Abstract
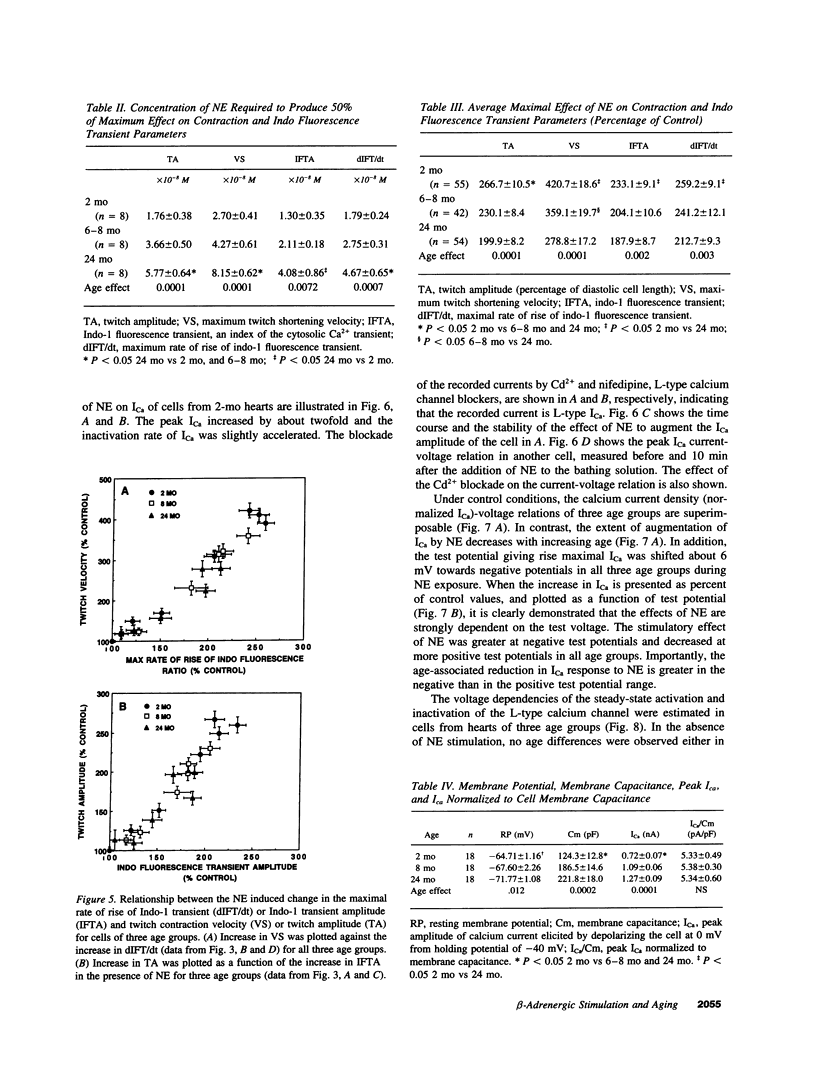
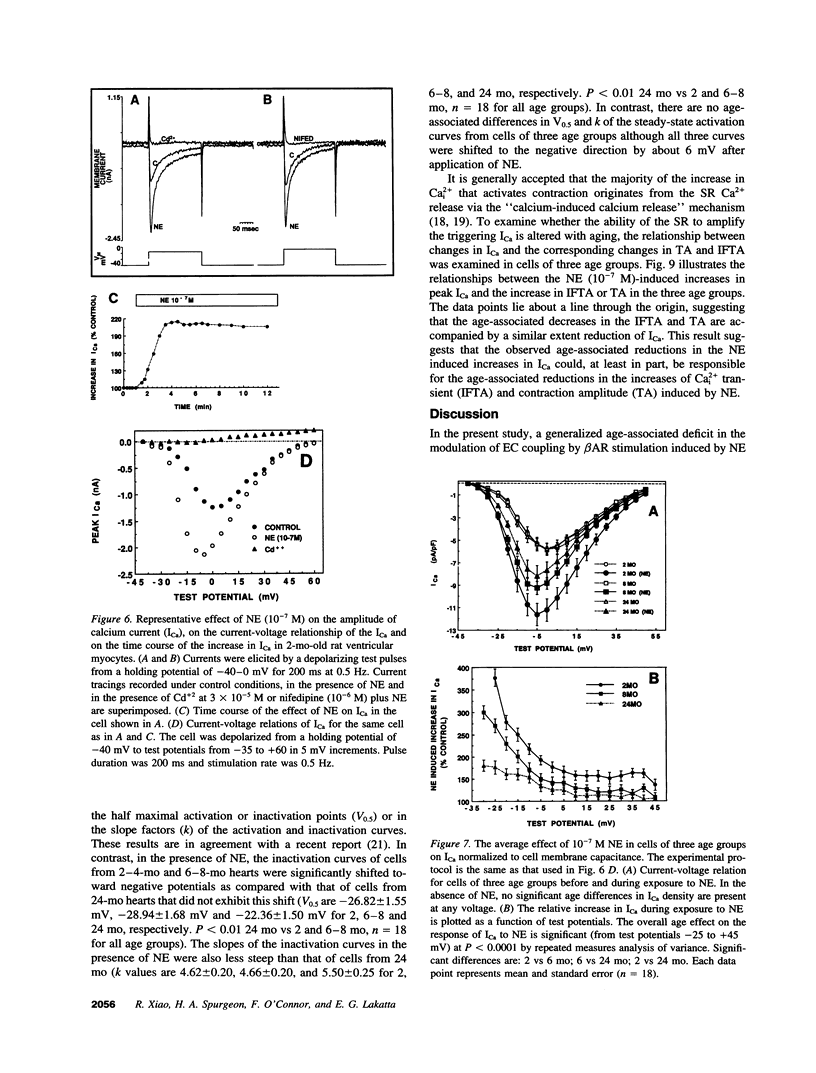
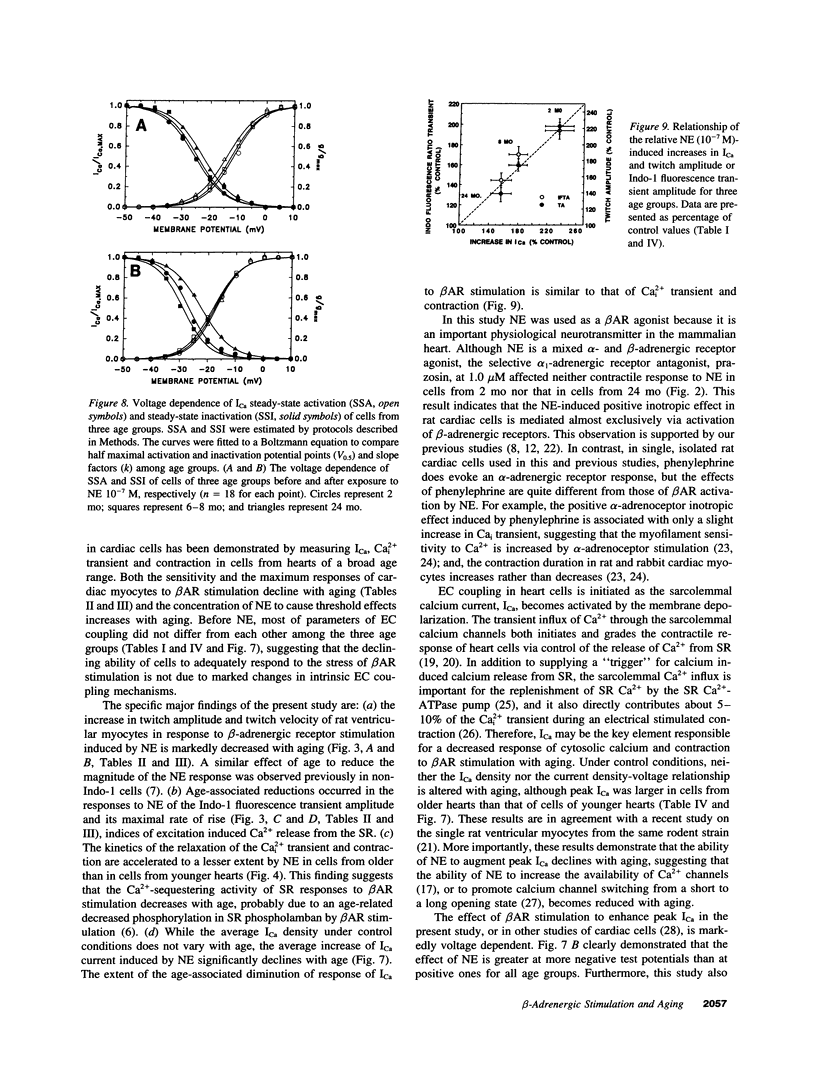
Previous studies have demonstrated that the ability of beta-adrenergic receptor (beta AR) stimulation to increase cardiac contractility declines with aging. In the present study, the control mechanisms of excitation-contraction (EC) coupling, including calcium current (ICa), cytosolic Ca2+ (Cai2+) transient and contraction in response to beta AR stimulation were investigated in ventricular myocytes isolated from rat hearts of a broad age range (2, 6-8, and 24 mo). While the baseline contractile performance and the Cai2+ transient did not differ markedly among cells from hearts of all age groups, the responses of the Cai2+ transient and contraction to beta-adrenergic stimulation by norepinephrine (NE) diminished with aging: the threshold concentration and the ED50 increased in rank order with aging; the maximum responses of contraction and Cai2+ transient decreased with aging. Furthermore, the efficacy of beta AR stimulation to increase ICa was significantly reduced with aging, and the diminished responses of the contraction and Cai2+ transient amplitudes to NE were proportional to the reductions in the ICa response. These findings suggest that the observed age-associated reduction in beta AR modulation of the cardiac contraction is, in part at least, due to a deficit in modulation of Cai2+, particularly the activity of L-type calcium channels.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P., Nowycky M. C., Tsien R. W. Beta-adrenergic modulation of calcium channels in frog ventricular heart cells. 1984 Jan 26-Feb 1Nature. 307(5949):371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- Bhatnagar G. M., Walford G. D., Beard E. S., Humphreys S., Lakatta E. G. ATPase activity and force production in myofibrils and twitch characteristics in intact muscle from neonatal, adult, and senescent rat myocardium. J Mol Cell Cardiol. 1984 Mar;16(3):203–218. doi: 10.1016/s0022-2828(84)80587-8. [DOI] [PubMed] [Google Scholar]
- Capogrossi M. C., Kachadorian W. A., Gambassi G., Spurgeon H. A., Lakatta E. G. Ca2+ dependence of alpha-adrenergic effects on the contractile properties and Ca2+ homeostasis of cardiac myocytes. Circ Res. 1991 Aug;69(2):540–550. doi: 10.1161/01.res.69.2.540. [DOI] [PubMed] [Google Scholar]
- Danziger R. S., Sakai M., Lakatta E. G., Hansford R. G. Interactive alpha- and beta-adrenergic actions of norepinephrine in rat cardiac myocytes. J Mol Cell Cardiol. 1990 Jan;22(1):111–123. doi: 10.1016/0022-2828(90)90976-9. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C. Ca2+ channel currents in rat sensory neurones: interaction between guanine nucleotides, cyclic AMP and Ca2+ channel ligands. J Physiol. 1991 Jan;432:23–43. doi: 10.1113/jphysiol.1991.sp018374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M., Blinks J. R. Actions of sympathomimetic amines on the Ca2+ transients and contractions of rabbit myocardium: reciprocal changes in myofibrillar responsiveness to Ca2+ mediated through alpha- and beta-adrenoceptors. Circ Res. 1988 Feb;62(2):247–265. doi: 10.1161/01.res.62.2.247. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleg J. L., Tzankoff S. P., Lakatta E. G. Age-related augmentation of plasma catecholamines during dynamic exercise in healthy males. J Appl Physiol (1985) 1985 Oct;59(4):1033–1039. doi: 10.1152/jappl.1985.59.4.1033. [DOI] [PubMed] [Google Scholar]
- Fraticelli A., Josephson R., Danziger R., Lakatta E., Spurgeon H. Morphological and contractile characteristics of rat cardiac myocytes from maturation to senescence. Am J Physiol. 1989 Jul;257(1 Pt 2):H259–H265. doi: 10.1152/ajpheart.1989.257.1.H259. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guarnieri T., Filburn C. R., Zitnik G., Roth G. S., Lakatta E. G. Contractile and biochemical correlates of beta-adrenergic stimulation of the aged heart. Am J Physiol. 1980 Oct;239(4):H501–H508. doi: 10.1152/ajpheart.1980.239.4.H501. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Houser S. R., Bahinski A., Silver L. H. Passive membrane properties of isolated feline ventricular myocytes. Am J Physiol. 1985 May;248(5 Pt 2):H622–H630. doi: 10.1152/ajpheart.1985.248.5.H622. [DOI] [PubMed] [Google Scholar]
- January C. T., Fozzard H. A. Delayed afterdepolarizations in heart muscle: mechanisms and relevance. Pharmacol Rev. 1988 Sep;40(3):219–227. [PubMed] [Google Scholar]
- January C. T., Riddle J. M., Salata J. J. A model for early afterdepolarizations: induction with the Ca2+ channel agonist Bay K 8644. Circ Res. 1988 Mar;62(3):563–571. doi: 10.1161/01.res.62.3.563. [DOI] [PubMed] [Google Scholar]
- Jiang M. T., Moffat M. P., Narayanan N. Age-related alterations in the phosphorylation of sarcoplasmic reticulum and myofibrillar proteins and diminished contractile response to isoproterenol in intact rat ventricle. Circ Res. 1993 Jan;72(1):102–111. doi: 10.1161/01.res.72.1.102. [DOI] [PubMed] [Google Scholar]
- Kalman D., O'Lague P. H., Erxleben C., Armstrong D. L. Calcium-dependent inactivation of the dihydropyridine-sensitive calcium channels in GH3 cells. J Gen Physiol. 1988 Oct;92(4):531–548. doi: 10.1085/jgp.92.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta E. G., Gerstenblith G., Angell C. S., Shock N. W., Weisfeldt M. L. Diminished inotropic response of aged myocardium to catecholamines. Circ Res. 1975 Feb;36(2):262–269. doi: 10.1161/01.res.36.2.262. [DOI] [PubMed] [Google Scholar]
- Niggli E., Lederer W. J. Voltage-independent calcium release in heart muscle. Science. 1990 Oct 26;250(4980):565–568. doi: 10.1126/science.2173135. [DOI] [PubMed] [Google Scholar]
- Pelzer D., Pelzer S., McDonald T. F. Properties and regulation of calcium channels in muscle cells. Rev Physiol Biochem Pharmacol. 1990;114:107–207. doi: 10.1007/BFb0031019. [DOI] [PubMed] [Google Scholar]
- Rodeheffer R. J., Gerstenblith G., Becker L. C., Fleg J. L., Weisfeldt M. L., Lakatta E. G. Exercise cardiac output is maintained with advancing age in healthy human subjects: cardiac dilatation and increased stroke volume compensate for a diminished heart rate. Circulation. 1984 Feb;69(2):203–213. doi: 10.1161/01.cir.69.2.203. [DOI] [PubMed] [Google Scholar]
- Sakai M., Danziger R. S., Staddon J. M., Lakatta E. G., Hansford R. G. Decrease with senescence in the norepinephrine-induced phosphorylation of myofilament proteins in isolated rat cardiac myocytes. J Mol Cell Cardiol. 1989 Dec;21(12):1327–1336. doi: 10.1016/0022-2828(89)90678-0. [DOI] [PubMed] [Google Scholar]
- Sakai M., Danziger R. S., Xiao R. P., Spurgeon H. A., Lakatta E. G. Contractile response of individual cardiac myocytes to norepinephrine declines with senescence. Am J Physiol. 1992 Jan;262(1 Pt 2):H184–H189. doi: 10.1152/ajpheart.1992.262.1.H184. [DOI] [PubMed] [Google Scholar]
- Sipido K. R., Wier W. G. Flux of Ca2+ across the sarcoplasmic reticulum of guinea-pig cardiac cells during excitation-contraction coupling. J Physiol. 1991 Apr;435:605–630. doi: 10.1113/jphysiol.1991.sp018528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgeon H. A., Stern M. D., Baartz G., Raffaeli S., Hansford R. G., Talo A., Lakatta E. G., Capogrossi M. C. Simultaneous measurement of Ca2+, contraction, and potential in cardiac myocytes. Am J Physiol. 1990 Feb;258(2 Pt 2):H574–H586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986 Jul;18(7):691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Varro A., Negretti N., Hester S. B., Eisner D. A. An estimate of the calcium content of the sarcoplasmic reticulum in rat ventricular myocytes. Pflugers Arch. 1993 Apr;423(1-2):158–160. doi: 10.1007/BF00374975. [DOI] [PubMed] [Google Scholar]
- Walker K. E., Lakatta E. G., Houser S. R. Age associated changes in membrane currents in rat ventricular myocytes. Cardiovasc Res. 1993 Nov;27(11):1968–1977. doi: 10.1093/cvr/27.11.1968. [DOI] [PubMed] [Google Scholar]
- Xiao R. P., Lakatta E. G. Beta 1-adrenoceptor stimulation and beta 2-adrenoceptor stimulation differ in their effects on contraction, cytosolic Ca2+, and Ca2+ current in single rat ventricular cells. Circ Res. 1993 Aug;73(2):286–300. doi: 10.1161/01.res.73.2.286. [DOI] [PubMed] [Google Scholar]
- Xiao R. P., Lakatta E. G. Deterioration of beta-adrenergic modulation of cardiovascular function with aging. Ann N Y Acad Sci. 1992 Dec 26;673:293–310. doi: 10.1111/j.1749-6632.1992.tb27465.x. [DOI] [PubMed] [Google Scholar]
- Yin F. C., Spurgeon H. A., Greene H. L., Lakatta E. G., Weisfeldt M. L. Age-associated decrease in heart rate response to isoproterenol in dogs. Mech Ageing Dev. 1979 Apr;10(1-2):17–25. doi: 10.1016/0047-6374(79)90067-8. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Herzig S., Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990 Jan;87(2):753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]



