Abstract
Background
The rate-limiting step that determines the dominant time constant (τD) of mammalian rod photoresponse recovery is the deactivation of the active phosphodiesterase (PDE6). Physiologically relevant Ca2+-dependent mechanisms that would affect the PDE inactivation have not been identified. However, recently it has been shown that τD is modulated by background light in mouse rods.
Methodology/Principal Findings
We used ex vivo ERG technique to record pharmacologically isolated photoreceptor responses (fast PIII component). We show a novel static effect of calcium on mouse rod phototransduction: Ca2+ shortens the dominant time constant (τD) of saturated photoresponse recovery, i.e., when extracellular free Ca2+ is decreased from 1 mM to ∼25 nM, the τD is reversibly increased ∼1.5–2-fold.
Conclusions
We conclude that the increase in τD during low Ca2+ treatment is not due to increased [cGMP], increased [Na+] or decreased [ATP] in rod outer segment (ROS). Also it cannot be due to protein translocation mechanisms. We suggest that a Ca2+-dependent mechanism controls the life time of active PDE.
Introduction
In all eukaryotic cells a very steep gradient of Ca2+ exists across the plasma membrane. The intracellular calcium concentration ([Ca2+]i) is generally in the order of 100 nM while the extracellular Ca2+ level ([Ca2+]o) is above 1 mM. The steady-state concentration difference is set by delicate control of calcium influx through various types of calcium channels and by the extrusion of calcium ions through Na+/Ca2+ exchange (the Na+/Ca2+ exchangers, NCXs, or the Na+/Ca2+-K+ exchangers, NCKXs) and Ca2+-ATPases. This kind of dynamic control principle ensures that all the changes in the intracellular [Ca2+] are transient in nature, no matter whether the release of calcium is from intracellular stores or from the extracellular space, and this allows the wide use of calcium ion as internal transmitter in various types of signalling mechanisms.
In vertebrate photoreceptors in darkness, calcium enters the outer segment through cGMP-gated channels and is extruded by the NCKX. Light closes cGMP-gated channels, which reduces the influx of calcium while the calcium extrusion by the NCKX is continued, leading to a decrease in the intracellular calcium concentration. These changes in [Ca2+]i during photoresponses are used as negative feedback signals in phototransduction. In mouse rods [Ca2+]i is ∼250 nM in darkness and declines to ∼20–50 nM during bright illumination [1], [2]. This decrease in Ca2+ accelerates both the synthesis of cGMP [3], [4] and the inactivation of activated rhodopsin, R* [5], [6]. Further, in many photoreceptors, especially in most cones and in amphibian rods, the reduction of [Ca2+]i is known to lower the affinity of the cGMP-gated channels to cGMP [7].
In addition to the dynamic effects described above, calcium ions also may have static effects on signalling. By “static effects” of calcium we refer to calcium-dependent mechanisms that participate in setting the operating point of a system (here phototransduction machinery) while moderate changes in the feedback signal (here [Ca2+]i) do not affect the output signal through the mechanisms of the static effect. In this study we demonstrate a novel effect of calcium on mouse rod phototransduction: calcium shortens the dominant time constant of saturated photoresponse recovery (τD). Our data suggests that in mouse rods the intracellular calcium concentration needed to generate this effect may be below the values of [Ca2+]i attained in bright light. Hence we call this effect of calcium on τD static. We show that τD is 1.5–2 times larger in very low free [Ca2+]o (25 nM) compared to that in physiological [Ca2+]o (1 mM). The effect of low external calcium was fully reversible, since the physiological value of τD was completely restored upon return to normal calcium even after long (>1 hour) exposures to low Ca2+. We show that the increase of τD in low Ca2+ was not due to high [cGMP]i that results from the extensive activation of guanylate cyclase (GC) in low Ca2+. Also we show that neither highly increased ATP consumption nor increased intracellular sodium concentration can explain the increase of τD in low Ca2+. We suggest that in mouse rods there is a Ca2+ -dependent mechanism that controls the life-time of active PDE, and that a certain minimum level of intracellular calcium is needed in setting the physiological value of τD.
Results
Determination of Dominant Time Constant by ERG from Isolated Retina Preparation
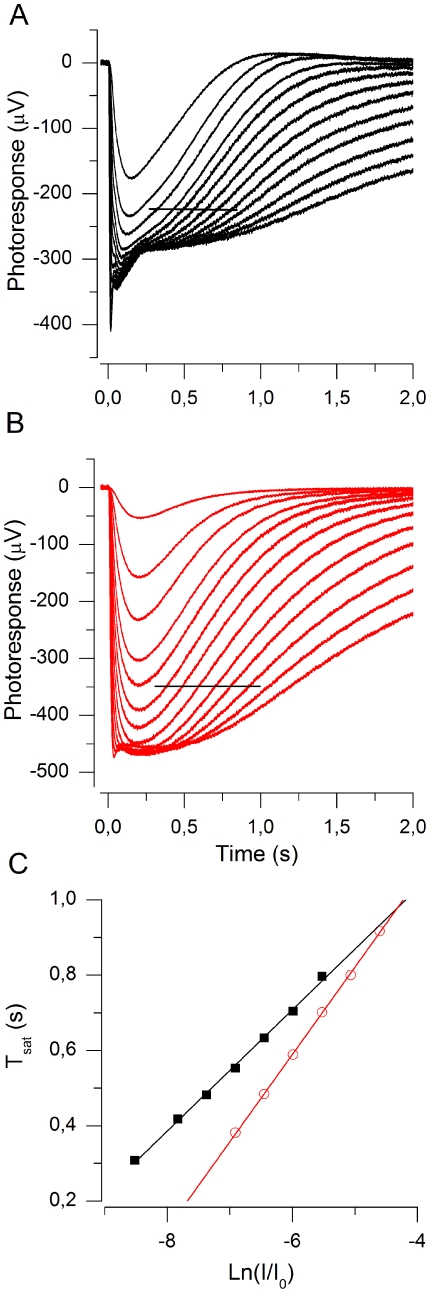
Fig. 1A shows a photoresponse family recorded by the ERG technique across an isolated dark-adapted mouse retina perfused with bicarbonate-buffered (see Methods) Ringer solution at 37°C, when the ON-bipolar cell activity was suppressed by 50 µM DL-AP4. The saturated responses reflect the activity of both rods and cones [8], but the lower sensitivity and faster photoresponse kinetics of cones lead to a faster turn-off of the cone responses compared to rods. Further, the cone population is only 3% of all photoreceptors, leading to a low cone contribution to the ERG signal. Therefore the recovery phase of saturated ERG responses can be expected to reflect pure rod activity, as suggested by the common plateau level during rod saturation. The initial recovery of near-saturated and saturated responses closely follows the same slope with stimulus strengths covering the range from ca. 100 to 3500 Rh*, very similarly to those obtained by suction pipette recording [9]. Fig. 1C plots the times from flash (Tsat) to the moment when the responses had recovered 20% from saturation (i.e. from the plateau level) as a function of stimulus strength in logarithmic scale. A linear regression line fitted to the data points (black squares) gives a dominant time constant τD of 161 ms (mean ± SEM: 166±12 ms, n = 4) which is comparable to τD-values obtained for mouse rods by the double-flash ERG method in vivo [10] and by suction electrode recording from single rods, τD = ∼0.2 s [6], [11], [12].
Figure 1. Determination of τD by the ERG method from isolated mouse retina.
Recordings made from a retina perfused with bicarbonate-buffered Ringer at 37°C. (A) Family of responses to flashes of light in normal Ringer with 1 mM [Ca2+]. Flash strengths: 28, 87, 174, 348, 551, 874, 1390, 2200, 3480, 5510, 8740, 13900, 22000 Rh*. Data points used to determine τD ranged from 174 to 3480 Rh* (indicated by the horizontal line). (B) Response family in solution with 25 nM [Ca2+]free. Flash strengths were the same as in A and data points used to determine τD ranged from 874 to 8740 Rh* (indicated by the horizontal line). (C) Times for photoresponses to recover 20% (Tsat) from saturation plotted as a function of the natural logarithm of normalized flash strength. Data points from A (▪, normal Ringer with 1 mM Ca2+) and B (red ○, low-Ca2+ solution, 25 nM Ca2+). τD was 161and 231 ms in normal Ringer and in low Ca2+, respectively.
The Dominant Time Constant of Photoresponse Recovery Increases in Low Ca2+
Fig. 1B shows a corresponding response family after most of the extracellular calcium ions were removed by exchanging the perfusate to EGTA-buffered low Ca2+ solution with ∼25 nM free [Ca2+] (see Methods). When the retina was exposed to low Ca2+ solution, the saturated response amplitude showed a large initial increase (up to ∼4-fold). However, at 37°C these large responses could not be maintained long, and the saturated response amplitudes dropped quite fast (within ∼10 min) to the values ∼50–100% larger compared to normal Ca2+. After this the responses continued to decrease more slowly, being only somewhat larger than in normal Ca2+ after about 30 minutes exposure to low Ca2+. The responses in panel B were recorded between 22–32 minutes after the solution change to low Ca2+. Fig. 1C illustrates the Tsat data from these responses (red circles), showing that τD increased from 161 in normal Ringer to 231 ms in low Ca2+. A similar increase was observed in all four similar experiments at 37°C (166±12 in 1 mM Ca2+ to 251±15 ms in 25 nM Ca2+, n = 4). This novel Ca2+-dependent effect on τD is investigated in the following sections.
Lowered Temperature Can Retain Large Responses in Low Calcium
The isolated mouse retina is a robust preparation that allows long-lasting treatments and experiments not easily attained with e.g. suction pipette recordings. However, the low external calcium concentration we used exerts a heavy metabolic load on photoreceptors: in these conditions intracellular calcium should go low enough to fully activate the calcium-controlled feedback mechanisms (guanylate cyclase through guanylate cyclase activating protein, GCAP, and rhodopsin through recoverin) in the rod outer segment even in darkness. The maximal guanylate cyclase activity leads to a substantial increase in [cGMP], and thus in the number of open cGMP-gated channels. Further, low external Ca2+ directly increases the single channel conductance of cGMP-gated channels [13], [14]. These metabolic factors together can most likely explain our observation that a steady-state could not be achieved in low calcium at 37°C, indicated by the continuous decay of saturated response amplitude. This decay, however, was largely absent at 25°C in HEPES-buffered Ringer. A further advantage of using HEPES-Ringer at 25°C is that, contrary to the bicarbonate-buffered Ringer at 37°C, the inner retinal contributions to the ERG can be very effectively pharmacologically removed (by 2 mM aspartate), as suggested by the simpler photoresponse shapes in Fig. 2A and C compared to the responses in bicarbonate-buffered Ringer at 37°C (Fig. 1A). We therefore chose to conduct the following experiments, tailored to investigate the Ca2+-dependent increase of τD, in HEPES-buffered Ringer at 25°C.
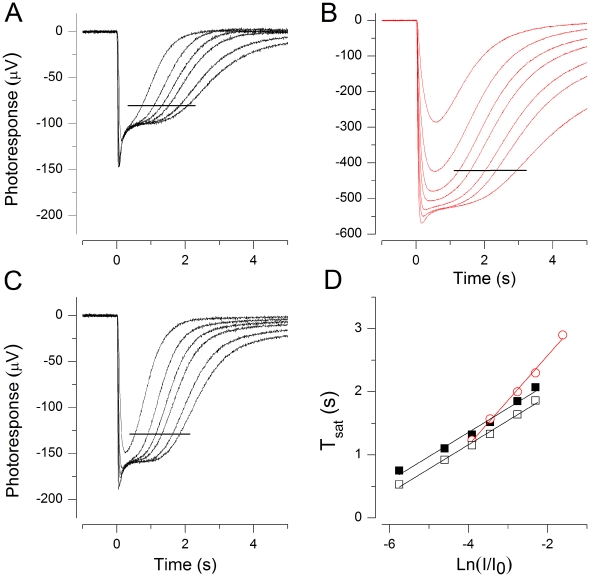
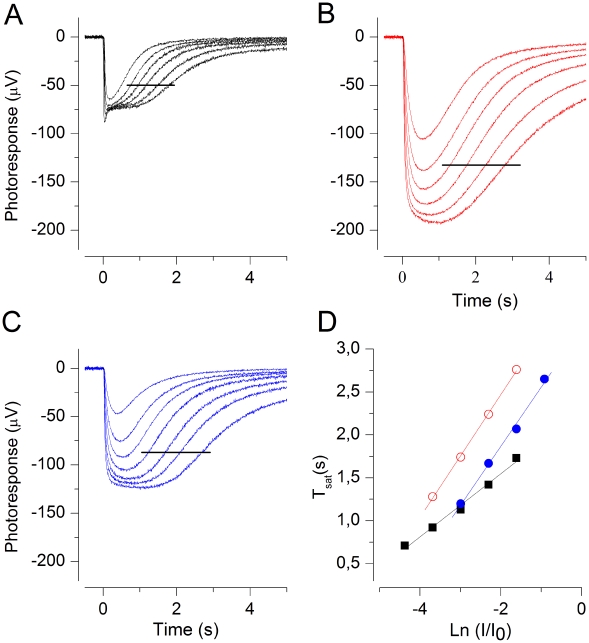
Figure 2. Dominant time constant τD is reversibly increased in low Ca2+.
(A–C) Mouse ERG flash responses at 25°C in normal Ringer (A and C) and in 25 nM free Ca2+ (B). Flash strengths were 173, 548, 1100, 1730, 3460, 5480, (11 000, only in B) Rh*. Data used for τD determinations are indicated by horizontal lines. (D) Pepperberg plots extracted from A–C: τD was 383 ms before (▪) and 382 ms after (□) low Ca2+ (25 nM) exposure. In low Ca2+ τD was 710 ms (red ○).
Fig. 2 shows data from a typical experiment at 25°C. When the retina was exposed to low Ca2+ solution, the saturated response amplitude showed a very large initial increase that was followed by a decrease to a steady-state value ca. 5 times larger than the saturated photoresponse in 1 mM Ca2+ (measured at the plateau level). In other experiments the steady-state increase varied between 2 and 5-fold being 2.6-fold on average (n = 12). Despite of this large increase in the response amplitudes, the recordings in low Ca2+ were extremely stable. After ca. 10 minutes needed to reach a steady state in low Ca2+, the response amplitudes and kinetics remained virtually unchanged for at least 1 hour in most of the experiments.
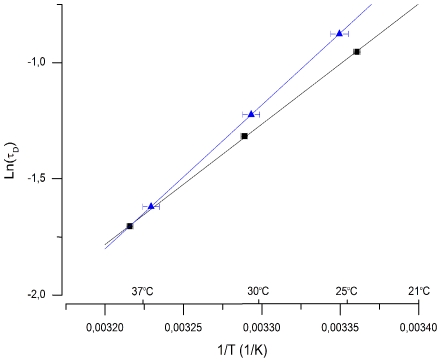
The semi-saturated and saturated photoresponses of Fig. 2 to identical sets of 20 ms light flashes were recorded in 1 mM external calcium (A; before low Ca2+ exposure and C; after low Ca2+ exposure) and in 25 nM [Ca2+]free (B). The collected Tsat data of these responses is presented in Fig. 2D. It shows that τD increased from 380 ms in 1 mM calcium to 710 ms in 25 nM Ca2+. The effects of low Ca2+ treatment on photoresponse kinetics, sensitivity and τD were always almost fully reversible, indicating that low Ca2+ treatment as such was not harmful to rods. As evident also in Fig. 2, the plateau amplitude often remained at a slightly larger level after the return to normal Ringer as compared to that before low-Ca2+ treatment. In this experiment the return to normal Ringer restored τD to its original value of 380 ms. In 12 experiments τD was (mean ± SEM) 342±28 ms in 1 mM calcium and 662±50 ms in 25 nM Ca2+, corresponding to 94% increase in τD when switched to low Ca2+. This value is somewhat larger than the observed 51% increase in τD at 37°C. To test whether the reason for this difference is that the molecular identity of the rate-limiting process of saturated photoresponse recovery changes (e.g. from PDE deactivation to rhodopsin deactivation) between 25°C and 37°C, we determined τD at three different temperatures (24–38°C) from two retinas in HEPES buffered Ringer. If the identity of the rate-limiting mechanism were different at 25°C and 37°C, and if these mechanisms had different activation energies, the slope of the Arrhenius plot would change between 25°C and 37°C. Fig. 3 shows that the temperature dependence followed accurately the simple Arrhenius equation (see Methods), suggesting that a single and same process is rate-limiting both at 25°C and 37°C. The activation energy of the process was about 47±4 kJ/mol (mean ± SEM, n = 2).
Figure 3. Temperature dependence of τD.
τD determined from the mouse rod response families at three different temperatures from two retinas. The natural logarithm of τD is plotted as a function of T−1. Ea was determined from the slope of the fitted straight lines (see Methods), giving 51.4 (blue ▴) and 43.0 (▪) kJ/mol, respectively.
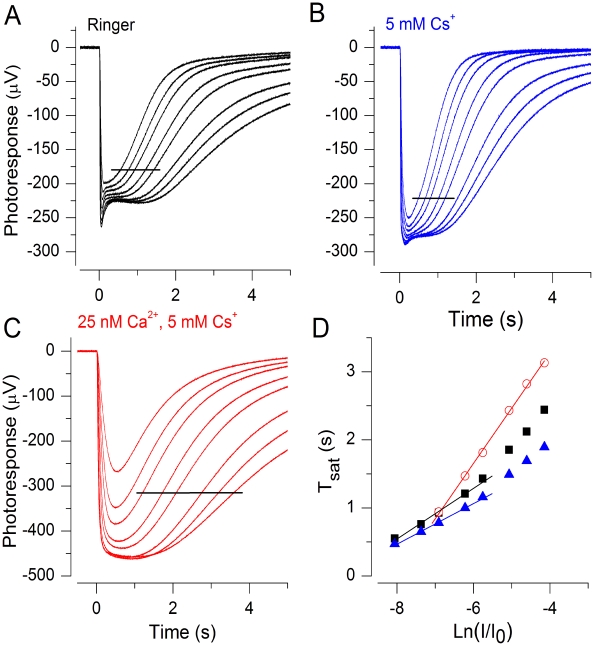
The Effect of Cs+ on τD
The saturated rod responses include a fast negative peak (see Figs. 1A, 2A, C), the nose, which originates from the action of h and probably Kx channels in the rod inner segment [15]. This nose disappears in low extracellular Ca2+ (Figs. 1B and 2B), revealing a small cone component in the early phase of the saturated responses. To confirm that the increase in τD in low Ca2+ could not be due to the disappearance of the voltage-dependent nose component, we applied 3–5 mM Cs+, a blocker of hyperpolarization-activated h channels, which removes the nose-like behaviour in the saturated rod responses [15]. Fig. 4 shows the result from one such experiment. The introduction of 5 mM Cs+ accelerated the recovery rate of semi-saturated and saturated responses. This acceleration was qualitatively similar to that found in toad rod membrane voltage responses when 2 mM CsCl was introduced [16]. The accelerated photoresponse decay was accompanied by a decrease in τD from 370 ms to 300 ms with the addition of 5 mM Cs+. Similar or smaller decreases in τD were observed in the two other experiments of this kind. The reason for this small decrease in τD is not known, but the results convincingly show, that disappearance of h channel activity cannot be the reason for the increase in τD when switched to low Ca2+ solution. Figs. 4C and D show that when Ca2+ is lowered to 25 nM in the presence of Cs+, over 2-fold increase in τD is evident. This further confirms that although the voltage-dependent h channels in the rod inner segment might shape the saturated ERG photoresponses, their effect cannot explain the ∼2-fold increase in τD when [Ca2+]o is lowered from 1 mM to 25 nM.
Figure 4. Effect of Cs+ on τD.
(A) Response family to flashes of light in HEPES-buffered Ringer containing 2 mM aspartate. Flash strengths: 140, 278, 441, 880, 1394, 2780, 4400, 7000 Rh*. (B) Response family to identical set of flashes as in A from the same retina after addition of 5 mM Cs+ to perfusion. (C) Identical stimulus set as in A and B when retina was exposed to low 25 nM free Ca2+ with 5 mM Cs+ present. Horizontal lines indicate the data points used in D. (D) Determination of τD from A (▪), B (blue ▴) and C (red ○).τD was 370, 300, 812 ms in Ringer, 5 mM Cs+ and in low Ca2+ with 5 mM Cs+, respectively.
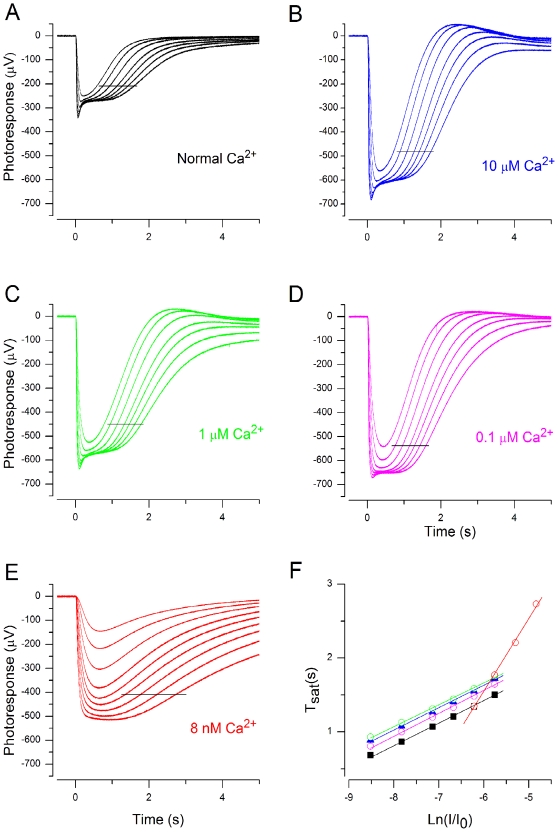
Calcium Concentration Dependence of τD
We did three experiments in which we studied the dependence of τD on external [Ca2+]. In these experiments no lengthening of τD was observed until the free extracellular [Ca2] was reduced below 1 µM, although a large increase in saturated photoresponse amplitude was present already at 100 µM [Ca2+]o. Fig. 5 illustrates one such experiment in which τD was ∼300 ms in 1 mM Ca2+ and did not change significantly until the [Ca2+]free in the perfusate was lowered to ∼10 nM. In the two other experiments τD was increased slightly (22 and 34%) at 100 nM free Ca2+, while the τD values at 10 nM [Ca2+]free were 68 and 56% above those in normal Ringer. Although we do not know how much the declines in [Ca2+]o reduce the intracellular calcium concentration at each [Ca2+]o, the large circulating current even at the lowest external calcium concentrations used in this study suggests, that there remains a substantial inward sodium gradient across the outer segment plasma membrane, that can drive the Na+/Ca2+-K+ exchanger to keep the intracellular calcium level below the [Ca2+]o. Therefore it is probable that the ∼two-fold increase in τD takes place only at intracellular calcium levels below those (20–50 nM, [1], [2]) attained in bright light.
Figure 5. Effect of external [Ca2+] on τD.
Photoresponses at 1 mM (A), 10 µM (B), 1 µM (C), 0.1 µM (D), and 8 nM (E) free [Ca2+]out. Flash strengths: 87, 174, 348, 694, 1100, 1744, 2764, (4380, 6942, only in E) Rh*. (F) Dominant time constant determination from A–E. Data points used were between 174–2764 in A–D, and 1744–6942 Rh* in E, indicated by black horizontal lines in A–E. τD was 306, 303, 297, 311 and 983 ms in A, B, C, D and E, respectively.
Increased cGMP Concentration Cannot Explain the Longer τD in Low Ca2+
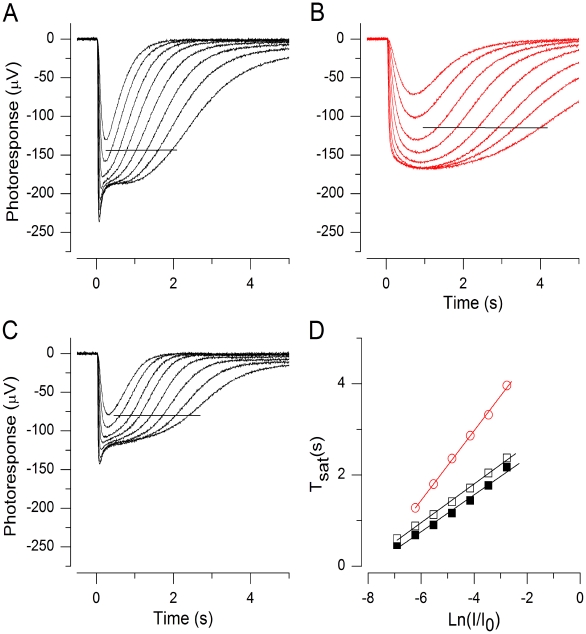
One of the major consequences of lowered external calcium is the increase in [cGMP] in the ROS. To investigate whether elevated [cGMP] could explain the increase in τD in low Ca2+, we used background lights to reduce [cGMP] during low Ca2+ exposures. Fig. 6 illustrates one such experiment. Panel A shows a response family recorded in 1 mM calcium in darkness. Then the retina was exposed to 25 nM external Ca2+, and a response family was recorded after the responses had stabilized (panel B). Finally, response families were recorded under backgrounds of increasing intensity (range from 20 to 6400 Rh*/s per rod), when a steady-state had been achieved in each background. Fig. 6C presents a response family recorded in low Ca2+ under a background producing 640 Rh*/s per rod. Panel D collects the results of panels A–C, and for this retina τD was 360 ms in 1 mM Ca2+, increased to 660 ms in 25 nM Ca2+, and was slightly shorter, 600 ms, in 25 nM Ca2+ under the background. In this experiment response families were recorded under backgrounds producing 20, 64, 200, 640, 2000 and 6400 Rh* s−1 per rod. The corresponding τD values were 690, 680, 780, 600, 580 and 540 ms, respectively. Under the strongest background (6400 Rh* s−1 per rod) the saturated photoresponse amplitude was already smaller than in normal Ringer in darkness. Since lowered calcium is known to strongly increase the conductance of cGMP-gated channels in rods [13], [14], it is expected that the [cGMP] was much smaller under the strongest backgrounds in low Ca2+ compared to that in normal Ringer without background. With backgrounds exceeding 640 Rh* s−1 τD shortened somewhat with increasing background intensity, but remained significantly larger compared to that in normal Ca2+. These results suggest that the increase in τD during low Ca2+ exposures is not due to a direct or an indirect effect of elevated [cGMP].
Figure 6. Background light does not prevent τD increase in low Ca2+.
(A) Photoresponse family recorded in normal Ringer. Flash strengths: 425, 848, 1693, 3378, 6739, 13500 Rh*. Data points used to determine τD ranged from 848 to 13500 Rh* (indicated by the horizontal line). (B) Photoresponse family in 25 nM free [Ca2+]o. Flash strengths same as in A. Data points used to determine τD indicated by the horizontal line. (C) Photoresponse family in 25 nM free [Ca2+]o during background illumination producing 640 Rh* s−1. Flash strengths: 425, 848, 1693, 3378, 6739, 13 500, 26800 Rh*. Data points used to determine τD ranged from 3378 to 26800 Rh* (indicated by the horizontal line). (D) Pepperberg plots extracted from A–C. τD increased from 360 ms (▪) to 660 ms (red ○) when retina was switched from normal Ringer to low Ca2+ solution. Background light producing 640 Rh* s−1 decreased τD to 600 ms (blue  ).
).
Our second approach to investigate the possible effect of elevated cGMP concentration on τD was to increase [cGMP] concentration in the presence of normal Ringer by inhibiting PDE with IBMX. IBMX is known to boost photoresponse amplitudes and decelerate photoresponse kinetics as low Ca2+ treatment does, but to our knowledge, the effect of IBMX on τD has not been investigated before. We did two experiments in which we used two concentrations of IBMX, 30 and 100 µM. Instead of increasing, IBMX decreased τD slightly, being 270 ms in normal Ringer, and 260 and 250 ms at 30 and 100 µM IBMX, respectively. The same experimental protocol was repeated with another retina with similar results. Our results indicate that the level of steady-state [cGMP] is not important in determining τD.
Increased [Na+]i or Decreased [ATP] in Outer Segment Cannot Explain the Longer τD in Low Ca2+
In dark-adapted mouse rods the major metabolic load in regard to ATP consumption comes from the extrusion of Na+ ions flowing into the ROS through the cGMP-gated channels [17]. In low Ca2+ this load is heavily raised: First, the guanylate cyclase is activated, leading to an increase in [cGMP] and thereby to an excess opening of cGMP-gated channels, and secondly, the conductance of single cGMP-gated channels is increased two- to threefold in low Ca2+ [13]. Consistently with this, Winkler and Riley have shown that in rat rods the Na+/K+ ATPase activity increases about 16-fold as the rat retina was switched from 2 mM Ca2+ to Ca2+-free media [18]. Thus, it seemed possible that in low Ca2+ the high ATP consumption might drop the ATP concentration in rods to a level low enough to slow down e.g. the phosphorylation processes involved in the phototransduction machinery, and thereby lead to an increase in τD. Further, the increased cGMP-gated conductance in lowered external Ca2+ most likely leads to an increase in [Na+]i that is assumed somehow to impair phototransduction [19]–[21].
To test the possible effects of increased ATP consumption and increased [Na+]i on τD, we decreased the external sodium concentration from 140 mM to 8 mM in our low Ca2+ solution by replacing sodium with membrane impermeable choline. Fig. 7 shows the result. A control response family recorded in our normal Ringer is shown in panel A, while a corresponding response family recorded in 25 nM Ca2+ and 8 mM Na+ is shown in panel B. In low Ca2+-low Na+ the response amplitudes were not much reduced compared to those recorded in our normal Ringer, in spite of the fact that a large fraction of the current carriers through the cGMP-gated channels were removed. The responses remained very stable in these conditions. In this experiment τD was 400 ms in normal Ringer, increased to 760 ms in low Ca2+-low Na+ solution, and returned to 420 ms when Ringer was restored. Similar results were obtained in two other experiments conducted with the same protocol. The average increase in τD was (mean ± SEM) 174±44% when the perfusate was changed from our normal Ringer to low Ca2+-low Na+ Ringer. These results show that even in conditions where sodium accumulation inside the rods and the excessive ATP consumption are prevented, the exposure of rods to 25 nM Ca2+ results in a similar (or larger) increase in τD as observed in the presence of normal Na+.
Figure 7. Lowered ATP/GTP and/or increased intracellular Na+ concentration does not explain the increase of τD in low Ca2+.
(A) Response family in normal Ringer. Flash strengths: 52, 104, 207, 413, 824, 1643, 3280, 6540 Rh*. (B) Response family in solutions containing low 25 nM Ca2+ and low 8 mM Na+. Same flash strengths as in A. (C) Response family in normal Ringer after low Ca2+/Na+ exposure. (D) Pepperperg plots from A–C, data points indicated by horizontal line in A–C. τD was 400 ms before (▪) and 420 ms after (□) the low Ca2+/low Na+ exposure. It was increased to 760 ms in low Ca2+/low Na+ solution (red ○).
Discussion
The Use of ERG Fast PIII Component in τD Determination
The canonical way to determine the dominant time constant of saturated photoresponse recovery is to suck either the outer or the inner segment of a photoreceptor into a tight-fitting suction pipette, and record the recovery kinetics of photoresponses to saturating light flashes of varying strength [22], [23]. To allow long and stable recordings under metabolically stressing conditions, and easy manipulation of the ionic environment around the entire photoreceptor cells, we instead used the isolated retina ERG technique for mouse rod τD-determinations. The use of transretinal ERG for this purpose, however, brings about several possible sources of error that have to be carefully considered. Firstly, the transretinal ERG contains components from several types of neurons that temporally overlap the rod photoresponse recovery phase. These components should be removed by blocking all synaptic transmission from photoreceptors to bipolar and horizontal cells. In our low-Ca conditions the synaptic transmission was completely blocked presynaptically simply by the lack of calcium. In the presence of physiological external calcium the mGluR6 agonist L-AP4 effectively blocks signal transmission to ON-bipolar cells and thus completely removes the b-wave, leaving transmission to OFF-bipolar and horizontal cells intact. The ERG components from these latter cells are generally suppressed by the use of cocktails of different glutamate-receptor agonists and antagonists. Another approach is to perfuse the retina with the glutamate analog aspartate in mM concentrations to saturate all the glutamate binding sites in the various glutamate receptors. We observed that at 37°C in bicarbonate- and HEPES-buffered Ringer the combination of 50 µM DL-AP4 +10 µM NBQX was more effective than 2–5 mM aspartate in removing the second- and higher-order components, while at 25°C in HEPES-buffered Ringer both treatments were equally effective. Another robust component temporally overlapping with the recovery phase of fast PIII photoresponses is the glial component, or slow PIII, generated by the Müller cells in response to extracellular potassium changes due to photoreceptor activity. The slow PIII appears as a rounded component of same polarity as the fast PIII (see e.g. Fig. 1 in [24]) and can be removed by blocking the potassium channels of Müller cells by e.g. barium. To remove the slow PIII as completely as possible with minimally affecting the potassium channels in photoreceptors, we used high (10 mM) BaCl at the proximal side of the retina while perfusing the photoreceptor side with Ringer not including barium. With this approach it was possible to remove virtually all slow PIII at 25°C as indicated by the extending, horizontal plateau with increasing saturating stimulus strength. Under treatments described above the mouse fast PIII rod photoresponses look very much like the corresponding suction pipette current recordings from single mouse rods, and both recording methods give similar results for sensitivity and photoresponse kinetics. The major difference between these two methods is that in ERG the rate of photoresponse recovery may be modified by the voltage-dependent channels in the rod inner segment, while the suction pipette responses are not voltage-dependent in the physiological voltage range.
The determination of τD from saturated photoresponses by plotting the time photoreceptors remain in saturation as a function of logarithmic stimulus strength is, however, a very robust method. It is based on three key assumptions [22]: 1) Intracellular [cGMP] is solely determined by the rates of synthesis and hydrolysis of cGMP, and the slowest deactivation step after light stimulus is assumed to follow first-order kinetics. 2) Photoresponse recovery from saturation starts when [cGMP] has reached a certain criterion level specific to the experimental conditions. The criterion level of cGMP may be dissimilar in different experimental conditions, but it should not affect the τD value if the other conditions are met. 3) Guanylate cyclase is fully activated, i.e. constant, during the phase of photoresponse recovery that is used for τD determination. This condition is met when the intracellular calcium level has reached a value low enough to enable maximal guanylate cyclase activation by GCAPs, and the fulfilment of this condition is verified as the same slope of photoresponse recovery for responses to stimuli of different strengths when read at a certain amplitude criterion level. There is nothing in the assumptions that should make the “Pepperberg plots” voltage-dependent. This conclusion is supported e.g by the data of Baylor and Nunn (1986) [25]: When determined from their Fig. 2, the τD values are practically equivalent whether obtained from either the current or voltage responses simultaneously recorded from salamander rods. Therefore, transretinal ERG is expected to give the same τD values as the suction pipette method (or membrane potential recording) taken that components other than those of photoreceptor origin are carefully abolished. This is in agreement with our ERG data that gives τD = 166±12 ms (n = 4) in 1 mM external calcium at 37°C in the C57Bl/6N mice compared to τD = 168...246 ms obtained with single rod suction pipette recordings at the same temperature in darkness [6], [11], [12].
Mouse rod photoresponses appear to be sensitive to the Ringer composition perfusing the rods (see e.g. the supplemental figure in [26]). Therefore the reason for the observation that our ERG gives slightly smaller τD values than suction pipette recordings may lie in the minor differences in the perfusates used in these experiments, especially in the differences in pH buffers. In this study we observed that our (ERG) τD values were somewhat larger (τD = 194±5 ms, n = 4) at 37°C when determined in HEPES-buffered Ringer compared to those obtained in the presence of bicarbonate-buffered Ringer (τD = 166±12 ms, n = 4). The comparison of these values to those obtained with suction pipette recordings is not straightforward, since in most cases the filling solution of the suction pipette, where the rod outer segment is sucked in, has been buffered with HEPES while the solution perfusing the rod inner segment was buffered with bicarbonate. The τD values obtained by us with ERG and literature values obtained by suction pipette recordings match so closely that there seems to be no reason to believe that they might reflect different rate-limiting mechanisms.
Effects of Low Ca2+ on Rods
Reducing intracellular Ca2+ to nM range has several effects on the phototransduction machinery in rod outer segments that potentially might cause the observed increase in τD in low calcium. The synthesis of cGMP by GC is strongly accelerated [3], [27], and the single channel conductance of cGMP-gated channels is increased [13], [14], [27]. Also the affinity of the cGMP-gated channel to cGMP is modulated by calcium [7], [28], but this effect seems to be negligible in mammalian rods [29]. These actions of low Ca2+ induce 1) elevation of intracellular [cGMP], 2) accumulation of Na+ inside rod outer segments [19]–[21] as well as 3) strongly increased ATP consumption [30], possibly leading to lowered intracellular ATP and GTP levels. In this study we focused ourselves to investigate whether these effects could explain the increased τD in low Ca2+.
The occupancy of the non-catalytic cGMP-binding sites of rod phosphodiesterase (PDE6) has been shown to control the GTPase activity of PDE, and the termination of PDE activity is slower when the non-catalytic binding sites are occupied and maximal when the sites are empty [31], [32]. The elevated [cGMP] in low calcium could, in principle, lead to full occupation of the non-catalytic binding sites and by that means increase τD. In this study, however, we showed that a significant increase of τD was evident also when [cGMP]i was strongly lowered (most probably well below the cGMP level in darkness in 1 mM external calcium) by background light during low Ca2+ exposure. This leads us to conclude that the occupation of PDE non-catalytic binding sites do not explain the increase of τD in low Ca2+.
Low Ca2+ supports very high inward Na+ current in ROS through cGMP channels. In toad the cGMP current increased transiently over 20-fold when switching from 1 mM Ca2+ to 0 Ca2+ plus 2 mM EGTA, but this dark current could not be maintained [19]. This suggests that Na+/K+ ATPase cannot maintain sodium gradient across plasma membrane in the presence of very high inward Na+ current, and that Na+ most probably is accumulated into the cell. Our experiments, however, suggest that elevated intracellular [Na+] itself does not increase τD in our low Ca2+ conditions. Firstly, the increase in τD is evident also in low Ca2+ Ringer containing only 8 mM Na+. Secondly, we did one experiment (not shown) in which we switched background light on just before introduction of low Ca2+ in order to suppress excessive cGMP-gated current. The intensity of the background light was chosen to keep the saturated response amplitude in low Ca2+ at a level approximately the same as in normal Ca2+ in darkness. Still τD increased in low Ca2+ (with background light) 67% from that in normal Ringer without background.
It has been calculated that the maintenance of ion gradients dominates the ATP consumption in darkness for mammalian rods even in normal Ca2+ [17]. Thus, the large circulating current in low Ca2+ must exert a very high metabolic load on mouse rods. In accordance with this, Winkler and Riley [18] have shown 16-fold increase in Na+/K+ ATPase activity in rat rods when exposed to low Ca2+. The high expenditure of ATP in low calcium may lead to the deficiency of ATP, which might retard phosphorylation processes, especially phosphorylation of active rhodopsin, and thus lengthen the lifetime of R*. Our experiments where we prevented the increase in the circulating current in low Ca2+ with 1) by lowering Na+ by more than one log unit or by 2) application of background light suggest that the larger τD in low Ca2+ is not due to the deficiency of ATP in mouse ROS.
One possible explanation for the increase in τD in low calcium could be the retardation of diffusion of the membrane proteins involved in phototransduction. This explanation, however, seems quite improbable: As a first approximation, instead of retardation one might expect acceleration of membrane protein diffusion, since the binding of the divalent calcium to the negative surface charges of disc membranes should rather increase than decrease the order parameter of the membranes, and thereby enhance the diffusion constants of the membrane proteins. Further evidence against this explanation comes from experiments on amphibian rods, where lowering the extracellular calcium level to nM range does not affect the diffusion constant of rhodopsin (Dr. V. Govardovskii, personal communication).
One further possibility for explaining the elevated τD in low Ca2+ is that the removal of calcium might induce translocation of a phototransduction protein or proteins between the outer and inner segments. To test this hypothesis we made fast solution change experiments (not shown) where we rapidly switched from 1 mM to 25 nM external calcium, recorded one response at a moment of about 30 s after the solution had changed at the retina, and immediately switched back to 1 mM calcium. These experiments showed that that τD had increased to its final value within 60 s after the solution change to low calcium. This approach ruled out the role of translocation of phototransduction proteins in τD increase.
Target of Calcium-dependent τD Modulation
The seminal study by Krispel et al. (2006) [11] tied the molecular identity of the rate-limiting step in mouse rod photoresponse recovery in physiological conditions to the deactivation of PDE. The increase of τD observed in our low calcium experiments could, in principle, be explained by the deceleration of either PDE or rhodopsin deactivation. If the latter case were true, it would mean that in low calcium conditions PDE deactivation would be faster than rhodopsin deactivation, and now rhodopsin deactivation should be the rate-limiting step. This seems to be very unlikely since calcium-bound recoverin inhibits phosphorylation of rhodopsin [33], and light-induced decrease in [Ca2+]i releases this inhibition and accelerates photoresponse recovery [34]. This is consistent with the observation that τD is the same in wild-type and recoverin knockout mice [34]. Therefore the most probable target behind the calcium-dependent modulation of τD seems to be PDE. The physiological significance of this novel mechanism may be that a certain amount of calcium is necessary in setting the operation point of the phototransduction machinery in regard to photoresponse recovery.
Materials and Methods
Preparation, Recording and Light Stimulation
Ethical Approval
The use and handling of all the animals in this study were in accordance with the Finnish Act on Animal Experimentation (62/2006) with guidelines of the Animal Experimentation Committee of Finland.
The ERG Experiments
Pigmented mice (C57Bl/6N) were dark-adapted overnight. The animals were sacrificed, the eyes were enucleated and bisected along the equator, and the retinas were detached in cooled Ringer under dim red light. The isolated retina was placed in a specimen holder [35], [36] with an active recording area of 1.2 mm or in later experiments 0.5 mm (diam.) at the flat-mounted central retina. The upper (photoreceptor) side was superfused with a constant flow (ca. 1.5 ml/min at 25°C and 3–5 ml/min at 37°C) of Ringer's solution. In the experiments at 25°C the Ringer contained (mM): Na+ 133.9, K+ 3.3, Mg2+ 2.0, Ca2+ 1.0; Cl− 143.2, glucose, 10.0; EDTA, 0.01; Hepes, 12.0, buffered to pH 7.5 (at room temperature) with 5.8 mM NaOH. Sodium-L-aspartate (2 mM) was added to block synaptic transmission to second-order neurons. In the experiments at 37°C the Ringer contained (mM): Na+, 115.3; K+, 3.3; Mg2+, 2.0; Ca2+, 1.0; Cl−, 124.6; glucose, 10.0; EDTA, 0.01; HEPES 10.0; NaOH, 4.8 mM; NaHCO3, 20 mM. DL-2-Amino-4-phosphonobutyric acid (DL-AP4; 50 µM) was used to block synaptic signaling from photoreceptors to ON-bipolar cells. The Ringer was pre-heated to ca. 37°C and bubbled with a mixture of 95% O2 and 5% CO2 Leibovitz culture medium L-15 (Sigma), 0.72 mg/ml, was added to improve the viability of the retina. In addition in the experiments at 25°C, BaCl2 (10 mM) was added in the lower electrode space, from where it would diffuse to the retina to suppress glial currents by blocking potassium channels located mainly at the endfeet of Müller cells [24]. In experiments at 37°C 50 µM of BaCl2 was added to the perfusate, instead, because the 10 mM BaCl2 in the lower electrode space seemed to be less effective probably due to higher perfusion rate at 37°C. Pharmacological manipulations of the standard solution are explained below. The temperature was controlled by a heat exchanger below the specimen holder and monitored with a thermistor in the bath close to the retina.
Recording and Light Stimulation
The transretinal voltage changes (ERG) were recorded with two Ag/AgCl pellet electrodes, one in the subretinal space and the other in chloride solution connected to the perfusion Ringer through a porous plug. The DC signal was sampled at 200–10000 Hz with a voltage resolution of 0.25 µV.
The flashes used to produce short flash stimuli with homogeneous full-field illumination to the distal side of the retina were provided by a dual-beam optical system adapted from the setup used in [24]. In brief, 20 ms and later 2 ms light pulses were generated with a 543.5 nm HeNe laser (Melles Griot 05 LGR 173, 0.8 mW) or with a 532 nm laser (Power Technology IQ5C(532–100)L74, ∼130 mW) and a Compur (for 543.5 nm laser) or Oriel (model #76992, for 532 nm laser) shutter for both laser paths, the midpoint of the flash indicating the zero-time for the recordings. The Gaussian profile of the laser beam was flattened by conducting the beam through a light guide with mixing fibers. The uniformity of the beam at the level of the retina was confirmed with a small aperture photodiode. The light intensity of each source was controlled separately with calibrated neutral density filters and wedges.
The absolute intensity of the unattenuated laser beam (photons µm−2 s−1) incident on the retina (I0) was measured in each experiment with a calibrated photodiode (EG&G HUV-1000B; calibration by the National Standards Laboratory of Finland). The amount of isomerisations (Rh*) produced by the stimulating flash light in individual rods was calculated as described earlier [8].
Chemicals and Pharmacological Manipulations
All the chemicals were purchased from Sigma-Aldrich Inc. In the lowest [Ca2+]free (7.5–100 nM) solutions calcium was buffered with EGTA, and the free [Ca2+] was calculated using an “EGTA calculator” [37] taking into account 2 mM [Mg2+] present in our Ringer's solution. The low Na+ solution was prepared by substituting 132 mM [choline chloride] for NaCl, yielding 8 mM [Na+].
Response Analysis
The dominant time constant τD was extracted by determining the time for semisaturated or saturated photoresponses to recover to a criterion level (Tsat) which was chosen as 20–30% of the saturated photoresponse amplitude measured at plateau level after the nose [15] (see Fig. 1). The Tsat values were plotted against the natural logarithm of normalized flash intensity (Ln(I/I0)) and τD was then the slope of the fitted straight line.
The Arrhenius equation
| (1) |
was fitted to ln(τD) data as a function of T−1 in the temperature range 24–38°C. There, k is the rate constant of the reaction, Ea is the activation energy of the reaction (J/mol), R is molar gas constant and T is the absolute temperature of the retina and A is a temperature-independent pre-exponential factor. Ea was calculated from the slope of the fitted straight line (Ea = R*slope).
Acknowledgments
We thank Ms. Elina Sahala for technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Academy of Finland (http://www.aka.fi/en-gb/A/), grant 111866. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Woodruff ML, Sampath AP, Matthews HR, Krasnoperova NV, Lem J, et al. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J Physiol. 2002;542:843–854. doi: 10.1113/jphysiol.2001.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodruff ML, Olshevskaya EV, Savchenko AB, Peshenko IV, Barrett R, et al. Constitutive excitation by Gly90Asp rhodopsin rescues rods from degeneration caused by elevated production of cGMP in the dark. J Neurosci. 2007;27:8805–8815. doi: 10.1523/JNEUROSCI.2751-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch KW, Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988;334:64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- 4.Lolley RN, Racz E. Calcium modulation of cyclic GMP synthesis in rat visual cells. Vision Res. 1982;22:1481–1486. doi: 10.1016/0042-6989(82)90213-9. [DOI] [PubMed] [Google Scholar]
- 5.Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 1993;362:855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- 6.Chen CK, Woodruff ML, Chen FS, Chen D, Fain GL. Background light produces a recoverin-dependent modulation of activated-rhodopsin lifetime in mouse rods. J Neurosci. 2010;30:1213–1220. doi: 10.1523/JNEUROSCI.4353-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu YT, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993;361:76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- 8.Heikkinen H, Nymark S, Koskelainen A. Mouse cone photoresponses obtained with electroretinogram from the isolated retina. Vision Res. 2008;48:264–272. doi: 10.1016/j.visres.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Chen CK, Burns ME, He W, Wensel TG, Baylor DA, et al. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature. 2000;403:557–560. doi: 10.1038/35000601. [DOI] [PubMed] [Google Scholar]
- 10.Lyubarsky AL, Pugh EN., Jr Recovery phase of the murine rod photoresponse reconstructed from electroretinographic recordings. J Neurosci. 1996;16:563–571. doi: 10.1523/JNEUROSCI.16-02-00563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krispel CM, Chen D, Melling N, Chen YJ, Martemyanov KA, et al. RGS expression rate-limits recovery of rod photoresponses. Neuron. 2006;51:409–416. doi: 10.1016/j.neuron.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Woodruff ML, Janisch KM, Peshenko IV, Dizhoor AM, Tsang SH, et al. Modulation of phosphodiesterase6 turnoff during background illumination in mouse rod photoreceptors. J Neurosci. 2008;28:2064–2074. doi: 10.1523/JNEUROSCI.2973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern JH, Knutsson H, MacLeish PR. Divalent cations directly affect the conductance of excised patches of rod photoreceptor membrane. Science. 1987;236:1674–1678. doi: 10.1126/science.3037695. [DOI] [PubMed] [Google Scholar]
- 14.Haynes LW, Kay AR, Yau KW. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986;321:66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- 15.Vinberg FJ, Strandman S, Koskelainen A. Origin of the fast negative ERG component from isolated aspartate-treated mouse retina. J Vis. 2009;9:9–17. doi: 10.1167/9.12.9. [DOI] [PubMed] [Google Scholar]
- 16.Fain GL, Quandt FN, Bastian BL, Gerschenfeld HM. Contribution of a caesium-sensitive conductance increase to the rod photoresponse. Nature. 1978;272:466–469. doi: 10.1038/272467a0. [DOI] [PubMed] [Google Scholar]
- 17.Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol. 2008;18:1917–1921. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkler BS, Riley MV. Na+-K+ and HCO-3 ATPase activity in retina: dependence on calcium and sodium. Invest Ophthalmol Vis Sci. 1977;16:1151–1154. [PubMed] [Google Scholar]
- 19.Yau KW, McNaughton PA, Hodgkin AL. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981;292:502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]
- 20.Matthews HR. Effects of lowered cytoplasmic calcium concentration and light on the responses of salamander rod photoreceptors. J Physiol. 1995;484:267–286. doi: 10.1113/jphysiol.1995.sp020664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodgkin AL, McNulty JA, Nunn BJ. Effects of changing Ca before and after light flashes in salamander rods. 54J Physiol. 1986;372 [Google Scholar]
- 22.Pepperberg DR, Cornwall MC, Kahlert M, Hofmann KP, Jin J, et al. Light-dependent delay in the falling phase of the retinal rod photoresponse. Vis Neurosci. 1992;8:9–18. doi: 10.1017/s0952523800006441. [DOI] [PubMed] [Google Scholar]
- 23.Pepperberg DR, Jin J, Jones GJ. Modulation of transduction gain in light adaptation of retinal rods. Vis Neurosci. 1994;11:53–62. doi: 10.1017/s095252380001110x. [DOI] [PubMed] [Google Scholar]
- 24.Nymark S, Heikkinen H, Haldin C, Donner K, Koskelainen A. Light responses and light adaptation in rat retinal rods at different temperatures. J Physiol. 2005;567:923–938. doi: 10.1113/jphysiol.2005.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baylor DA, Nunn BJ. Electrical properties of the light-sensitive conductance of rods of the salamander Ambystoma tigrinum. J Physiol. 1986;371:115–145. doi: 10.1113/jphysiol.1986.sp015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doan T, Azevedo AW, Hurley JB, Rieke F. Arrestin competition influences the kinetics and variability of the single-photon responses of mammalian rod photoreceptors. J Neurosci. 2009;29:11867–79. doi: 10.1523/JNEUROSCI.0819-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koutalos Y, Nakatani K, Tamura T, Yau KW. Characterization of guanylate cyclase activity in single retinal rod outer segments. J Gen Physiol. 1995;106:863–890. doi: 10.1085/jgp.106.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen TY, Illing M, Molday LL, Hsu YT, Yau KW, et al. Subunit 2 (or beta) of retinal rod cGMP-gated cation channel is a component of the 240-kDa channel-associated protein and mediates Ca(2+)-calmodulin modulation. Proc Natl Ac Sci. 1994;91:11757–11761. doi: 10.1073/pnas.91.24.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebrik TI, Korenbrot JI. In intact mammalian photoreceptors, Ca2+-dependent modulation of cGMP-gated ion channels is detectable in cones but not in rods. J Gen Physiol. 2004;123:63–75. doi: 10.1085/jgp.200308952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winkler BS, Riley MV. Influence of calcium on retinal ATPases. Invest Ophthalmol Vis Sci. 1980;19:562–564. [PubMed] [Google Scholar]
- 31.Arshavsky VY, Dumke CL, Bownds MD. Noncatalytic cGMP-binding sites of amphibian rod cGMP phosphodiesterase control interaction with its inhibitory gamma- subunits. A putative regulatory mechanism of the rod photoresponse. J Biol Chem. 1992;267:24501–24507. [PubMed] [Google Scholar]
- 32.Zhang XJ, Cahill KB, Elfenbein A, Arshavsky VY, Cote RH. Direct allosteric regulation between the GAF domain and catalytic domain of photoreceptor phosphodiesterase PDE6. J Biol Chem. 2008;283:29699–29705. doi: 10.1074/jbc.M803948200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen CK, Inglese J, Lefkowitz RJ, Hurley JB. Ca(2+)-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem. 1995;270:18060–18066. doi: 10.1074/jbc.270.30.18060. [DOI] [PubMed] [Google Scholar]
- 34.Makino CL, Dodd RL, Chen J, Burns ME, Roca A, et al. Recoverin regulates light-dependent phosphodiesterase activity in retinal rods. [erratum appears in J Gen Physiol. 2005 Jul;126(1):81]. J Gen Physiol. 2004;123:729–741. doi: 10.1085/jgp.200308994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nymark S, Haldin C, Tenhu H, Koskelainen A. A new method for measuring free drug concentration: retinal tissue as a biosensor. Invest Ophthalmol Vis Sci. 2006;47:2583–2588. doi: 10.1167/iovs.05-1116. [DOI] [PubMed] [Google Scholar]
- 36.Donner K, Hemilä S, Koskelainen A. Temperature-dependence of rod photoresponses from the aspartate- treated retina of the frog (Rana temporaria). Acta Physiol Scand. 1988;134:535–541. doi: 10.1111/j.1748-1716.1998.tb08528.x. [DOI] [PubMed] [Google Scholar]
- 37.Portzehl H, Caldwell PC, Rueegg JC. The dependence of contraction and relaxation of muscle fibres from the crab maia squinado on the internal concentartion of free calcium ions. Biochim Biophys Acta. 1964;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]