3). These compounds also do not affect 65Zn2+ influx, and where determined, have a very low Kd for Zn2+ (Martell, 1995; Sheline et al., 2004). TPEN also reduced the levels of background and control fluorescence.
Figure 3. [Zn2+]i was increased by SD and oxidant exposures.
Near-pure neuronal cultures were cultured for 7–8 days after which the neurons were loaded for 30 minutes with 2.5 µM FluoZin3-AM (Zn2+ fluorescent indicator). A. These cultures were then washed and exposed to SD and oxidants as indicated and identical exposure fluorescence photo-micrographs were taken at 200x magnification, bar represents 50 microns. B. The fluorescence intensity of the cells under these conditions were quantitated using Metamorph and are presented as the percentage of baseline fluorescence. The effects of therapeutic concentrations of compounds against ethacrynic acid induced increases in [Zn2+]i were also tested as indicated. * indicates difference from 1 hr control exposure at P<0.05; # indicates difference from 5 hr ethacrynic acid exposure at P<0.05
Compounds which restore [NAD+]i attenuated SD and oxidant neurotoxicities, while 3-AP potentiated toxicities
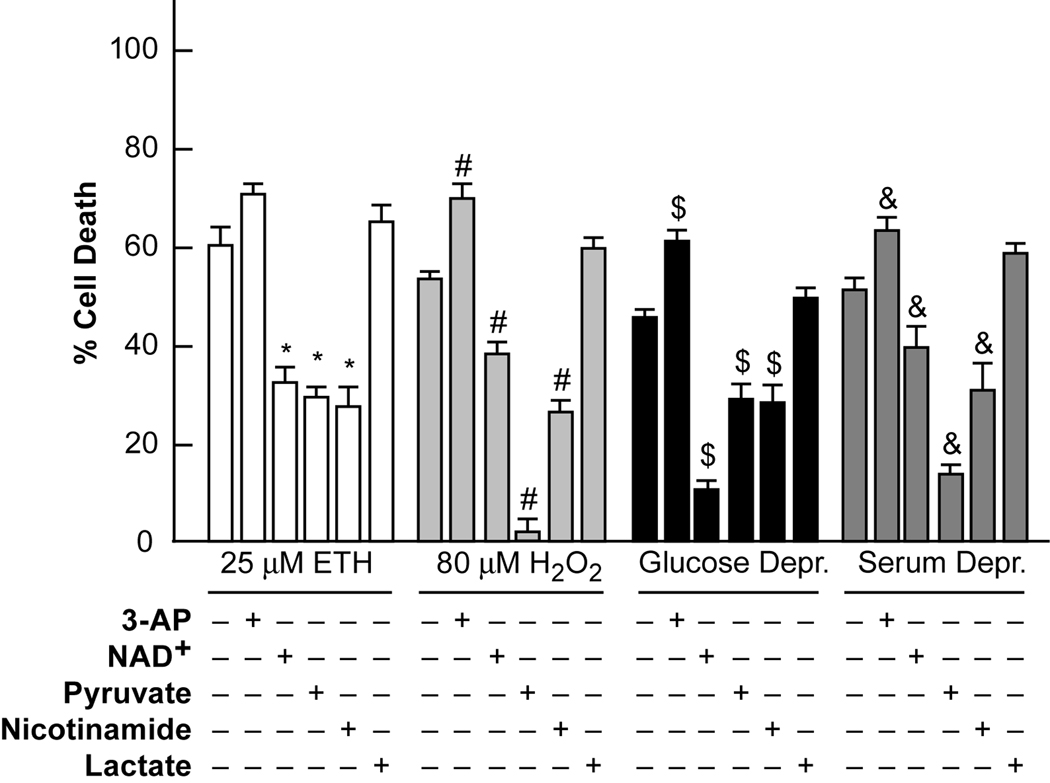
We investigated the effects on ETH, H2O2, serum and glucose deprivation mediated neurotoxicities of decreasing or increasing intracellular NAD+ levels ([NAD+]i) using pretreatment with 3-acetyl pyridine (3-AP, 50 µM) to generate inactive NAD+, or addition of exogenous pyruvate, nicotinamide or NAD+ to increase [NAD+]i. Ethacrynic acid is being used as an inducer of intracellular ROS, as it is thought to both induce intracellular ROS, and reduce levels of glutathione (Rizzardini et al., 2003). H2O2 is being used as an inducer of extracellular ROS. As shown in figure 4, 3-AP potentiated most of these neurotoxicities, and exogenous pyruvate, nicotinamide, and NAD+ (6–10 mM) reduced these neurotoxicities to varying extents. Lactate was ineffective or detrimental. Pyruvate and ZVAD (100 µM) attenuated the basal death of control cultures (normally occurring apoptosis of PNC cultures), whereas the other compounds did not have significant basal effects. The death induced by oxidants was only mildly attenuated by inhibitors of apoptosis or autophagy, whereas glucose and serum deprivation induced death were significantly attenuated (Supplementary Figure 2A–B).
Figure 4. 3-AP potentiated SD and oxidant neurotoxicity, and increased [NAD+]i attenuated them; lactate was ineffective.
Near-pure cortical neuronal cultures were exposed to the indicated conditions. Neuronal death was determined 24 hrs later by propidium iodide staining scaled to the level associated with near complete death produced by exposure to 20 µM A23187 for 24 hrs, = 100 (mean ± SEM, n = 9–18 cultures per condition). 3-AP indicates the addition of 50 µM 3-acetyl pyridine, NAD+ indicates the addition of 6 mM NAD+. Pyruvate, nicotinamide and lactate were present as indicated at 10 mM. * indicates difference from ethacrynic acid (ETH) exposure, # indicates difference from H2O2 exposure, $ indicates difference from glucose deprivation, and & indicates difference from serum deprivation at P < 0.05 by one-way ANOVA followed by a Bonferroni test.
Pyruvate, nicotinamide and NAD+ restored [NAD+]i, and glycolytic flux that was inhibited by oxidant neurotoxicities
Pyruvate, nicotinamide, and NAD+ have been previously shown to restore [NAD+]i and glycolytic flux, and to attenuate both chronic and acute Zn2+ neurotoxicity, while 3-AP potentiated Zn2+ neurotoxicities (Cai et al., 2006; Sheline et al., 2000). A 30–70% loss of NAD+ levels occurred before the onset of SD and oxidant neurotoxicities, and pyruvate, nicotinamide, and TPEN restored [NAD+]i. Furthermore, levels of FBP were elevated by these exposures suggesting glycolytic inhibition, and were restored by pyruvate, nicotinamide or TPEN (Table 1).
Near-pure neuronal cultures from PARP-1 and MT-III knockouts were susceptible to ethacrynic acid mediated neurotoxicity, but H2O2-, and SD-mediated toxicities were reduced; Wlds cultures were somewhat resistant to ETH, and SD
NAD+ levels were reduced prior to oxidant-induced neurotoxicity, and compounds which restored [NAD+]i attenuated toxicity. This led us to study the most prevalent NAD+-catabolic enzyme, PARP-1. PARP-1 induces poly-ADP ribosylation (PAR) of proteins using NAD+ as substrate in response to DNA nicking caused by oxidative stress. Oxidative injuries which target DNA (peroxynitrite, superoxide, and MNNG) have been shown to be very responsive to poly-ADP ribose polymerase (PARP-1) inhibition or knockout (see above). We previously demonstrated by Western blots that exogenous Zn2+ exposure did not induce PAR in neurons, but did induce PAR in glia (Sheline et al., 2003). Wlds animals overexpress the NMNAT-1 protein, and have been suggested to better maintain NAD+ (Bedalov & Simon, 2004). MT-III knockouts have reduced brain Zn2+, and therefore should have less oxidant releasable Zn2+ (Erickson et al., 1997). Near pure neuronal cultures from PARP-1 knockouts (and it’s control 129) and MT-III knockouts or Wlds (and their control B6), were exposed to ethacrynic acid, H2O2, or serum deprivation. Neuronal death was determined to be modestly reduced for Wlds compared to controls for ETH, and was reduced for MT-III and PARP-1 knockouts and controls for H2O2. All 3 mouse lines showed significantly less death after serum deprivation (Figure 5). Larger effects for these genetic variants (Wlds, MT-III KO, and PARP-1 KO) have been reported for in vivo mediated neuronal injuries such as Wallerian degeneration, seizures, and ischemias respectively. The lesser effects seen in vitro could be due to lower levels of expression of the target proteins in cultures from embryonic tissue with a limited number of days in vitro for development.
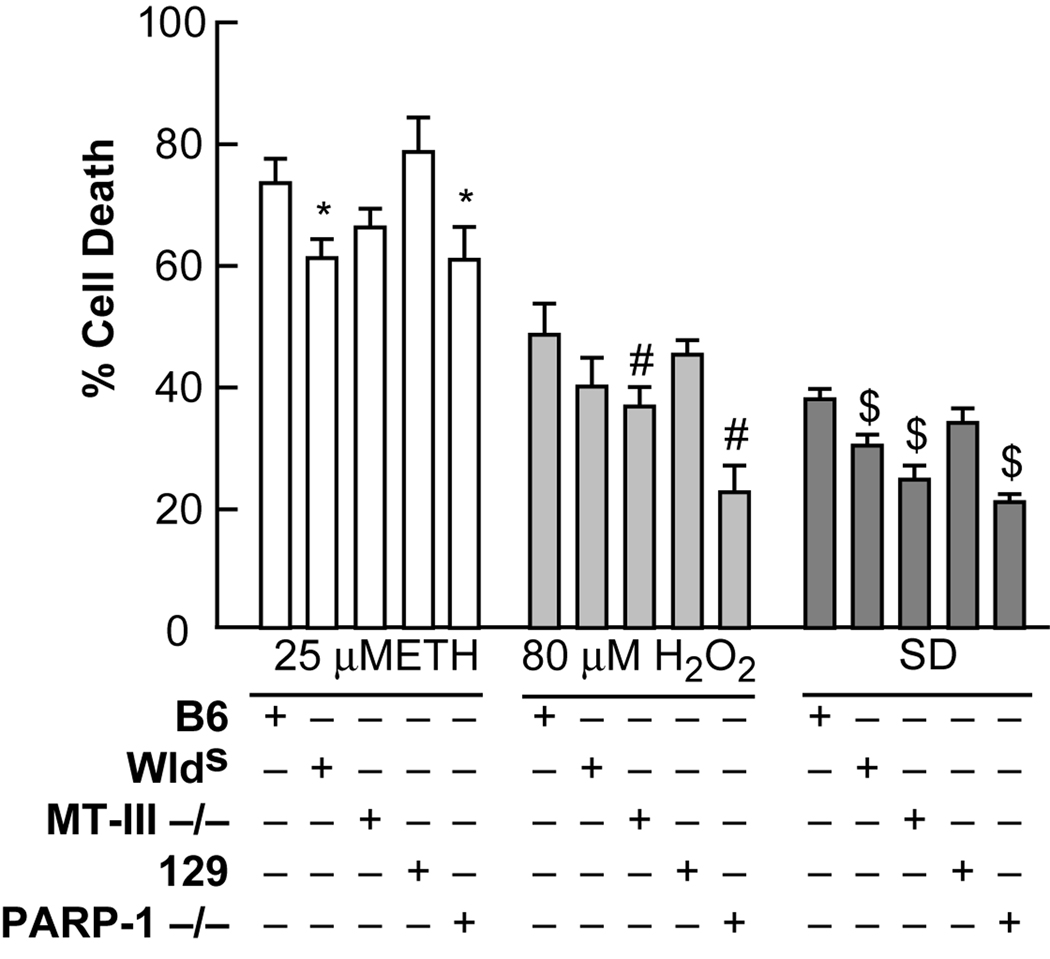
Figure 5. Neuronal cultures from MT-III and PARP-1 knockouts and Wlds were differentially sensitive to SD and oxidant neurotoxicity.
Near-pure cortical neuronal cultures from MT-III and PARP-1 knockouts, Wlds and control cultures were exposed to the indicated conditions. Neuronal death was determined 24 hrs later by lactate dehydrogenase release to the medium. * indicates difference between B6 control cultures and Wlds cultures after ETH exposure at P < 0.05; # indicates difference between control cultures and MT-III or PARP-1 knockouts after H2O2 exposure at P< 0.05. $ indicates difference between control cultures and MT-III or PARP-1 knockouts, or Wlds cultures after serum deprivation at P< 0.05. B6 is the appropriate control for MT-III −/− and Wlds, and 129 is the appropriate control for PARP-1 −/−.
Neuronal death induced by ROS generators, and serum or glucose deprivations were attenuated by sirtuin inhibitors, and potentiated by sirtuin activators
The sirtuin pathway catalyzes the NAD+-dependent deacetylation of proteins resulting in modulation of their function; the substrates for the reaction are acetylated protein (at lysines) and NAD+ with the products being O-acetyl ADP-ribose, nicotinamide, and de-acetylated protein. We utilized chronic administration of the sirtuin-pathway inhibitors sirtinol and its precursor, 2- hydroxynaphthaldehyde at 10 µM to attenuate SD and oxidant neurotoxicity. We also utilized a 3 hr pretreatment with the sirtuin-pathway activators resveratrol and fisetin at 10 µM to potentiate SD and oxidant neurotoxicity (Figure 6). Activators of the sirtuin pathway further reduced NAD+ levels (Table 1). In a manner similar to that seen in exogenous Zn2+ toxicity (Cai et al., 2006), sirtinol did not significantly restore NAD+ levels after ROS or SD neurotoxicities.
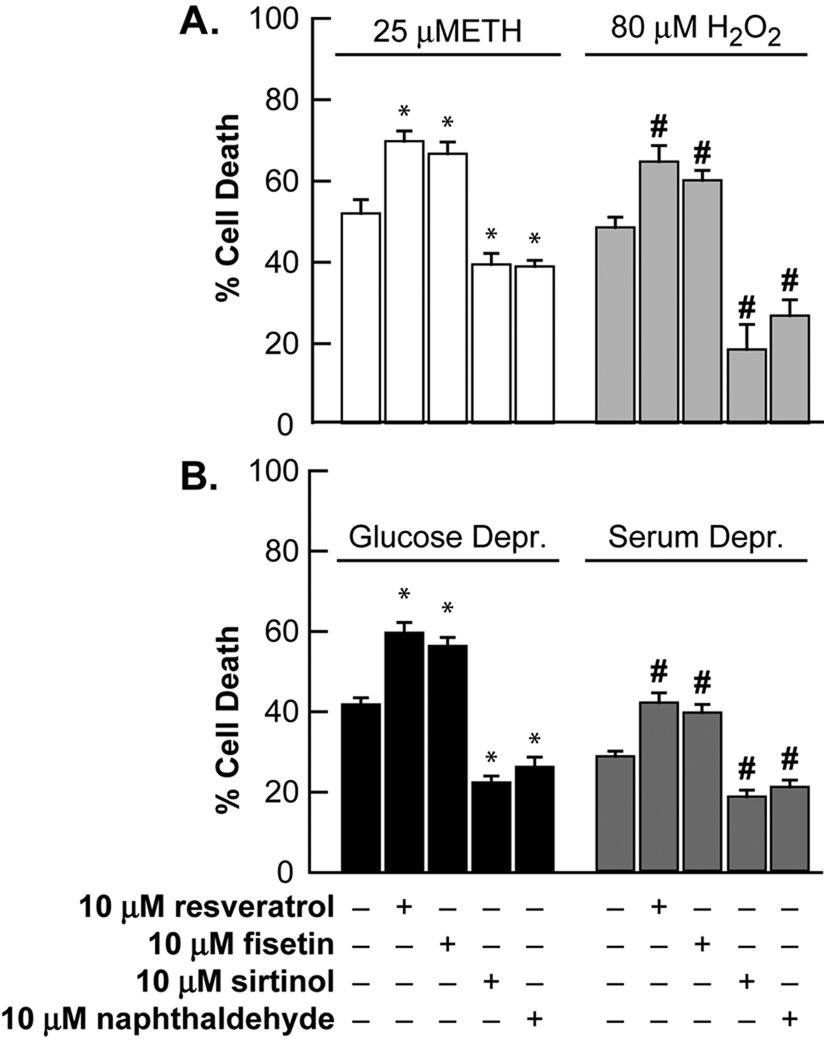
Figure 6. Ethacrynic acid, H2O2, serum or glucose deprivation mediated neuronal injuries were attenuated by sirtinol or 2-hydroxynaphthaldehyde, and these injuries were potentiated by resveratrol or fisetin.
Near-pure neuronal cultures were exposed to A) 25 µM ethacrynic acid (ETH), or 80 µM H2O2 for 24 hrs, or B) to 5 hrs of glucose deprivation, or continuous serum deprivation (36 hrs) and neuronal death was assessed by LDH release to the bathing medium (mean ± SEM, n = 6–18 cultures per condition). Fisetin and resveratrol exposures were a 3 hr pretreatment at 10 µM. Sirtinol and 2-hydroxynaphthaldehyde were present chronically as indicated at 10 µM. * indicates difference from 25 µM ETH; # indicates difference from 80 µM H2O2; $ indicates difference from 5 hours of glucose deprivation exposure; and & indicates difference from serum deprivation exposure at P < 0.05 by one-way ANOVA followed by a Bonferroni test.
Visual cortex ablation induces Zn2+ staining and death in lateral geniculate nucleus neurons; LGNd neuronal death in pyruvate-, nicotinamide-, or reduced zinc diet-treated or Wlds animals was attenuated
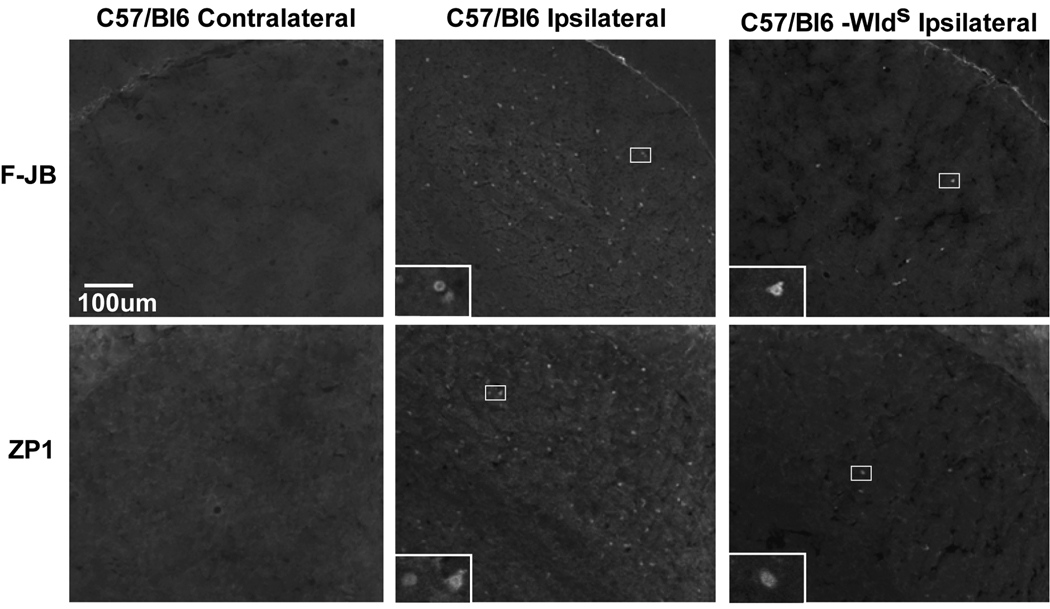
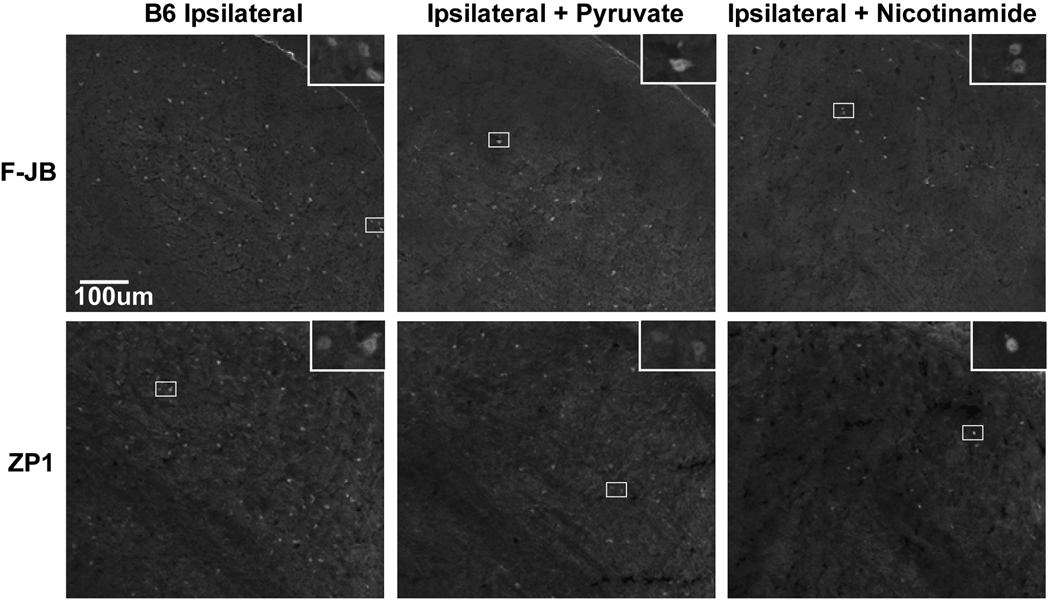
Recently, target deprivation of dorsal lateral geniculate nucleus (LGNd) neurons by ablation of the visual cortex was shown to induce intracellular Zn2+ staining, and LGNd neuronal death. LGNd neurons are normally devoid of synaptic/stainable Zn2+ suggesting intracellular Zn2+ release (Land & Aizenman, 2005). We have now reproduced the results of Land, and examined LGNd Zn2+ staining (ZP1) and death (FluoroJadeB) after cortical ablation in C57/Bl6 controls −/+ intraperitoneal injection of pyruvate or nicotinamide, and in Wlds mice (Figures 7 & 8, and Table 2). Both ZP1 and FluoroJadeB staining of LGNd neurons increased only on the ipsilateral (injured) side 3 or 7 days after the visual cortex ablation in wildtype animals. Pyruvate or nicotinamide (500 mg/kg i.p. 3×/week) attenuated LGN neuronal death while the number of LGNd neurons staining for Zn2+ remained elevated. A similar lactate injection was ineffective. In addition, a reduced zinc diet (1ppm versus 50 ppm) attenuated LGN neuronal death and Zn2+ staining after VCA (Table 2). The number of dead LGNd neurons in Wlds mice was reduced to 15% compared to wildtype mice, and Zn2+ staining was reduced as well. This dramatic attenuation of LGNd death and Zn2+ staining by the Wlds protein perhaps suggests that axonal/synaptic protection may play a role, and that Wlds may attenuate the mechanisms of Zn2+ accumulation. ZnT3, and MT3 knockout mice had a similar susceptibility to VCA compared to controls, whereas LGNd death in PARP1 knockouts was mildly attenuated (Figure 7 and Table 2).
Figure 7. Ipsilateral LGNd neurons die after visual cortex ablation; Wlds mice were resistant.
Unilateral V1 ablation was performed and animals were sacrificed after 7–11 days, and the LGNd was stained for Zn2+ using ZP1, or death using FluoroJadeB. Identical exposure fluorescence photo-micrographs were taken at 100x magnification, bar represents 100 microns. Insets are the boxed area at 400x magnification, and the bar represents 25 microns.
Figure 8. Only ipsilateral LGNd neurons stain for Zn2+ and die after visual cortex ablation; pyruvate, or nicotinamide treated mice were resistant.
Unilateral V1 ablation was performed and animals were sacrificed after 7–11 days, and the LGNd was stained for Zn2+ using ZP1, or death using FluoroJadeB. Identical exposure fluorescence photo-micrographs were taken at 100x magnification, bar represents 100 microns. Insets are the boxed area at 400x magnification, and the bar represents 25 microns.
Table 2. Quantitation of LGNd neuronal death after unilateral visual cortex ablation; Pyruvate and nicotinamide attenuated death, and Wlds mice or mice pre-fed a zinc deficient diet were resistant to injury.
C57/Bl6 with or without pyruvate, or nicotinamide, or lactate (500 mg/kg i.p. 3×/week) or C57/Bl6/Wlds, or ZnT3, MT3, or PARP1 knockout animals (n=4–10) had a unilateral V1 ablation and 7–11 days later the animals were sacrificed. Brains were removed, fresh frozen, sectioned coronally at 16 um through the injury, and every fifth section was ZP1 stained for Zn2+, or post-fixed and stained for FluoroJadeB (cell death). Photomicrographs of the LGNd were taken of 20 sections spanning the entire injury, and stained neurons were counted by a rater blinded to the genotype. The average of the total number of stained neurons in 20 sections is presented ± SEM. Genotype or treatment condition is as stated.
| Animal | # Neurons Staining for Zn2+ (Injured Side) |
# Neurons Staining for Zn2+ (Control Side) |
# Dead Neurons (Injured Side) |
# Dead Neurons (Control Side) |
|---|---|---|---|---|
| C57/Bl6 | 550 ± 47 | 2 ± 2 | 2512 ± 186 | 2 ± 2 |
|
C57/Bl6 + Pyruvate |
450 ± 29 | 1 ± 1 | 1871 ± 136 * | 1 ± 1 |
|
C57/Bl6 + Lactate |
520 ± 37 | NP | 2399 ± 108 | NP |
|
C57/Bl6 + Nicotinamide |
480 ± 32 | NP | 1819 ± 285 * | NP |
|
C57/Bl6 + 1ppm Zn2+ Diet |
300 ± 19 * | NP | 1845 ± 142 * | NP |
| C57/Bl6/Ola | 420 ± 31 | 2 ± 2 | 1957 ± 117 | 4 ± 2 |
| C57/Bl6/Ola/Wlds | 285 ± 27 * | NP | 480 ± 127 * | NP |
| ZnT3 −/− | 461 ± 35 | NP | 2118 ± 270 | NP |
| MT3 −/− | 492 ± 32 | NP | 2646 ± 412 | NP |
| PARP1 −/− | 520 ± 39 | NP | 2032 ± 179 * | NP |
indicates difference between B6 control and treatment or genotype conditions at P < 0.05. ZnD diet denotes that animals were given 1 ppm total zinc in the diet (rather than 50 ppm) for 3 weeks before the surgery, and maintained on this diet after the injury. NP means not performed.
DISCUSSION
In these studies we show that our neuronal cultures normally have reduced Zn2+, and Zn2+ proficiency increased sensitivity to SD and oxidative injuries, and a Zn2+ chelator attenuated these injuries (Figure 2, Supplementary Figure 1). SD and oxidative injuries induced an increase in [Zn2+]i (Figure 3). SD and oxidative injuries induced a loss of NAD+ resulting in an increase in FBP; restoration of NAD+ using exogenous NAD+, pyruvate, or nicotinamide attenuated the increase in FBP and these injuries, and potentiation of NAD+ loss potentiated injury (Figure 4, Table 1, Supplementary Figure 2). Genetically modified or inbred mouse strains which reduce intracellular Zn2+ content (MT-III knockout), reduce NAD+ catabolism (PARP-1 knockout), or increase NAD+ synthesis (Wlds) attenuated SD and oxidative injuries to different extents (Figure 5). Inhibitors of the sirtuin pathway attenuated SD and oxidant neurotoxicity, and activators potentiated these injuries (Figure 6). Ipsilateral LGNd neuronal death after visual cortex ablation was particularly reduced in Wlds mice, and substantially reduced in pyruvate, nicotinamide, or reduced Zn2+ diet treated mice, but lactate was ineffective (Figures 7, 8, and Table 2).
Injury Models and Intracellular Zn2+
Some in vivo neuronal injuries may involve ROS-mediated intracellular Zn2+ release as opposed to synaptic Zn2+ release. These neuronal injuries may include kainate-induced seizure, target deprivation, and MCAO (Land & Aizenman, 2005; Lee et al., 2002; Lee et al., 2003). Kainate-induced seizure mediated CA1 and thalamic neuronal Zn2+ staining and death were not responsive to knockout of ZnT3 (no synaptic Zn2+), but were responsive to knockout of MT-III. This suggests that intracellular Zn2+ stores (especially MT-III) are more important than synaptic stores for seizure mediated CA1 and thalamic neuronal death. This result is in contrast to the equivalent sensitivity of MT-III knockout versus wildtype thalamic neurons to VCA reported here. One possible explanation is that MT3 is also an antioxidant, and its antioxidant function is more dominant in VCA in vivo than in seizure mediated injury or in vitro. It was suggested that Zn2+ was released from intracellular stores such as the mitochondrion or MT-III (Aravindakumar et al., 1999; Pal et al., 2004). Overexpression of metallothionein in glial cells provided for both an increased buffering capacity for [Zn2+]i but also an increased pool of intracellular Zn2+ able to be released by oxidant exposure (Malaiyandi et al., 2004). Other pools of Zn2+ are present inside neurons including ribosomal, lysosomal, mitochondrial, and nuclear Zn2+, and Zn2+ bound to proteins other than MT-III.
LGNd neurons lack synaptic Zn2+ suggesting that the increased [Zn2+]i after VCA results from intracellular release (Mengual et al., 2001). The Zn2+ staining after VCA started at 2–3 days, preceding caspase-3 activation at 4–5 days and neuronal death at 5–7 days (Land & Aizenman, 2005). In contrast, other injuries, including global and retinal ischemias, and hypoglycemia, appear to result from synaptic release and reuptake of Zn2+ (Koh et al., 1996; Suh et al., 2005; Suh et al., 2007b; Suh et al., 2008; Yoo et al., 2004). Zn2+ neurotoxicity has also been implicated in neuronal oxidant-mediated injuries in vitro using a thiol oxidizing agent, peroxynitrite, or chemical glutathione depletion (Aizenman et al., 2000; Ho et al., 2008; Zhang et al., 2004). Alternate Zn2+ neurotoxicity mechanisms involving ROS, mitochondria, and activation of kinase cascades have also been proposed (Kim et al., 1999; Kim & Koh, 2002; Noh et al., 1999; Redman et al., 2009; Sensi et al., 2003; Sensi et al., 2000; Zhang et al., 2007).
NAD+ Loss and Zn2+ neurotoxicity
Similar to what we show in this manuscript on intracellular Zn2+ release, we have previously demonstrated that nicotinamide adenine dinucleotide (NAD+) levels are reduced upon Zn2+ exposures, and NAD+ restoration attenuated exogenous Zn2+ neurotoxicity, whereas [NAD+]i reduction potentiated Zn2+ neurotoxicity . The Zn2+-induced loss of NAD+ levels resulted in a block of glycolysis at GAPDH (increased levels of FBP/DHAP) in both neurons and glia, and under both chronic and acute Zn2+ exposure conditions; NAD+ restoration unblocked GAPDH (Cai et al., 2006; Sheline et al., 2000). In addition, NAD+ levels were shown to be reduced, and [Zn2+]i increased in the rat permanent global ischemia model, after hypoglycemia, and other oxidative injuries (Alano et al., 2004; Plaschke et al., 2000; Suh et al., 2003; Virag et al., 2003; Ying et al., 2001).
We measure NAD+ levels in total cellular lysates, and NAD+ is small enough to permeate through the nuclear pores. Nuclear [NAD+] has been reported to be 70 µM suggesting this to be the cytoplasmic concentration as well (Fjeld et al., 2003). Since exogenous NAD+ was neuroprotective and neurons can take up NAD+, but mitochondria are not thought to take up NAD+, then restoration of cytoplasmic/nuclear NAD+ concentrations may be sufficient to attenuate Zn2+, oxidative, and serum or target deprivation neurotoxicities (this manuscript, and Cai et al., 2006). Also, the efficacy of NAD+ and nicotinamide against oxidative neurotoxicities were similar (Figure 3), but only nicotinamide is a catabolism inhibitor. This suggests that [NAD+]i levels may be the critical determinant rather than inhibition of catabolism.
We previously examined the effects of Zn2+ on the predominant catabolizer of NAD+, PARP, by measuring levels of poly-ADP ribosylation (PAR). In cortical cultures, only glia had significant PAR formation which was only induced by an acute 400 µM Zn2+ exposure, not a chronic 40 µM Zn2+ exposure. Cortical neuronal cultures (neurons and glia) from PARP-1 −/− mice were found to be partially resistant to an acute 400 µM Zn2+ exposure, but appeared equally sensitive to a chronic 40 µM Zn2+ exposure (Sheline et al., 2003). PARP knockout or inhibition has been shown to attenuate oxidative injuries that induce DNA strand breaks (peroxynitrite, MNNG, and superoxide/hypoglycemia) (Alano et al., 2004; Suh et al., 2003; Virag et al., 2003; Ying et al., 2001). To the extent that PARP knockout spares [NAD+]i, then it would be expected to attenuate Zn2+ neurotoxicity, and does mildly attenuate VCA (Table 2, and Araki et al., 2004). In addition, Zn2+ induction of PARP-1 in astrocytes causes glycolytic inhibition and inhibition of glutamate uptake both of which would be detrimental to their role of supporting neurons (Sheline et al., 2004; Suh et al., 2007a). We recently demonstrated that neurons from slow Wallerian degeneration mice (Wlds) were resistant to exogenous Zn2+ neurotoxicity (Cai et al., 2006).
Wlds mice contain a spontaneous autosomal dominant recombination triplication mutation resulting in the overexpression of NMNAT-1 an important enzyme in the synthesis of NAD+. The CA1, CA2 and caudate nucleus of Wlds mice showed substantially less death after transient global ischemia and reperfusion, or oligodendrocyte death after spinal cord injury (Dong et al., 2003; Gillingwater et al., 2004). It has now been shown that the NMNAT-1 portion of the fusion protein is requisite for axonal protection (Araki et al., 2004), that NMNAT catalytic activity is required, and cytoplasmic overexpression of NMNAT1 is sufficient for Wlds mediated axonal- and neuro- protection (Sasaki et al., 2009a; Sasaki et al., 2009b). The therapeutic effects in cultures derived from Wlds embryonic tissue was less than that demonstrated for pharmacologic neuroprotectants, and much less than that demonstrated in adult Wlds mice subjected to VCA (Figure 4, Table 2). This could be because the effects of the Wlds phenotype require protein expression, or loss of protein expression which may not occur in embryonically derived neuronal cultures with a limited number of days in culture (Gillingwater et al., 2006). The large reduction in LGNd neuronal death after VCA in Wlds mice, compared to the efficacy of pyruvate or nicotinamide, suggests that in addition to effects on NAD+ levels, overexpression of NMNAT1 is especially beneficial in vivo perhaps through additional effects on axonal/synaptic integrity or Zn2+ accumulation. We recently demonstrated that exogenous Zn2+ neurotoxicity could also be attenuated by inhibition of another NAD+ catabolic pathway, the sirtuin pathway (Cai et al., 2006).
The sirtuin family of proteins are NAD+-dependent protein deacetylases which catalyze the removal of acetyl groups from lysine amino acids of histones and transcription factors resulting in their modulation. They have been implicated in genomic stability, transcriptional silencing, transcriptional regulation, mitochondrial regulation, and lifespan extension through effects on p53, forkhead transcription factors and histones (for review see Blander & Guarente, 2004). The inhibitors sirtinol and 2-hydroxy napthaldehyde, have been reported to be specific for sirtuin proteins and to not affect histone deacetylase or PARP activities (Grozinger et al., 2001). Previous neuroprotective effects of sirtuins were mediated through the deacetylation of target proteins, especially transcription factors (Blander & Guarente, 2004; Brunet et al., 2004; Lantz & Kaestner, 2005). These results are in contrast to our results where activation of the sirtuin pathway was detrimental. In Zn2+, oxidant, or serum deprivation neurotoxicities, NAD+, pyruvate or nicotinamide were not therapeutic if only a pretreatment was performed (data not shown, and Cai et al., 2006). Pretreatment with NAD+ and SIRT-1 activation were required for the beneficial effects on Wallerian degeneration, which occurred through effects on the transcription/translation of target genes (Araki et al., 2004). This suggests that the mechanism involved in the negative effects of sirtuins may be NAD+ loss. However, NAD+ levels were not significantly restored by sirtuin inhibition, suggesting sirtuins may have another role, or that sirtinol inhibits deacetylation but not NAD+ catabolism.
Pyruvate or nicotinamide also reduce death after many in vivo injuries including diabetes, global, focal, and retinal ischemias, and hypoglycemia (Chang et al., 2003; Hassan & Janjua, 2001; Lee et al., 2001; Suh et al., 2003; Suh et al., 2005; Visalli et al., 1986; Yamada et al., 1982; Yoo et al., 2004; Cai et al., 2006). These injuries all involve Zn2+ toxicity, and these compounds would be expected to ameliorate Zn2+ toxicity by restoring NAD+ levels. There have been fewer reports of in vivo therapeutic efficacy of inhibitors of alternate Zn2+ neurotoxicity mechanisms such as kinase cascades, autophagy, or mitochondrial dysfunction. Pyruvate is converted to lactate by lactate dehydrogenase regenerating NAD+ at the expense of NADH. Lactate is ineffective even harmful against exogenous Zn2+, serum or glucose deprivation, or oxidant mediated neurotoxicities in vitro, or against global or focal ischemias, target deprivation, or hypoglycemia mediated neuronal death in vivo (Table 2, data not shown, and Cai et al., 2006; Koh et al., 1996; Sheline et al., 2000; Suh et al., 2005). This shows that NAD+ restoration is required for efficacy, not availability of energy substrates. Beyond NAD+ restoration, pyruvate can hyperpolarize and activate mitochondria, and is a crucial, permeable, endogenous α-keto acid which can inactivate H2O2 thereby acting as a sink for oxygen radicals (Holleman, 1904; Kauppinen & Nicholls, 1986). In addition to those injuries cited above, pyruvate has also been shown to be cytoprotective against: heart transplant (Kojima et al., 1993), quinolinic acid injection (Ryu et al., 2003), NMDA (Maus et al., 1999), H2O2 (Desagher et al., 1997), Zn2+, ROS, SD, GD, and VCA (this manuscript and Sheline et al., 2000).
Nicotinamide can induce increased synthesis of NAD+, or decrease its degradation by NAD+-catabolizing enzymes. All NAD+-catabolizing reactions that have nicotinamide as a leaving group would be inhibited by nicotinamide. Nicotinamide has also been shown to induce an increase in basal levels of NAD+, and to restore NAD+ and mitochondrial function after injury (Chong et al., 2004; Klaidman et al., 2003). It has been suggested to prevent PARP-activation and NAD+ depletion, thereby reducing apoptosis of neurons induced by DNA damage (reviewed in Szabo & Dawson, 1998). Other “PARP inhibitors” were shown to be effective against global and focal ischemia (Endres et al., 1997; Plaschke et al., 2000). These “PARP inhibitors” are designed to look like nicotinamide, and therefore could be inhibiting any enzyme whose reaction involves nicotinamide as a leaving group including sirtuins.
These experiments have demonstrated that Zn2+, NAD+, and the sirtuin pathway are involved in target deprivation and oxidant-induced neurotoxicity. We believe the specific enzymes involved in the NAD+ loss and neurotoxicity of exogenous Zn2+ and Zn2+ released intracellularly may be the same. Furthermore, these studies have validated and suggested mechanisms of action for pyruvate and nicotinamide as therapeutic compounds against target deprivation, hopefully spurring interest in clinical trials. Finally, since target deprivation occurs in many injury and neurodegenerative models, these compounds and pathways should be tested in these other models.
Supplementary Material
Near-pure neuronal cultures were cultured for 8 days - or + an additional 10 µM Zn2+ after which the neurons were exposed chronically to the indicated conditions. Neuronal death was determined 24 hrs later by lactate dehydrogenase release to the medium scaled to the level associated with near complete death produced by exposure to 20 µM A23187 for 24 hrs, = 100 (mean ± SEM, n = 8–18 cultures per condition). * indicates difference from control, # indicates difference from 25 µM ethacrynic acid at P<0.05
A. Near-pure neuronal cultures were exposed as indicated for 24 hrs, and neuronal death was assessed by lactate dehydrogenase release to the medium. The basal effects of these therapeutics were subtracted from their effects on oxidant injuries (Figures 2, 4, 5). * indicates difference from control cultures at P<0.05 B. Neuronal death was determined 24-36 hrs later by lactate dehydrogenase release to the medium after exposure as indicated. Death was scaled to the level associated with near complete death produced by exposure to 20 µM A23187 for 24 hrs, = 100 (mean ± SEM, n = 6–12 cultures per condition). * # $ indicate difference from appropriate oxidant exposure at P<0.05
Acknowledgments
This work was supported by NIH grants NS 030337, and DK 073446 (CTS). Abstracts have appeared (Sheline et al., 2006; Sheline et al., 2008)
Abbreviations
- SD
serum deprivation
- LGNd
dorsal lateral geniculate nucleus
- [Zn2+]i
intracellular Zn2+ levels
- [NAD+]i
intracellular NAD+ levels
- VCA
visual cortex ablation
- MT-III
metallothionein-3
- PCD
programmed cell death
- ROS
Reactive oxygen species
- BDNF
brain derived neurotrophic factor
- NAD+
Nicotinamide adenine dinucleotide
- TSQ
6-Methoxy-(8-p-toluenesulfonamido)quinoline
- ZP1
zinpyr-1
- ZnT3
zinc transporter 3
- ETH
ethacrynic acid
- DeNO
diethyl amine NONOate
- Wlds
Slow Wallerian degeneration mouse
- PAR
poly-ADP ribosylation
- PARP-1
poly-ADP ribose polymerase
- TPEN
N,N,N'N'-tetrakis(−)[2-pyridylmethyl]-ethylenediamine
- 3-AP
3-acetyl pyridine
- NMNAT
Nicotinamide mononucleotide adenyl transferase
References
- Agarwala S, Kalil RE. Axotomy-induced neuronal death and reactive astrogliosis in the lateral geniculate nucleus following a lesion of the visual cortex in the rat. J Comp Neurol. 1998;392:252–263. doi: 10.1002/(sici)1096-9861(19980309)392:2<252::aid-cne7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- Al-Abdulla NA, Portera-Cailliau C, Martin LJ. Occipital cortex ablation in adult rat causes retrograde neuronal death in the lateral geniculate nucleus that resembles apoptosis. Neuroscience. 1998;86:191–209. doi: 10.1016/s0306-4522(98)00014-1. [DOI] [PubMed] [Google Scholar]
- Alano CC, Ying W, Swanson RA. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J Biol Chem. 2004;279:18895–18902. doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Aravindakumar CT, Ceulemans J, De Ley M. Nitric oxide induces Zn2+ release from metallothionein by destroying zinc-sulphur clusters without concomitant formation of S-nitrosothiol. Biochem J. 1999;344(Pt 1):253–258. doi: 10.1042/0264-6021:3440253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedalov A, Simon JA. Neuroscience. NAD to the rescue. Science. 2004;305:954–955. doi: 10.1126/science.1102497. [DOI] [PubMed] [Google Scholar]
- Berardi N, Maffei L. From visual experience to visual function: roles of neurotrophins. J Neurobiol. 1999;41:119–126. [PubMed] [Google Scholar]
- Blander G, Guarente L. The sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Cai AL, Zipfel GJ, Sheline CT. Zinc neurotoxicity is dependent on intracellular NAD levels and the sirtuin pathway. Eur J Neurosci. 2006;24:2169–2176. doi: 10.1111/j.1460-9568.2006.05110.x. [DOI] [PubMed] [Google Scholar]
- Caleo M, Medini P, von Bartheld CS, Maffei L. Provision of brain-derived neurotrophic factor via anterograde transport from the eye preserves the physiological responses of axotomized geniculate neurons. J Neurosci. 2003;23:287–296. doi: 10.1523/JNEUROSCI.23-01-00287.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesur S, Kocaturk PA, Kavas GO, Aksaray S, Tezeren D, Ciftci U. Serum copper and zinc concentrations in patients with brucellosis. Journal of Infection. 2005;50:31–33. doi: 10.1016/j.jinf.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Chang I, Cho N, Koh JY, Lee MS. Pyruvate inhibits zinc-mediated pancreatic islet cell death and diabetes. Diabetologia. 2003;46:1220–1227. doi: 10.1007/s00125-003-1171-z. [DOI] [PubMed] [Google Scholar]
- Chang LK, Putcha GV, Deshmukh M, Johnson JEM. Mitochondrial involvement in the point of no return in neuronal apoptosis. Biochimie. 2002;84:223–231. doi: 10.1016/s0300-9084(02)01372-x. [DOI] [PubMed] [Google Scholar]
- Cho E, Hwang JJ, Han SH, Chung SJ, Koh JY, Lee JY. Endogenous zinc mediates apoptotic programmed cell death in the developing brain. Neurotox Res. 17:156–166. doi: 10.1007/s12640-009-9085-2. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004;24:728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- Davis SR, McMahon RJ, Cousins RJ. Metallothionein Knockout and Transgenic Mice Exhibit Altered Intestinal Processing of Zinc with Uniform Zinc-Dependent Zinc Transporter-1 Expression. J. Nutr. 1998;128:825–831. doi: 10.1093/jn/128.5.825. [DOI] [PubMed] [Google Scholar]
- Desagher S, Glowinski J, Premont J. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J Neurosci. 1997;17:9060–9067. doi: 10.1523/JNEUROSCI.17-23-09060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh M, Johnson EM., Jr Programmed cell death in neurons: focus on the pathway of nerve growth factor deprivation-induced death of sympathetic neurons. Mol Pharmacol. 1997;51:897–906. doi: 10.1124/mol.51.6.897. [DOI] [PubMed] [Google Scholar]
- Dong H, Fazzaro A, Xiang C, Korsmeyer SJ, Jacquin MF, McDonald JW. Enhanced oligodendrocyte survival after spinal cord injury in Bax-deficient mice and mice with delayed Wallerian degeneration. J Neurosci. 2003;23:8682–8691. doi: 10.1523/JNEUROSCI.23-25-08682.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RE, Yuan JY, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Endres M, Wang ZQ, Namura S, Waeber C, Moskowitz MA. Ischemic brain injury is mediated by the activation of poly(ADP- ribose)polymerase. J Cereb Blood Flow Metab. 1997;17:1143–1151. doi: 10.1097/00004647-199711000-00002. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Hollopeter G, Thomas SA, Froelick GJ, Palmiter RD. Disruption of the metallothionein-III gene in mice: analysis of brain zinc, behavior, and neuron vulnerability to metals, aging, and seizures. J Neurosci. 1997;17:1271–1281. doi: 10.1523/JNEUROSCI.17-04-01271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjeld CC, Birdsong WT, Goodman RH. Differential binding of NAD+ and NADH allows the transcriptional corepressor carboxyl-terminal binding protein to serve as a metabolic sensor. Proc Natl Acad Sci U S A. 2003;100:9202–9207. doi: 10.1073/pnas.1633591100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingwater TH, Haley JE, Ribchester RR, Horsburgh K. Neuroprotection after transient global cerebral ischemia in Wld(s) mutant mice. J Cereb Blood Flow Metab. 2004;24:62–66. doi: 10.1097/01.WCB.0000095798.98378.34. [DOI] [PubMed] [Google Scholar]
- Gillingwater TH, Wishart TM, Chen PE, Haley JE, Robertson K, Macdonald SH, Middleton S, Wawrowski K, Shipston MJ, Melmed S, Wyllie DJ, Skehel PA, Coleman MP, Ribchester RR. The Neuroprotective Wlds Gene Regulates Expression of PTTG1 and Erythroid Differentiation Regulator 1-Like Gene in Mice and Human Cells. Hum Mol Genet. 2006 doi: 10.1093/hmg/ddi478. [DOI] [PubMed] [Google Scholar]
- Gottron FJ, Ying HS, Choi DW. Caspase inhibition selectively reduces the apoptotic component of oxygen-glucose deprivation-induced cortical neuronal cell death. Mol Cell Neurosci. 1997;9:159–169. doi: 10.1006/mcne.1997.0618. [DOI] [PubMed] [Google Scholar]
- Grozinger CM, Chao ED, Blackwell HE, Moazed D, Schreiber SL. Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J Biol Chem. 2001;276:38837–38843. doi: 10.1074/jbc.M106779200. [DOI] [PubMed] [Google Scholar]
- Hassan N, Janjua MZ. The optimum dose of nicotinamide for protection of pancreatic beta- cells against the cytotoxic effect of streptozotocin in albino rat. J Ayub Med Coll Abbottabad. 2001;13:26–30. [PubMed] [Google Scholar]
- Ho Y, Samarasinghe R, Knoch ME, Lewis M, Aizenman E, DeFranco DB. Selective inhibition of mitogen-activated protein kinase phosphatases by zinc accounts for extracellular signal-regulated kinase 1/2-dependent oxidative neuronal cell death. Mol Pharmacol. 2008;74:1141–1151. doi: 10.1124/mol.108.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleman MAF. Notice sur l'action de l'eau oxygénée sur les acides a-cétoniques et sur les dicétones 1.2. Recl. Trav. Chim. Pays Bas. Belg. 1904;23:169–171. [Google Scholar]
- Hwang JJ, Lee SJ, Kim TY, Cho JH, Koh JY. Zinc and 4-hydroxy-2-nonenal mediate lysosomal membrane permeabilization induced by H2O2 in cultured hippocampal neurons. J Neurosci. 2008;28:3114–3122. doi: 10.1523/JNEUROSCI.0199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen RA, Nicholls DG. Synaptosomal bioenergetics. The role of glycolysis, pyruvate oxidation and responses to hypoglycaemia. Eur J Biochem. 1986;158:159–165. doi: 10.1111/j.1432-1033.1986.tb09733.x. [DOI] [PubMed] [Google Scholar]
- Kim YH, Kim EY, Gwag BJ, Sohn S, Koh JY. Zinc-induced cortical neuronal death with features of apoptosis and necrosis: mediation by free radicals. Neuroscience. 1999;89:175–182. doi: 10.1016/s0306-4522(98)00313-3. [DOI] [PubMed] [Google Scholar]
- Kim YH, Koh JY. The role of NADPH oxidase and neuronal nitric oxide synthase in zinc- induced poly(ADP-ribose) polymerase activation and cell death in cortical culture. Exp Neurol. 2002;177:407–418. doi: 10.1006/exnr.2002.7990. [DOI] [PubMed] [Google Scholar]
- Klaidman L, Morales M, Kem S, Yang J, Chang ML, Adams JD., Jr Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology. 2003;69:150–157. doi: 10.1159/000072668. [DOI] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Kojima S, Wu ST, Wikman-Coffelt J, Parmley WW. Eighteen-hour preservation of rat hearts with hexanol and pyruvate cardioplegia. J Am Coll Cardiol. 1993;21:1238–1244. doi: 10.1016/0735-1097(93)90252-v. [DOI] [PubMed] [Google Scholar]
- Land PW, Aizenman E. Zinc accumulation after target loss: an early event in retrograde degeneration of thalamic neurons. Eur J Neurosci. 2005;21:647–657. doi: 10.1111/j.1460-9568.2005.03903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz KA, Kaestner KH. Winged-helix transcription factors and pancreatic development. Clin Sci (Lond) 2005 doi: 10.1042/CS20040309. [DOI] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Park KH, He YY, Hsu CY, Choi DW. Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience. 2002;115:871–878. doi: 10.1016/s0306-4522(02)00513-4. [DOI] [PubMed] [Google Scholar]
- Lee JY, Hwang JJ, Park MH, Koh JY. Cytosolic labile zinc: a marker for apoptosis in the developing rat brain. Eur J Neurosci. 2006;23:435–442. doi: 10.1111/j.1460-9568.2005.04553.x. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kim JH, Palmiter RD, Koh JY. Zinc released from metallothionein-iii may contribute to hippocampal CA1 and thalamic neuronal death following acute brain injury. Exp Neurol. 2003;184:337–347. doi: 10.1016/s0014-4886(03)00382-0. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kim YH, Koh JY. Protection by pyruvate against transient forebrain ischemia in rats. J Neurosci. 2001;21:RC171. doi: 10.1523/JNEUROSCI.21-20-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SS, Manchester JK, Gordon JI. Enhanced gluconeogenesis and increased energy storage as hallmarks of aging in Saccharomyces cerevisiae. J Biol Chem. 2001;276:36000–36007. doi: 10.1074/jbc.M103509200. [DOI] [PubMed] [Google Scholar]
- Malaiyandi LM, Dineley KE, Reynolds IJ. Divergent consequences arise from metallothionein overexpression in astrocytes: zinc buffering and oxidant-induced zinc release. Glia. 2004;45:346–353. doi: 10.1002/glia.10332. [DOI] [PubMed] [Google Scholar]
- Martell AM, Smith RM. Critically selected stability constants of metal complexes. Gaithersburg, MD: N.I.S.T; 1995. [Google Scholar]
- Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res Bull. 1998;46:281–309. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Price AC, McClendon KB, Al-Abdulla NA, Subramaniam JR, Wong PC, Liu Z. Early events of target deprivation/axotomy-induced neuronal apoptosis in vivo: oxidative stress, DNA damage, p53 phosphorylation and subcellular redistribution of death proteins. J Neurochem. 2003;85:234–247. doi: 10.1046/j.1471-4159.2003.01659.x. [DOI] [PubMed] [Google Scholar]
- Maus M, Marin P, Israel M, Glowinski J, Premont J. Pyruvate and lactate protect striatal neurons against N-methyl-D-aspartate-induced neurotoxicity. Eur J Neurosci. 1999;11:3215–3224. doi: 10.1046/j.1460-9568.1999.00745.x. [DOI] [PubMed] [Google Scholar]
- Mengual E, Casanovas-Aguilar C, Perez-Clausell J, Gimenez-Amaya JM. Thalamic distribution of zinc-rich terminal fields and neurons of origin in the rat. Neuroscience. 2001;102:863–884. doi: 10.1016/s0306-4522(00)00472-3. [DOI] [PubMed] [Google Scholar]
- Muessel MJ, Berman NE, Klein RM. Early and specific expression of monocyte chemoattractant protein-1 in the thalamus induced by cortical injury. Brain Res. 2000;870:211–221. doi: 10.1016/s0006-8993(00)02450-1. [DOI] [PubMed] [Google Scholar]
- Noh KM, Kim YH, Koh JY. Mediation by membrane protein kinase C of zinc-induced oxidative neuronal injury in mouse cortical cultures. J Neurochem. 1999;72:1609–1616. doi: 10.1046/j.1471-4159.1999.721609.x. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annual Review of Neuroscience. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Pal S, He K, Aizenman E. Nitrosative stress and potassium channel-mediated neuronal apoptosis: is zinc the link? Pflugers Arch. 2004;448:296–303. doi: 10.1007/s00424-004-1256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passonneau JV, Lowry OH. Enzymatic Analysis A Practical Guide. NJ: Humana Press; 1993. [Google Scholar]
- Plaschke K, Kopitz J, Weigand MA, Martin E, Bardenheuer HJ. The neuroprotective effect of cerebral poly(ADP-ribose)polymerase inhibition in a rat model of global ischemia. Neurosci Lett. 2000;284:109–112. doi: 10.1016/s0304-3940(00)00988-5. [DOI] [PubMed] [Google Scholar]
- Purves D, Snider WD, Voyvodic JT. Trophic regulation of nerve cell morphology and innervation in the autonomic nervous system. Nature. 1988;336:123–128. doi: 10.1038/336123a0. [DOI] [PubMed] [Google Scholar]
- Redman PT, Hartnett KA, Aras MA, Levitan ES, Aizenman E. Regulation of apoptotic potassium currents by coordinated zinc-dependent signalling. J Physiol. 2009;587:4393–4404. doi: 10.1113/jphysiol.2009.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzardini M, Lupi M, Bernasconi S, Mangolini A, Cantoni L. Mitochondrial dysfunction and death in motor neurons exposed to the glutathione-depleting agent ethacrynic acid. Journal of the Neurological Sciences. 2003;207:51–58. doi: 10.1016/s0022-510x(02)00357-x. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Kim SU, McLarnon JG. Neuroprotective effects of pyruvate in the quinolinic acid rat model of Huntington's disease. Exp Neurol. 2003;183:700–704. doi: 10.1016/s0014-4886(03)00214-0. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Baloh RH, Milbrandt J. Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. J Neurosci. 2009a;29:6526–6534. doi: 10.1523/JNEUROSCI.1429-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Lund FE, Milbrandt J. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci. 2009b;29:5525–5535. doi: 10.1523/JNEUROSCI.5469-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, Weiss JH. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc Natl Acad Sci U S A. 2003;100:6157–6162. doi: 10.1073/pnas.1031598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Yin HZ, Weiss JH. AMPA/kainate receptor-triggered Zn2+ entry into cortical neurons induces mitochondrial Zn2+ uptake and persistent mitochondrial dysfunction. Eur J Neurosci. 2000;12:3813–3818. doi: 10.1046/j.1460-9568.2000.00277.x. [DOI] [PubMed] [Google Scholar]
- Sheline CT, Behrens MM, Choi DW. Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD(+) and inhibition of glycolysis. J Neurosci. 2000;20:3139–3146. doi: 10.1523/JNEUROSCI.20-09-03139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline CT, Choi DW. Neuronal death in cultured murine cortical cells is induced by inhibition of GAPDH and triosephosphate isomerase. Neurobiol Dis. 1998;5:47–54. doi: 10.1006/nbdi.1998.0177. [DOI] [PubMed] [Google Scholar]
- Sheline CT, Shi C, Cai A. Society for Neurosciences Online Itinerary Planner. Washington, DC, Atlanta, GA: SFN; 2006. Oxidative neuronal injuries and target deprivation: role of Zn2+, NAD+, and the sirtuin pathway. pp. #186.10. [Google Scholar]
- Sheline CT, Takata T, Ying H, Canzoniero LM, Yang A, Yu SP, Choi DW. Potassium attenuates zinc-induced death of cultured cortical astrocytes. Glia. 2004;46:18–27. doi: 10.1002/glia.10313. [DOI] [PubMed] [Google Scholar]
- Sheline CT, Wang H, Cai AL, Dawson VL, Choi DW. Involvement of poly ADP ribosyl polymerase-1 in acute but not chronic zinc toxicity. Eur J Neurosci. 2003;18:1402–1409. doi: 10.1046/j.1460-9568.2003.02865.x. [DOI] [PubMed] [Google Scholar]
- Sheline CT, Wei L, Shi C, Cai AL. Society for Neurosciences Online Itinerary Planner. Washington, DC, Washington, DC: SFN; 2008. Trophic factor deprivation: roles of oxidation, zinc, and NAD+ pp. #555.15. [Google Scholar]
- Suh SW, Aoyama K, Alano CC, Anderson CM, Hamby AM, Swanson RA. Zinc inhibits astrocyte glutamate uptake by activation of poly(ADP-ribose) polymerase-1. Mol Med. 2007a;13:344–349. doi: 10.2119/2007-00043.Suh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SW, Aoyama K, Chen Y, Garnier P, Matsumori Y, Gum E, Liu J, Swanson RA. Hypoglycemic neuronal death and cognitive impairment are prevented by poly(ADP-ribose) polymerase inhibitors administered after hypoglycemia. J Neurosci. 2003;23:10681–10690. doi: 10.1523/JNEUROSCI.23-33-10681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SW, Aoyama K, Matsumori Y, Liu J, Swanson RA. Pyruvate administered after severe hypoglycemia reduces neuronal death and cognitive impairment. Diabetes. 2005;54:1452–1458. doi: 10.2337/diabetes.54.5.1452. [DOI] [PubMed] [Google Scholar]
- Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007b;117:910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SW, Hamby AM, Gum ET, Shin BS, Won SJ, Sheline CT, Chan PH, Swanson RA. Sequential release of nitric oxide, zinc, and superoxide in hypoglycemic neuronal death. J Cereb Blood Flow Metab. 2008;28:1697–1706. doi: 10.1038/jcbfm.2008.61. [DOI] [PubMed] [Google Scholar]
- Szabo C, Dawson VL. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol Sci. 1998;19:287–298. doi: 10.1016/s0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- Virag L, Szabo E, Gergely P, Szabo C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett. 2003;140–141:113–124. doi: 10.1016/s0378-4274(02)00508-8. [DOI] [PubMed] [Google Scholar]
- Visalli N, Costa C, Natoli A. [Hypoprolactinemia in subjects with various pathologies of the reproductive system] Clin Ter. 1986;116:421–423. [PubMed] [Google Scholar]
- Wang XQ, Xiao AY, Sheline C, Hyrc K, Yang A, Goldberg MP, Choi DW, Yu SP. Apoptotic insults impair Na+, K+-ATPase activity as a mechanism of neuronal death mediated by concurrent ATP deficiency and oxidant stress. J Cell Sci. 2003;116:2099–2110. doi: 10.1242/jcs.00420. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nonaka K, Hanafusa T, Miyazaki A, Toyoshima H, Tarui S. Preventive and therapeutic effects of large-dose nicotinamide injections on diabetes associated with insulitis. An observation in nonobese diabetic (NOD) mice. Diabetes. 1982;31:749–753. doi: 10.2337/diab.31.9.749. [DOI] [PubMed] [Google Scholar]
- Ying W, Sevigny MB, Chen Y, Swanson RA. Poly(ADP-ribose) glycohydrolase mediates oxidative and excitotoxic neuronal death. Proc Natl Acad Sci U S A. 2001;98:12227–12232. doi: 10.1073/pnas.211202598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo MH, Lee JY, Lee SE, Koh JY, Yoon YH. Protection by pyruvate of rat retinal cells against zinc toxicity in vitro, and pressure-induced ischemia in vivo. Invest Ophthalmol Vis Sci. 2004;45:1523–1530. doi: 10.1167/iovs.03-1315. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Aizenman E, DeFranco DB, Rosenberg PA. Intracellular zinc release, 12-lipoxygenase activation and MAPK dependent neuronal and oligodendroglial death. Mol Med. 2007;13:350–355. doi: 10.2119/2007-00042.Zhang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang H, Li J, Jimenez DA, Levitan ES, Aizenman E, Rosenberg PA. Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J Neurosci. 2004;24:10616–10627. doi: 10.1523/JNEUROSCI.2469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Near-pure neuronal cultures were cultured for 8 days - or + an additional 10 µM Zn2+ after which the neurons were exposed chronically to the indicated conditions. Neuronal death was determined 24 hrs later by lactate dehydrogenase release to the medium scaled to the level associated with near complete death produced by exposure to 20 µM A23187 for 24 hrs, = 100 (mean ± SEM, n = 8–18 cultures per condition). * indicates difference from control, # indicates difference from 25 µM ethacrynic acid at P<0.05
A. Near-pure neuronal cultures were exposed as indicated for 24 hrs, and neuronal death was assessed by lactate dehydrogenase release to the medium. The basal effects of these therapeutics were subtracted from their effects on oxidant injuries (Figures 2, 4, 5). * indicates difference from control cultures at P<0.05 B. Neuronal death was determined 24-36 hrs later by lactate dehydrogenase release to the medium after exposure as indicated. Death was scaled to the level associated with near complete death produced by exposure to 20 µM A23187 for 24 hrs, = 100 (mean ± SEM, n = 6–12 cultures per condition). * # $ indicate difference from appropriate oxidant exposure at P<0.05