Abstract
While astrocytes produce key inflammatory mediators following exposure to neurotropic non-segmented negative-sense RNA viruses such as rabies virus and measles virus, the mechanisms by which resident central nervous system (CNS) cells perceive such viral challenges have not been defined. Recently, several cytosolic DExD/H box RNA helicases including retinoic acid-inducible gene I (RIG-I) have been described that function as intracellular sensors of replicative RNA viruses. Here, we demonstrate that primary human astrocytes constitutively express RIG-I and show that such expression is elevated following exposure to a model neurotropic RNA virus, vesicular stomatitis virus (VSV). Evidence for the functional nature of RIG-I expression in these cells comes from the observation that this molecule associates with its downstream effector molecule, interferon promoter stimulator-1, following VSV infection and from the finding that a specific ligand for RIG-I elicits astrocyte immune responses. Importantly, RIG-I knockdown significantly reduces inflammatory cytokine production by VSV-infected astrocytes and inhibits the production of soluble neurotoxic mediators by these cells. These findings directly implicate RIG-I in the initiation of inflammatory immune responses by human glial cells and provide a potential mechanism underlying the neuronal cell death associated with acute viral CNS infections.
Keywords: RNA viruses, Astrocytes, CNS inflammation, RIG-I
INTRODUCTION
The order Mononegavirales includes numerous etiological agents of human disease and members of this order include medically important pathogens with central nervous system (CNS) involvement such as rabies virus (RV) and measles virus (Fishbein et al, 1993). Virally induced CNS inflammation can be initiated by the elevated expression of inflammatory mediators including tumor necrosis factor-α (TNF-α) and interleukin (IL)-6. Importantly, elevated CNS levels of TNF-α (Nuovo et al, 2005; Solanki et al, 2009) and IL-6 (Phares et al, 2006) have been reported following RNA virus infection, and TNF-α has been implicated as a key factor in disease pathogenesis (Camelo et al, 2000; Nuovo et al, 2005). Like RV, vesicular stomatitis virus (VSV) is a negative-sense single-stranded RNA virus belonging to the Rhabdoviridae family. VSV has the ability to infect neurons and generate acute encephalitis in mice (Huneycutt et al, 1993) and has therefore proven to be a useful model for this and other non-segmented, negative-sense RNA viruses. As described in work by Dr. Carol Reiss and others (Bi et al, 1995; Lundh et al, 1987; 1990; Miyoshi et al, 1971), intranasal inoculation of VSV in mice leads to infection of the olfactory bulb via the olfactory neurons and subsequently spreads throughout the CNS. This infection is associated with acute encephalitis, breakdown of the blood-brain barrier, and a high degree of mortality (Honeycutt et al, 1993). Interestingly, VSV-induced encephalitis appears to be T-cell independent, having been observed in athymic mice after viral administration (Frei et al, 1989). As such, it is likely that the innate immune functions of resident CNS cells play an important role in the rapid inflammatory response following VSV infection.
There is growing appreciation that astrocytes can initiate and augment inflammation following infection (see Dong and Benveniste, 2001). When activated, these glial cells assume immune effector functions, including the production of TNF-α and IL-6 (Streit et al, 1998). Various RNA viruses have been reported to infect human and murine astrocytes in vitro (Ray et al, 1997) and in vivo (Garg et al, 2008; Wong et al, 2009), and the presence of RV and Hendra virus antigens have been observed in astrocytes following in vivo infection (Jogai et al, 2000; Wong et al, 2009). Similarly, we have recently demonstrated that VSV can infect and replicate within cultured primary murine astrocytes (Chauhan et al, 2010). Astrocytes appear to respond to VSV as CNS infections are associated with their proliferation and increased cell surface MHC class II molecule expression (Bi et al, 1995). Furthermore, we have demonstrated that astrocytes express TNF-α and IL-6 following VSV challenge, and that such production is dependent upon active viral replication (Chauhan et al, 2010). While the ability of astrocytes to be infected by and respond to non-segmented negative-sense RNA viruses has been established, the mechanisms underlying the activation of this important CNS cell type have not been defined.
Recently, a newly described group of molecules have been shown to function as intracellular sensors for replicative viral RNA (Takeuchi and Akira, 2007; Yoneyama et al, 2004; Yoneyama and Fujita, 2007). A member of the retinoic acid-inducible gene (RIG)-I like receptor (RLR) family, RIG-I is a soluble protein found in the cytosol of many cell types and has been shown to mediate innate immune responses to viral RNA. Responses initiated via this cytosolic sensor include the production of antiviral cytokines as well as proinflammatory cytokines such as TNF-α and IL-6 (as reviewed in Meylan and Tschopp, 2006, and Rehwinkel and Reis e Sousa, 2010). Genomic RNA from several viruses in the order Mononegavirales, including VSV, has been demonstrated to specifically activate immune responses via RIG-I (Kato et al, 2006). Importantly, we have recently demonstrated that murine astrocytes express RIG-I in conjunction with its downstream effector molecule, interferon promoter stimulator-1 (IPS-1) (Furr et al, 2008). In the present study, we demonstrate that primary human astrocytes functionally express RIG-I and we have begun to define the relative importance of this molecule to astrocyte responses to non-segmented negative-sense RNA viruses.
MATERIALS AND METHODS
Cell culture
Primary astrocytes isolated from human brain tissue were purchased from ScienCell (Carlsbad, CA). Cells were maintained in a specially formulated medium supplemented with fetal bovine serum (FBS), growth supplement, and penicillin/streptomycin solution, purchased from the supplier. These cells have been characterized as authentic astrocytes by the vendor according to their expression of glial fibrillary acidic protein as determined by immunofluorescent analyses. The human cortical neuron cell line, HCN-1A (Ronnett et al, 1990), was obtained from ATCC (CRL-10442; Manassas, VA). Cells were maintained in Dulbecco’s modified Eagle’s media containing 10% FBS with penicillin and streptomycin.
Preparation of viral stocks and in vitro infection with VSV
Viral stocks of wild type VSV (Indiana strain) were prepared and astrocytes infected as previously described by our laboratory (Furr et al, 2008). VSV-GFP is VSV (Indiana strain) encoding green fluorescent protein as an extra gene between G and L genes (Das et al, 2006) and was kindly provided by Dr. Asit K. Pattnaik (University of Nebraska). VSV-GFP was utilized to verify the absence of infectious particles in conditioned medium following filtration.
Isolation of RNA and semi-quantitative reverse transcribed PCR
Poly(A) + RNA was isolated from human astrocytes and reverse transcribed as previously described (Bowman et al, 2003). Polymerase chain reactions (PCR) were performed to determine the expression of mRNA encoding RIG-I. Positive and negative strand PCR primers used, respectively, were 5′-GTGCAAAGCCTTGGCATGT-3′ and 5′-TGGCTTGGGATGTGGTCTACTC-3′ to amplify mRNA encoding human RIG-1 (115 bp fragment). Primers were designed by using OligoCalc (Kibbe, 2007: www.basic.northwestern.edu/biotools/oligocalc.html) based on their location in different exons of the genomic sequence and their lack of significant homology to sequences present in GenBank (MacVector Sequence analysis software, IBI, New Haven, CT). The identity of the PCR amplified fragments was verified by size comparison with DNA standards (Promega). The input RNA was normalized to the expression of the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (G3PDH).
Western blot analyses for RIG-1, IPS-1, and NF-kB p65 (RelA)
Nuclear and cytoplasmic protein extracts were prepared from astrocytes by suspension in lysis buffer containing 10 mM KCl, 1.5 mM MgCl2, 10 mM HEPES, 0.5 mM dithiothreitol, and protease inhibitor cocktail for 20 min at 4°C. The nuclei and other fragments were then separated by centrifugation and supernatants were retained as cytoplasmic fractions. Nuclei were lysed by exposure to a high salt buffer containing 420 mM NaCl, 15 mM MgCl2, 20 mM HEPES, 0.2 mM EDTA, 20% glycerol, 0.5 mM phenylmethylsulphonylfluoride, 1 mM dithiothreitol, 1% NP-40, and 2.5 ug/ml leupeptin for 20 min at 4°C. Samples were then cleared of cellular debris by centrifugation and supernatants containing the nuclear fraction collected.
Western blot analyses for the presence of RIG-1, IPS-1, and NF-kB in human astrocytes were performed as described previously by our laboratory (Bowman et al, 2003) using a rabbit polyclonal antibody directed against human RIG-1 (Abgent, San Diego, CA), a polyclonal antibody directed against IPS-1 (Abcam, Cambridge, MA), and a polyclonal antibody directed against the p65 (RelA) subunit of NF-kB (Millipore, Billerica, MA). To assess total protein loading in each well, immunoblots were reprobed with a goat anti-mouse β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
Co-immunoprecipitation
Briefly, astrocytes (2 × 106) infected with VSV were washed with ice-cold PBS and lysed at 4°C at 1 hour following infection in Tris-buffered saline with EDTA (150 mM NaCl, 5 mM EDTA, 20 mM Tris, pH 7.5) plus 1% Brij-97 (Sigma-Aldrich) and 10 units/mL aprotinin (Calbiochem, San Diego, CA), 1 mM PMSF, and 1 ug/mL pepstatin A. The lysates were incubated with protein A agarose beads (Pierce Endogen) conjugated with antibodies directed against IPS-1 for 18 hours at 4°C. The immunoprecipitated material was subsequently subjected to immunoblot analysis for RIG-I. To assess total protein loading in each well, immunoblots were probed for the presence of total IPS-1 content in the immunoprecipitated material.
Quantification of IL-6 and TNF-α secretion in astrocyte culture supernatants
Levels of human IL-6 protein in culture supernatants were measured by specific capture ELISA as previously described (Furr et al, 2008) using capture and detection anti-human cytokine antibodies purchased from BD Biosciences (San Diego, CA; clone MQ2-13A5 and biotinylated clone MQ2-39C3). A commercially available ELISA kit (Ready-Set-Go!; eBioscience, San Diego, CA) was used to assay human TNF-α levels according to the manufacturer’s instructions.
In vitro stimulation of astrocytes with the RIG-I ligand, 5′-triphosphate single stranded RNA
To generate 5′-triphosphate single stranded RNA (5′ppp-ssRNA), we created a linear template for run-off in vitro transcription by digesting the pGEM-SeV-NP plasmid (previously described in Curran and Kolakofsky, 1991) with EcoRV. EcoRV cuts pGEM-SeV-NP at a single site allowing a 342 base RNA to be generated when using the SP6 promoter. RNA was generated using the MAXIscript In Vitro Transcription Kit (Ambion) at 37°C for 2.5 hours in a final volume of 200 ul containing approximately 1 ug of linearized pGEM-SeV-NP, all four NTPs, transcription buffer, and 200 U of SP6 enzyme mix. The reaction was then separated into two fractions and one was twice treated with 2 U calf intestinal alkaline phosphatase (CIAP; Promega) at 37°C for 15 minutes to dephosphorylate the RNA for use as a negative control in our studies. Both CIAP-treated and untreated RNAs were then incubated at 37°C for 20 minutes in the presence of 10 U Turbo DNase (Ambion) to remove the plasmid template. Following addition of EDTA (35 mM), the samples were phenol/chloroform extracted and ethanol precipitated. Precipitated RNA was resuspended in RNase free water to a final concentration of 200-250 ng/ul. The final RNA products were analyzed by electrophoresis on a 2.5% agarose gel.
5′ppp-ssRNA or dephosphorylated ssRNA was introduced intracellularly into human astrocytes using Tfx-20 transfection reagent (Promega) at a charge ratio of 1:4 for one hour. For comparison purposes, cells were exposed to Tfx-20 reagent alone or 5′ppp-ssRNA in the absence of Tfx-20.
siRNA-mediated RIG-I knockdown
Three validated Stealth RNAi™ siRNA duplexes targeting human RIG-I, as well as universal negative control siRNA not homologous to anything in the vertebrate transcriptome, were purchased from Invitrogen (Carlsbad, CA). Astrocytes were transfected with each siRNA duplex individually or in concert using Tfx-20 Reagent (Promega) according to the manufacturer’s instructions. Antibiotic-free media was replaced with complete media at 6 hours following transfection. At 24, 48 and 72 hours after transfection, whole cell lysates were collected for immunoblot analyses to confirm RIG-I expression knockdown.
Assessment of soluble neurotoxic mediator production by infected human astrocytes
Transfected or untransfected astrocytes were uninfected or infected with VSV and non-adherent viral particles were removed by repeated washing after 1 hour. At 24 hours following infection, medium was collected and filtered with a 0.1-mm syringe filter (Sterlitech, Kent,WA) to remove residual viral particles (0.1 to 0.4 mm in length) prior to addition to uninfected neuronal cells. The complete removal of infectious viral particles using this filtering protocol was verified using GFP-expressing VSV. Glial cells were infected for 24 hours with GFP-VSV (MOIs of 1 and 10 PFU per cell) and medium from these cells was unfiltered or filtered, and placed on HCN-1A cultures. At 12 hours following exposure, virus associated GFP fluorescence was visualized and was only present in cells treated with unfiltered conditioned media.
At varying time points (4, 8, and 12 hours) following addition of conditioned media, the numbers of adherent HCN-1A cells were counted in ten microscopy fields and viability was assessed by trypan blue exclusion.
Densitometric analyses
Densitometric analyses of immunoblots were performed using ImageJ (obtained from the NIH Web site http://rsbweb.nih.gov/ij/download.html). Results are presented as mean values of arbitrary densitometric units corrected for background intensity normalized to the expression of β-actin, or as fold increases over levels in unstimulated cells.
Statistical analyses
Results of the present studies were tested statistically by ANOVA and Tukey’s post hoc test using commercially available software (SAS Institute, Cary, NC). Results were considered statistically significant when a P value of less than 0.05 was obtained.
RESULTS
Primary human astrocytes constitutively express the cytoplasmic viral RNA sensor, RIG-I, and such expression is elevated following viral challenge
To determine whether primary human astrocytes express this newly described viral pattern recognition receptor, we have assessed the level of RIG-I mRNA expression in cultures of these cells at rest and following VSV challenge. Human astrocytes were untreated or exposed to VSV at varying MOIs prior to RNA isolation at 4 and 8 hours post-infection (p.i.). Semi-quantitative RT-PCR was performed to assess the presence of mRNA encoding RIG-I. As shown in Figure 1A, astrocytes constitutively express mRNA encoding RIG-I. Interestingly, such expression is rapidly elevated at 4 hours p.i. but such elevations are transient and are not apparent at 8 hours p.i. (Figure 1A).
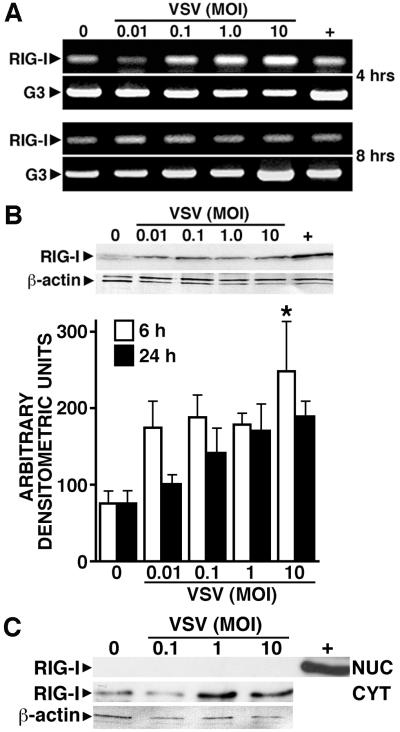
FIGURE 1.
Primary human astrocytes constitutively express the cytoplasmic viral RNA sensor, RIG-I, and such expression is elevated following viral challenge. Panel A: Cultured astrocytes (2 × 106) were untreated (0) or infected with VSV (MOI of 0.01, 0.1, 1, and 10). At 4 and 8 hours p.i., the level of RIG-I and G3PDH (G3) mRNA expression was determined. For comparison purposes, RIG-I mRNA levels in a similar number of HeLa cells are shown (+). Representative results of three separate experiments are shown. Panel B: Cells (2 × 106) were untreated (0) or infected with VSV, and RIG-I protein expression determined at 6 and 24 hours p.i. A representative immunoblot of protein isolates prepared at 6 hours p.i. is shown in the upper panel, and the average densitometric values of three separate experiments at 6 and 24 hours p.i. normalized to β-actin expression is shown below. Data is expressed as mean +/− SEM and an asterisk indicates a statistically significant difference from uninfected cells (p < 0.05). For comparison purposes, RIG-I protein expression in a similar number of resting HeLa cells is shown in the representative immunoblot (+). Panel C: Cells (2 × 106) were untreated (0) or infected with VSV. At 12 hours p.i., nuclear and cytoplasmic extracts were prepared and RIG-I expression assessed. For comparison purposes, RIG-I protein expression in HeLa cells is shown (+). A representative immunoblot of three separate experiments is shown.
We next performed immunoblot analyses to assess RIG-I protein levels in human astrocytes. Consistent with RIG-I mRNA expression, human astrocytes constitutively express detectable levels of RIG-I protein (Figure 1B). Furthermore, exposure of astrocytes to VSV elevates expression of this receptor as rapidly as 6 hours p.i with a maximal 3.3 fold induction at an MOI of 10 PFU/cell. Importantly, constitutive and VSV-induced RIG-I expression is restricted to the cytoplasm of these cells with an absence of detectable RIG-I protein in the nucleus (Figure 1C), consistent with its role as a cytoplasmic viral pattern recognition receptor.
Primary human astrocytes constitutively and functionally express IPS-1, a downstream effector of RIG-I
To begin to determine whether RIG-I is functional in human astrocytes, we have investigated whether these cells express interferon promoter stimulator-1 (IPS-1), a critical downstream effector molecule in RIG-I signaling (Kumar et al, 2006). As shown in Figure 2A, astrocytes constitutively express robust levels of IPS-1 but VSV challenge does not significantly elevate IPS-1 expression at 12 hours p.i. at any MOI used (Figure 2A). Next, we examined whether RIG-I interacts with IPS-1 following viral challenge using co-immunoprecipitation techniques. As shown in Figure 2B, infection of human astrocytes with VSV induces association of RIG-I with IPS-1 within one hour of infection.
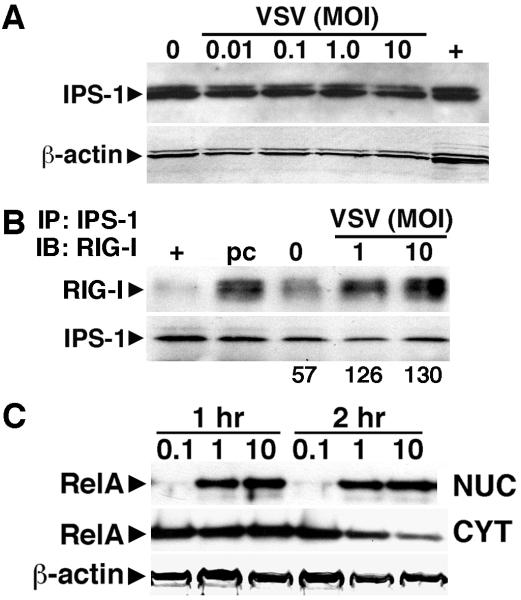
FIGURE 2.
VSV induces the activation of RIG-I associated signaling pathways in primary human astrocytes. Panel A: Cells (2 × 106) were untreated (0) or infected with VSV (MOI of 0.01, 0.1, 1, and 10). At 12 hours p.i., expression of the RIG-I downstream effector molecule IPS-1, was determined in whole cell protein isolates. IPS-1 expression in HeLa cells (+) is shown for comparison purposes. Panel B: Cells (2 × 106) were untreated (0) or infected with VSV (MOI of 1 and 10). At 1 hour p.i., association of RIG-I with IPS-1 was assessed using co-immunoprecipitation techniques. RIG-I content in samples following the pre-clearing step (pc) is shown as a control and RIG-I expression in a similar number of resting HeLa cells is shown (+) for comparison purposes. Representative results are shown for one of three separate experiments. The relative level of RIG-I/IPS-1 complex formation in this experiment was determined by densitometric analysis normalized to total IPS-1 content in the precipitated material and is shown below the immunoblot. Panel C: Cells (2 × 106) were untreated (0) or infected with VSV (MOI of 0.1, 1, and 10). At 1 and 2 hours p.i., nuclear (NUC) and cytoplasmic (CYT) extracts were prepared and RelA expression determined. A representative immunoblot of three separate experiments is shown.
Interaction of RIG-I with IPS-1 has been previously demonstrated to elicit activation of the pivotal inflammatory transcription factor NF-kB in other cell types (Lui et al, 2008). We have therefore correlated the observed interaction of RIG-I and IPS-1 with activation of NF-kB in astrocytes as assessed by the nuclear translocation of the NF-kB p65 (RelA) subunit. As shown in Figure 2C, infection of human astrocytes with VSV elicits nuclear translocation of RelA at 1-2 hours p.i. Such rapid responses are consistent with the observed constitutive expression of both RIG-I and IPS-1 in these primary glial cells.
Single stranded RNA induces RIG-I expression and inflammatory mediator production by primary human astrocytes
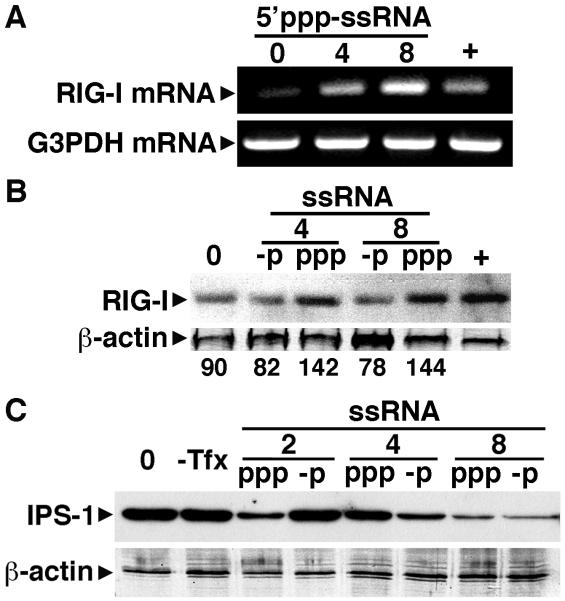
Viral genomes have been reported to serve as the major trigger for RIG-I in cells infected with negative-sense single-stranded RNA viruses (Rehwinkel et al, 2010) confirming earlier suggestions that ssRNAs bearing 5′-ppp generated in vitro, perhaps with base-pairing at the 5′-end, are effective agonists for RIG-I (Hornung et al., 2006 and Pichlmair et al., 2006). Consistent with this notion, treatment with calf intestinal phosphatase, which removes 5′-phosphates, has been shown to abolish stimulatory activity (Hornung et al., 2006 and Pichlmair et al., 2006). To confirm the functional nature of RIG-I expression in primary human astrocytes, we have investigated the effects of intracellular administration of in vitro generated uncapped 5′ppp-ssRNA. As shown in Figure 3A, administration of 5′ppp-ssRNA significantly increases RIG-I mRNA levels. Similarly, this ligand induced a modest but discernable elevation in RIG-I protein levels over those in cells receiving only transfection reagent (1.6 fold), an effect that was not seen in cells transfected with dephosphorylated ssRNA control (Figure 3B). Interestingly, and in agreement with the effects of VSV (Figure 2A), 5′ppp-ssRNA failed to consistently elicit increases in IPS-1 expression (Figure 3C). While IPS-1 levels decreased with increasing concentrations of 5′ppp-ssRNA, this effect does not appear to be specific as dephosphorylated ssRNA similarly decreased IPS-1 expression (Figure 3C).
FIGURE 3.
5′ triphosphate single stranded RNA, a specific ligand for RIG-I, upregulates RIG-I expression but not IPS-1 in primary human astrocytes. Panel A: Cells (2 × 106) were untreated (0) or exposed to 5′ triphosphate ssRNA (5′ppp-ssRNA: 4 or 8 ug/ml) in the presence of Tfx-20 transfection reagent. At 2 hrs following transfection, RIG-I and G3PDH mRNA levels were assessed. For comparison purposes, RIG-I mRNA levels in a similar number of resting HeLa cells are shown (+). Representative results of three separate experiments are shown. Panel B: Cells (2 × 106) were treated with Tfx-20 alone (0), 5′ triphosphate ssRNA (ppp: 4 or 8 ug/ml), or unphosphorylated RNA (-p: 4 or 8 ug/ml). At 6 hours following transfection, RIG-I protein expression was assessed in whole cell lysates. For comparison purposes, RIG-I protein expression in a similar number of resting HeLa cells is shown (+). Representative results are shown for one of three separate experiments and densitometric analysis of this experiment normalized to β-actin expression is shown below the immunoblot. Panel C: Cells (2 × 106) were untreated (0), or exposed to 5′ triphosphate ssRNA (ppp: 2, 4, or 8 ug/ml) or unphosphorylated ssRNA (-p: 2, 4, or 8 ug/ml) as a control in the presence of Tfx-20, or without the addition of the transfection reagent (−Tfx). At 6 hours following transfection, IPS-1 protein expression was determined in whole cell lysates. Representative results are shown of one of three separate experiments.
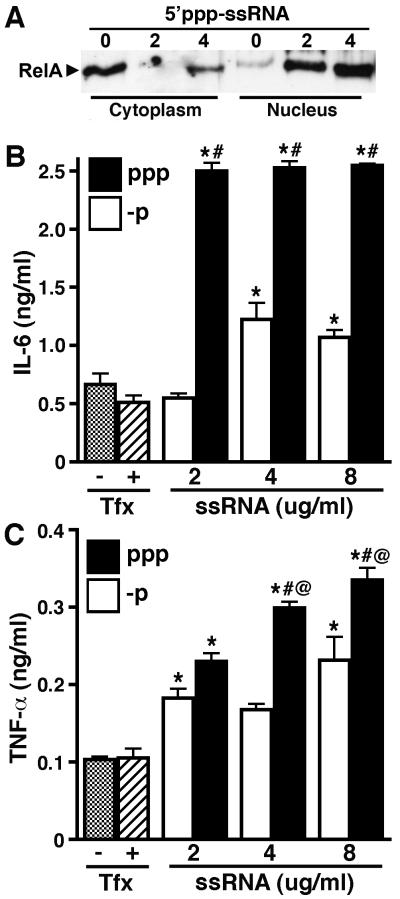
Furthermore, we have determined that 5′ppp-ssRNA can elicit inflammatory immune responses in primary human astrocytes. As shown in Figure 4A, 5′ppp-ssRNA induces nuclear translocation of NF-kB (RelA) in a dose dependent manner. Such NF-kB activation is consistent with the ability of this ligand to induce the production of the inflammatory cytokines IL-6 (Figure 4B) and TNF-α (Figure 4C). Importantly, these effects were significantly attenuated when the ligand was dephosphorylated (Figures 4B and C), consistent with the specific recognition of 5′ppp-ssRNA by RIG-I. Taken together, these data indicate that RIG-I is functionally expressed in primary human astrocytes and recognizes replicative viral RNA motifs.
FIGURE 4.
5′ppp-ssRNA induces NF-kB activation and inflammatory cytokine production in primary human astrocytes. Panel A: Cells (2 × 106) were untreated (0) or exposed to 5′ppp-ssRNA (2 or 4 ug/ml) in the presence of Tfx-20. At 1 hour following transfection, nuclear and cytoplasmic extracts were prepared and RelA levels assessed. Panels B and C: Cells (2 × 106) were exposed to tri-phosphorylated (ppp) or dephosphorylated (-p) recombinant ssRNA (ssRNA; 2, 4, or 8 ug/ml) in the presence of Tfx-20, or were unstimulated in the presence (+) or absence (−) of Tfx-20 alone. At 6 hrs following transfection, levels of IL-6 (Panel B) and TNF-α (Panel C) secretion were determined. Data is expressed as mean +/− SEM (n = 6). An asterisk indicates a statistically significant difference from unstimulated cells, pound symbol indicates a statistically significant difference from the equivalent concentration of dephosphorylated RNA, and asperand indicates a statistically significant difference from the preceding lower dose of each stimulus (p < 0.05).
RIG-I knockdown attenuates VSV-induced inflammatory cytokine production by primary human astrocytes
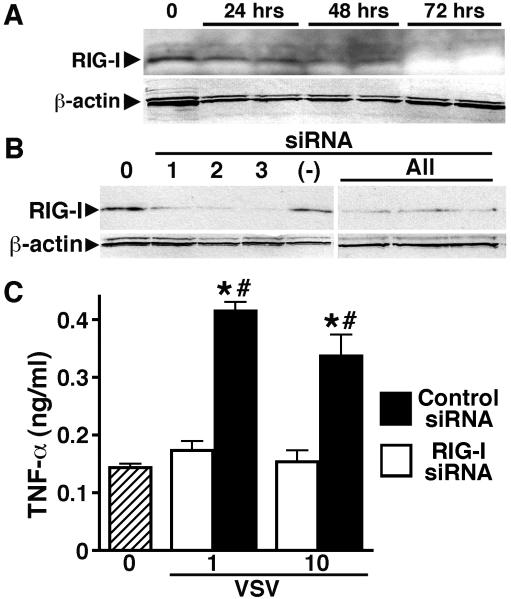
To begin to establish the relative importance of RIG-I in the initiation of VSV-mediated astrocyte immune responses, we employed silencing RNA techniques (siRNA) to knock down the expression of this viral sensor. As shown in Figure 5A, the use of a combination of three siRNA duplex oligomers effectively knocked down the expression of RIG-I in a time dependent manner with a maximal reduction observed at 72 hours post-transfection. We then optimized our knockdown protocol by selecting the most effective siRNA duplex (DDX58-HSS177513; Invitrogen) for use in our subsequent studies (Figure 5B; duplex 3). Importantly, we determined that transfection of human astrocytes with this siRNA duplex significantly inhibited VSV-induced inflammatory cytokine production relative to that produced by cells transfected with non-specific scrambled siRNA (Figure 5C). Together, these data indicate that RIG-I is an important component in the initiation of human astrocyte inflammatory immune responses to negative sense single-stranded RNA viruses.
FIGURE 5.
RIG-I knockdown attenuates VSV-induced inflammatory cytokine production by primary human astrocytes. Panel A: To determine knockdown efficiency, cells were untreated (0) or transfected with a combination of three different siRNA oligonucleotide pairs targeting RIG-I and cultured for 24, 48, or 72 hours prior to immunoblot analysis for RIG-I and β-actin. Panel B: To verify the effectiveness of the siRNA oligonucleotide pairs, cells were untreated (0) or transfected with each siRNA pair (1, 2, or 3), or a combination of the three (All), and cultured for 72 hours prior to analysis for RIG-I and β-actin. The relative level of RIG-I in astrocytes transfected with scrambled siRNA (−) is shown for comparison purposes. Panel C: Cells were untreated (0), or transfected with one siRNA pair (pair 3) targeting RIG-I (RIGI siRNA) or scrambled siRNA (Control siRNA). At 72 hours following transfection, cells were infected with VSV (MOI of 0.01, 0.1, 1, and 10) and TNF-α secretion measured at 12 hours p.i. Data is expressed as mean +/− SEM (n = 3). An asterisk indicates a statistically significant difference from unstimulated cells; a pound symbol indicates a statistically significant difference between cells transfected with siRNA directed against RIG-I versus scrambled siRNA (p < 0.05).
RIG-I is required for virally-induced production of neurotoxic mediators by human astrocytes
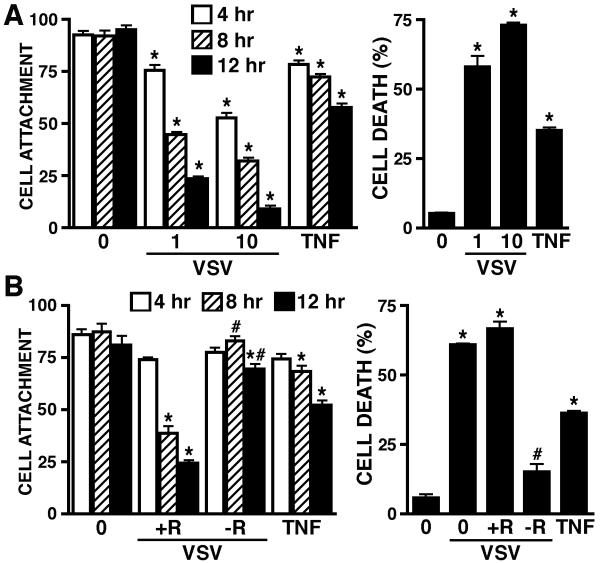
To begin to establish a role for RIG-I in the inflammatory CNS damage associated with neurotropic non-segmented, negative-sense RNA viral infections, we have assessed the effect of RIG-I knockdown on the production of soluble mediators that can elicit neuronal cell death by VSV-infected human astrocytes. As shown in Figure 6A, VSV induces the production of soluble mediators by primary human astrocytes that can elicit decreases in the viability of HCN-1A human neuronal-like cells, as assessed by changes in cell attachment and trypan blue exclusion, in a MOI and time dependent manner. Importantly, the production of such neurotoxic mediators was significantly attenuated in cells transfected with siRNA targeting RIG-I (−R) as compared to cells that were transfected with scrambled non-specific siRNA control (+R) (Figure 6B). Together, these data point to a key role for RIG-I in the inflammatory immune responses of human astrocytes to non-segmented, negative-sense RNA viral infection and may represent a key component in the inflammatory damage associated with virally induced encephalitis.
FIGURE 6.
VSV induces the production of a soluble factor(s) by human astrocytes that elicit neuronal cell death in a RIG-I dependent manner. Panel A: Human astrocytes were untreated (0) or infected with VSV (MOI of 0.01, 0.1, 1, and 10). At 24 hours p.i., filtered conditioned media from these infected cells or media spiked with recombinant TNF-α (63 pg/ml; TNF) was placed on HCN-1A cells. Changes in viability of these human neuronal-like cells were assessed at 4, 8 and 12 hours by quantification of cell attachment and at 12 hours by trypan blue exclusion. Panel B: Human astrocytes were untreated (0) or transfected with siRNA targeting RIG-I (−R) or scrambled siRNA (+R). At 72 hours following the transfection protocol, cells were uninfected or infected with VSV (MOI of 1) and cultured for a further 24 hours. Filtered conditioned media from these cells was placed on HCN-1A cells. As a positive control, media was spiked with recombinant TNF-α (63 pg/ml; TNF) and placed on HCN-1A cells. Changes in viability of these human neuronal-like cells were assessed at 4, 8 and 12 hours by quantification of cell attachment and at 12 hours by trypan blue exclusion. For comparison purposes, cell death of HCN-1A cells was assessed following exposure to conditioned medium from untransfected astrocytes infected with VSV (1). Data is expressed as mean +/− SEM (n = 3). An asterisk indicates a statistically significant difference from unstimulated cells; a pound symbol indicates a statistically significant difference between cells transfected with siRNA directed against RIG-I versus scrambled siRNA (p < 0.05).
DISCUSSION
The order Mononegavirales consists of viruses containing a non-segmented, negative-sense RNA genome and includes the causative agents for a number of established (rabies, measles, mumps) and emerging (Ebola, Borna, Hendra, Nipah) human diseases (Pringle, 1997). Some members of this order, such as RV and Newcastle disease virus, have the ability to circumvent the blood-brain barrier to establish CNS infection resulting in a severe and often fatal inflammation of brain tissue (Leung et al, 2007; Seal et al, 2000). VSV has proven to be a useful model for neurotropic non-segmented, negative-sense RNA viruses that cause human CNS infections due to its ability to gain access to the CNS and to elicit acute encephalitis (Honeycutt et al, 1993). VSV-associated CNS inflammation is rapid, occurring within days of intranasal inoculation (Honeycutt et al, 1993), and this rapid onset is indicative of an innate immune response that is likely to involve resident CNS cells. Such a hypothesis is supported by the observation that astrocytes exhibit immune functions in situ following VSV infection (Bi et al, 1995; Honeycutt et al, 1993; Leung et al, 2007). In a recent study, we have demonstrated that isolated murine astrocytes produce key soluble inflammatory mediators including TNF-α and IL-6 following exposure to VSV, and that such production is dependent upon viral replication (Chauhan et al, 2010). The recent description of RLRs, cytosolic DExD/H box RNA helicases that can recognize early viral replicative intermediates (Takeuchi and Akira, 2007), raises the intriguing possibility that such molecules could play an important role in the detection of RNA viruses by astrocytes.
In the present study, we describe the expression of RIG-I, an RLR that serves as an intracellular viral sensor, in primary cultures of human astrocytes. We show that these cells constitutively express robust levels of mRNA encoding RIG-I. Importantly, we demonstrate detectable levels of these proteins in resting astrocytes and we show that such expression is restricted to the cytoplasm of these cells. These data are in agreement with a recent study demonstrating the expression of mRNA encoding RIG-I and the detection of RIG-I protein in human astrocytoma cells, and the presence of RIG-I protein in uninfected primary human astrocytes as determined by immunocytochemical techniques (Yoshida et al, 2007). In addition, the present studies are consistent with our recent demonstration that primary murine astrocytes constitutively express this RLR (Furr et al, 2008). Interestingly, we show that the expression of RIG-I in the cytoplasm of primary human astrocytes is elevated following infection with the model RNA virus, VSV. Importantly, the present study provides the first demonstration that intracellular administration of in vitro generated 5′ppp-ssRNA, a reported ligand for RIG-I, induces RIG-I expression in primary human astrocytes. As such, the present demonstration of robust levels of RIG-I in resting human astrocytes is consistent with the ability of these cells to mount rapid inflammatory immune responses following viral challenge. Furthermore, the inducible nature of such expression by intact viral particles and a specific ligand for RIG-I suggests that astrocytes may become sensitized to the presence of intracellular viral motifs generated during viral replication in a feed-forward manner.
Perhaps more importantly, in the present study we provide the first demonstration that this RLR plays an important role in astrocyte inflammatory immune responses to a non-segmented negative-sense RNA virus. First, we have demonstrated that primary human astrocytes constitutively express robust levels of IPS-1, a critical downstream adaptor molecule for RIG-I signaling. The caspase activation and recruitment domains, or CARDs, of RIG-I interact with IPS-1 to induce type I interferon gene expression in mouse embryonic fibroblasts via TRAF3 and IRF3/IRF-7 pathways (Kumar et al, 2006), and activate NF-kB in a caspase-8 and 10-dependent manner to induce inflammatory cytokine production in immune cells such as macrophages (Hiscott, 2007). Consistent with this mechanism of action, we have demonstrated that VSV challenge initiates an association between RIG-I and IPS-1 in human astrocytes, and we have shown that this virus induces the translocation of the RelA subunit of NF-kB to the nucleus. Furthermore, we have demonstrated that 5′ppp-ssRNA delivered intracellularly into primary human astrocytes similarly elicits NF-kB activation and inflammatory cytokine production. We have confirmed the specificity of the actions of this RIG-I ligand by demonstrating that dephosphorylated RNA oligomers fail to elicit such marked cytokine responses. Finally, we have confirmed a role for this intracellular viral sensor in astrocyte immune responses to this model neurotropic rhabdovirus by demonstrating that RIG-I knockdown specifically and significantly inhibits VSV-induced cytokine production.
Neuronal loss has been reported in human cases of RV (Juntrakul et al, 2005) Hendra virus (Wong et al, 2009), and measles virus (Garg et al, 2008; McQuaid et al, 1997) infection. However, at least one study has indicated that rhabdoviruses do not have an intrinsic ability to induce apoptosis in isolated neuron-like cells (Baloul and Lafon, 2003) suggesting that these viruses elicit neuronal cell death in an indirect manner. Similarly, the pathogenesis of CNS measles virus infections has been suggested to result from neuronal apoptosis occurring either as a direct result of viral infection or as an indirect consequence of cytokine-mediated effects (McQuaid et al, 1997). Interestingly, the level of cytotoxic factors including TNF-α has been found to correlate with alterations in neuronal function and CNS damage in a murine model of RV infection (Marquette et al, 1996), and a high level of TNF-α has also been associated with poor prognosis in patients with other acute viral encephalopathies (Anlar et al, 2001; Ravi et al, 1997). In the present study we have demonstrated that human astrocytes produce robust levels of TNF-α in response to VSV in a RIG-I dependent manner. Furthermore, we have shown that soluble factors released by VSV-infected human astrocytes elicit cell damage/death of a human neuronal cell line, and that the production of these factors is RIG-I dependent. These findings directly implicate RIG-I in the initiation of inflammatory immune responses by human glial cells and provide a potential mechanism underlying the neuronal cell loss associated with acute viral CNS infections.
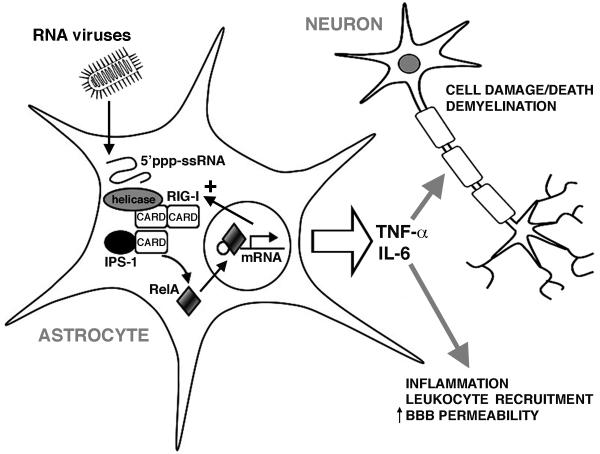
Based upon our results, we propose the model shown in Figure 7. We suggest that non-segmented negative-sense RNA viruses infect astrocytes and replicate within them, generating uncapped genomic RNAs with phosphorylated 5′-ends. These motifs are recognized by RIG-I and such recognition leads to an association of the CARD domains of this molecule with those of the downstream effector molecule, IPS-1. Activated IPS-1 ultimately liberates the RelA subunit of NF-kB allowing it to translocate to the nucleus and to initiate the de novo production of soluble inflammatory mediators. Cytokines such as TNF-α and IL-6 are well recognized to initiate inflammation and to increase blood-brain barrier permeability, facilitating leukocyte recruitment into the CNS. In addition, such soluble mediators can also initiate neuronal cell loss, either directly or via activation of resident/infiltrating myeloid cells. The expression of this RLR by human astrocytes may therefore represent an important innate immune mechanism underlying the rapid and potentially lethal CNS inflammation associated with neurotropic RNA virus infections.
FIGURE 7.
Proposed model by which astrocytes recognize neurotropic RNA viruses and elicit inflammatory CNS damage. Replicating RNA viruses generate phosphorylated single stranded RNA (5′ppp-ssRNA) that act as a ligand for RIG-I. RIG-I associates with IPS-1, which subsequently activates NF-kB by liberating the RelA subunit. RelA translocates to the nucleus and initiates the production of inflammatory mediators including TNF-α and IL-6. These soluble mediators, and others, could then promote inflammation, increase blood-brain-barrier (BBB) permeability, recruit leukocytes into the CNS, and directly or indirectly initiate neuronal cell damage and/or death.
Acknowledgments
This work is supported by grants NS050325 and NS057434 to IM, and grant NS064035 to VZG from the National Institutes of Health.
REFERENCES
- Anlar B, Söylemezoğlu F, Aysun S, Köse G, Belen D, Yalaz K. Tissue inflammatory response in subacute sclerosing panencephalitis (SSPE) J Child Neurol. 2001;16:895–900. doi: 10.1177/088307380101601206. [DOI] [PubMed] [Google Scholar]
- Baloul L, Lafon M. Apoptosis and rabies virus neuroinvasion. Biochimie. 2003;85:777–788. doi: 10.1016/s0300-9084(03)00137-8. [DOI] [PubMed] [Google Scholar]
- Bi Z, Barna M, Komatsu T, Reiss CS. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol. 1995;69:6466–6472. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express Toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- Camelo S, Lafage M, Lafon M. Absence of the p55 Kd TNF-alpha receptor promotes survival in rabies virus acute encephalitis. J Neurovirol. 2000;6:507–518. doi: 10.3109/13550280009091951. [DOI] [PubMed] [Google Scholar]
- Chauhan VS, Furr SR, Sterka D, Jr, Marriott I, Grdzelishvili G. Vesicular stomatitis virus infects resident cells of the central nervous system and induces replication-dependent inflammatory responses. Virology. 2010 doi: 10.1016/j.virol.2010.01.025. In press. [DOI] [PubMed] [Google Scholar]
- Christian AY, Barna M, Bi Z, Reiss CS. Host immune response to vesicular stomatitis virus infection of the central nervous system in C57BL/6 mice. Viral Immunol. 1996;9:195–205. doi: 10.1089/vim.1996.9.195. [DOI] [PubMed] [Google Scholar]
- Curran JA, Kolakofsky D. Rescue of a Sendai virus DI genome by other parainfluenza viruses: implications for genome replication. Virology. 1991;182:168–176. doi: 10.1016/0042-6822(91)90660-4. [DOI] [PubMed] [Google Scholar]
- Das SC, Nayak D, Zhou Y, Pattnaik AK. Visualization of intracellular transport of vesicular stomatitis virus nucleocapsids in living cells. J Virol. 2006;80:6368–6377. doi: 10.1128/JVI.00211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–90. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Fishbein DB, Robinson LE. Rabies. N Engl J Med. 1993;329:1632–1638. doi: 10.1056/NEJM199311253292208. [DOI] [PubMed] [Google Scholar]
- Frei K, Malipiero UV, Leist TP, Zinkernagel RM, Schwab ME, Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol. 1989;19:689–694. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- Furr SR, Chauhan VS, Sterka D, Jr, Grdzelishvili V, Marriott I. Characterization of retinoic acid-inducible gene-I expression in primary murine glia following exposure to vesicular stomatitis virus. J Neurovirol. 2008;7:1–11. doi: 10.1080/13550280802337217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg RK. Subacute sclerosing panencephalitis. J Neurol. 2008;255:1861–1871. doi: 10.1007/s00415-008-0032-6. [DOI] [PubMed] [Google Scholar]
- Hiscott J. Convergence of the NF-kappaB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 2007;18:483–490. doi: 10.1016/j.cytogfr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Huneycutt BS, Bi Z, Aoki CJ, Reiss CS. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J Virol. 1993;67:6698–6706. doi: 10.1128/jvi.67.11.6698-6706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogai S, Radotra BD, Banerjee AK. Immunohistochemical study of human rabies. Neuropathology. 2000;20:197–203. doi: 10.1046/j.1440-1789.2000.00332.x. [DOI] [PubMed] [Google Scholar]
- Juntrakul S, Ruangvejvorachai P, Shuangshoti S, Wacharapluesadee S, Hemachudha T. Mechanisms of escape phenomenon of spinal cord and brainstem in human rabies. BMC Infect Dis. 2005;16:104. doi: 10.1186/1471-2334-5-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Kato H, Sato S, Takahashi K, Coban C, Yamamoto M, Uematsu S, Ishii KJ, Takeuchi O, Akira S. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med. 2006;203:1795–1803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AK, Davies HD, Hon KL. Rabies: epidemiology, pathogenesis, and prophylaxis. Adv Ther. 2007;24:1340–1347. doi: 10.1007/BF02877781. [DOI] [PubMed] [Google Scholar]
- Lui P, Li K, Garofalo RP, Brasier AR. Respiratory syncytial virus induces RelA release from cytoplasmic 100-kDa NF-kappa B2 complexes via a novel retinoic acid-inducible gene-I NF-kappa B-inducing kinase signaling pathway. J Biol Chem. 2008;283:23169–23178. doi: 10.1074/jbc.M802729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh B, Kristensson K, Norrby E. Selective infections of olfactory and respiratory epithelium by vesicular stomatitis and Sendai viruses. Neuropathol Appl Neurobiol. 1987;13:111–122. doi: 10.1111/j.1365-2990.1987.tb00175.x. [DOI] [PubMed] [Google Scholar]
- Lundh B. Spread of vesicular stomatitis virus along the visual pathways after retinal infection in the mouse. Acta Neuropathol. 1990;79:395–401. doi: 10.1007/BF00308715. [DOI] [PubMed] [Google Scholar]
- Marquette C, Van Dam AM, Ceccaldi PE, Weber P, Haour F, Tsiang H. Induction of immunoreactive interleukin-1 beta and tumor necrosis factor-alpha in the brains of rabies virus infected rats. J Neuroimmunol. 1996;68:45–51. doi: 10.1016/0165-5728(96)00056-2. [DOI] [PubMed] [Google Scholar]
- McQuaid S, McMahon J, Herron B, Cosby SL. Apoptosis in measles virus-infected human central nervous system tissues. Neuropathol Appl Neurobiol. 1997;23:218–224. [PubMed] [Google Scholar]
- Meylan E, Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol Cell. 2006;22:561–569. doi: 10.1016/j.molcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Harter DH, Hsu KC. Neuropathological and immunofluorescence studies of experimental vesicular stomatitis virus encephalitis in mice. J Neuropathol Exp Neurol. 1971;30:266–277. doi: 10.1097/00005072-197104000-00008. [DOI] [PubMed] [Google Scholar]
- Nuovo GJ, Defaria DL, Chanona-Vilchi JG, Zhang Y. Molecular detection of rabies encephalitis and correlation with cytokine expression. Mod Pathol. 2005;18:62–67. doi: 10.1038/modpathol.3800274. [DOI] [PubMed] [Google Scholar]
- Phares TW, Kean RB, Mikheeva T, Hooper DC. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol. 2006;176:7666–7675. doi: 10.4049/jimmunol.176.12.7666. [DOI] [PubMed] [Google Scholar]
- Pichlmair A, Schulz O, Tan CP, Näslund TI, Liljeström P, Weber F, Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Pringle CR. The order Mononegavirales-current status. Arch Virol. 1997;142:2321–2326. [PubMed] [Google Scholar]
- Ravi V, Parida S, Desai A, Chandramuki A, Gourie-Devi M, Grau GE. Correlation of tumor necrosis factor levels in the serum and cerebrospinal fluid with clinical outcome in Japanese encephalitis patients. J Med Virol. 1997;51:132–136. [PubMed] [Google Scholar]
- Ray NB, Power C, Lynch WP, Ewalt LC, Lodmell DL. Rabies viruses infect primary cultures of murine, feline, and human microglia and astrocytes. Arch Virol. 1997;142:1011–1019. doi: 10.1007/s007050050136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehwinkel J, Tan CP, Goubau D, Schulz O, Pichlmair A, Bier K, Robb N, Vreede F, Barclay W, Fodor E, Reis e Sousa C. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell. 2010;140:397–408. doi: 10.1016/j.cell.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Rehwinkel J, Reis e Sousa C. RIGorous detection: exposing virus through RNA sensing. Science. 2010;327:284–286. doi: 10.1126/science.1185068. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Hester LD, Nye JS, Connors K, Snyder SH. Human cortical neuronal cell line: establishment from a patient with unilateral megalencephaly. Science. 1990;248:603–605. doi: 10.1126/science.1692158. [DOI] [PubMed] [Google Scholar]
- Saito T, Gale M., Jr. Differential recognition of double-stranded RNA by RIG-I-like receptors in antiviral immunity. J Exp Med. 2008;205:1523–1527. doi: 10.1084/jem.20081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal BS, King DJ, Sellers HS. The avian response to Newcastle disease virus. Dev Comp Immunol. 2000;24:257–268. doi: 10.1016/s0145-305x(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Solanki A, Radotra BD, Vasishta RK. Correlation of cytokine expression with rabies virus distribution in rabies encephalitis. J Neuroimmunol. 2009 doi: 10.1016/j.jneuroim.2009.09.019. In press. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT. Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol. 1998;152:74–87. doi: 10.1006/exnr.1998.6835. [DOI] [PubMed] [Google Scholar]
- Takahasi K, Yoneyama M, Nishihori T, Hirai R, Kumeta H, Narita R, Gale M, Jr, Inagaki F, Fujita T. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol Cell. 2008;29:428–440. doi: 10.1016/j.molcel.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol Rev. 2007;220:214–224. doi: 10.1111/j.1600-065X.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- Wong KT, Robertson T, Ong BB, Chong JW, Yaiw KC, Wang LF, Ansford AJ, Tannenberg A. Human Hendra virus infection causes acute and relapsing encephalitis. Neuropathol Appl Neurobiol. 2009;35:296–305. doi: 10.1111/j.1365-2990.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. RIG-I family RNA helicases: cytoplasmic sensor for antiviral innate immunity. Cytokine Growth Factor Rev. 2007;18:545–551. doi: 10.1016/j.cytogfr.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Imaizumi T, Lee SJ, Tanji K, Sakaki H, Matsumiya T, Ishikawa A, Taima K, Yuzawa E, Mori F, Wakabayashi K, Kimura H, Satoh K. Retinoic acid-inducible gene-I mediates RANTES/CCL5 expression in U373MG human astrocytoma cells stimulated with double-stranded RNA. Neurosci Res. 2007;58:199–206. doi: 10.1016/j.neures.2007.02.017. [DOI] [PubMed] [Google Scholar]