Abstract
Purpose
Understanding the spatial distribution of opioid abuse at the local level may facilitate public health interventions.
Methods
Using patient-level data from addiction treatment facilities in New Mexico from ASI-MV® Connect, we applied geographic information system in combination with a spatial scan statistics to generate risk maps of prescription opioid abuse and identify clusters of product- and compound-specific abuse. Prescribed opioid volume data was used to determine whether identified clusters are beyond geographic differences in availability.
Results
Data on 24,452 patients residing in New Mexico was collected. Among those patients, 1779 (7.3%) reported abusing any prescription opioid (past 30 days). According to opioid type, 979 patients (4.0%) reported abuse of any hydrocodone, 1007 (4.1%) for any oxycodone, 108 (0.4%) for morphine, 507 (2.1%) for Vicodin® or generic equivalent, 390 (1.6%) for OxyContin®, and 63 (0.2%) for MS Contin® or generic equivalent. Highest rates of abuse were found in the area surrounding Albuquerque with 8.6 patients indicating abuse per 100 interviewed patients. We found clustering of abuse around Albuquerque (P=0.001; Relative Risk=1.35 and a radius of 146 km). At the compound level, we found that drug availability was partly responsible for clustering of prescription opioid abuse. After accounting for drug availability, we identified a second foci of Vicodin® abuse in the southern rural portion of the state near Las Cruces, NM and El Paso, Texas and bordering Mexico (RR=2.1; P=0.001).
Conclusions
A better understanding of local risk distribution may have implications for response strategies to future introductions of prescription opioids.
Keywords: Cluster Detection, Geography, Opioid abuse, Post-marketing Surveillance
Introduction
The abuse of prescription opioids contributes to significant population-wide morbidity and mortality, increasing significantly since the early 1990s in the United States 1–3. This is believed to have occurred through increased medical use of opioids in primary care, the introduction of multiple potent and modified-release formulations, including OxyContin®, and the increased use of methadone in outpatient pain management. However, the public health impact of nonmedical use of prescription opioids is not homogeneous across the United States 4–6. Certain geographic regions have displayed elevated abuse and drug diversion rates 7. For instance, important differences have been identified in the use and abuse of prescriptions between urban and rural areas 8–10. A rural preference for nonmedical use of opioids, compared to heroin and other illicit drugs, has been observed in cross-sectional studies of prison and community samples, as well as from Medicaid databases. Prescription opioid abuse also occurs in metropolitan areas where most individuals with a history of opioid dependency live 11. Opioid prescribing is routine in urban areas, as are established networks of drug trafficking organizations.
Although large scale patterns of prescription opioid abuse and dependency have been described 5, 6, local-scale variability, particularly at the product-level, is not well understood 12, 13. Consideration of the spatial distribution of opioid abuse at this level of specificity may facilitate the application of public intervention strategies and give direction to the manufacturers in terms of geographically specific risk management responses. Furthermore, whether risk patterns are simply due to increased availability of prescription opioids or whether there are inherently susceptible populations/areas is unknown. This knowledge gap is partly related to lack of product specific, geographically detailed and timely data on prescription opioid abuse.
Patient-level data from addiction treatment facilities can be used for real-time automated electronic surveillance, offering new opportunities for the surveillance of prescription opioids at the local scale. In particular, the epidemiology of prescription opioid abuse can be displayed in the form of a risk map for a specific region where specific signals or clusters can be identified. Furthermore, limited field resources (such as sparse distribution of treatment facilities) can be vastly supplemented using spatial statistical methods in order to estimate risk at unsampled locations 14–16. Such mapping enables the focusing of surveillance and control efforts 14.
Because the probability of occurrence in one location is not independent of occurrence in neighboring locations, surveillance data is often spatially autocorrelated 17. Spatial statistics make use of the assumption of spatial autocorrelation. Although this phenomenon precludes the use of parametric models as the number of degrees of freedom reduces and the chance of a type I error increases, it does allow us to analyze and visualize spatial patterns of risk 18. In particular, cluster techniques can provide early detection of increased risk. These methods also distinguish true clusters from apparent clusters arising by chance.
In this study, we have applied a geographic information system (GIS) in combination with spatial statistics to generate a local risk map of prescription opioid abuse and aid in the identification of hotspots of product-specific abuse. We attempted to determine whether our identified clusters are beyond what is expected based on drug availability using local level prescription data. For this analysis, we leverage a comprehensive risk management system, called the National Addictions Vigilance Intervention and Prevention Program or NAVIPPRO™. We focus the analysis on the state of New Mexico for which there is a history of prescription opioid problems 19 and where the system has complete coverage. A better understanding of risk distribution may have implications for response strategies to future introductions of prescription opioids.
Methods
Study Population
Data for the spatial analysis were obtained from ASI-MV-Connect®, part of the National Addictions Vigilance Intervention and Prevention Program (NAVIPPRO™ ), a comprehensive risk management program for prescription opioids, stimulants, and other Schedule II or III therapeutic agents. Data from ASI-MVR® Connect 4 represents a real-time data stream of recent use and abuse (past 30 days) in adult individuals (18 years old or older) entering treatment for substance use disorders. Data on treatment admissions for substance dependency collected by the ASI-MVR® Connect (e.g., demographics, medical problems, employment status, alcohol and drug use history and patterns, legal, family and psychiatric problems) can help identify and characterize subpopulations of substance abusers who enter treatment, such as those who engage in nonmedical use of prescription opioids.
Data Collection of Prescription Opioid Abuse
The ASI is a semi-structured interview designed for use on admission to a drug and alcohol program that assesses severity of addiction and the need for treatment. The ASI–MV® interview is “conducted” by onscreen interviewers who present the questions according to a tree-logic that asks follow up questions only when appropriate, much as a live interviewer might. ASI questions are presented in both text and audio for those who cannot read. The ASI–MV® performs well and has excellent psychometrics 20, 21. The ASI–MV® has been used at more than 400 treatment sites in 48 states, and more than 200,000 ASI-MV®s have been administered since its original launch in 1999. In addition to measuring key problem areas associated with substance use disorders, patients who have abused prescription medications in the past 30 days are queried about specific products they abused (i.e., “used not in a way prescribed by your doctor, that is, for the way it makes you feel and not for pain relief”). The interview collects information on product-specific data using screens with names (brand, generic, and slang names) and pictures of the pharmaceutical products. When a product is selected, information about route of administration and source of the drug is collected. The ASI-MV® is Web-enabled allowing for automated real-time uploads of HIPAA compliant data to a central data center, where the aggregate, patient-specific data at the patient home 3-digit ZIP code level for products and drug class are available for analysis and surveillance monitoring.
We chose the state of New Mexico as our study region given the complete coverage of treatment facilities by the NAVIPPRO™ system and the history of prescription opioid abuse in the region (Figure 1). We obtained data on all visits over a 2-year period from October 1, 2006 to September 30, 2008 from 95 facilities. Using data collected from the ASI-MV® Connect network, we determined which patients had abused any prescription opioid in the 30 days prior to their admission to treatment. We examined results by active ingredient (hydrocodone, oxycodone, morphine) and specific branded products (OxyContin®, and Vicodin®, MS Contin®). We calculated the proportion of patients reporting abuse in a 3-digit ZIP code over the total number in treatment from that 3-digit ZIP code.
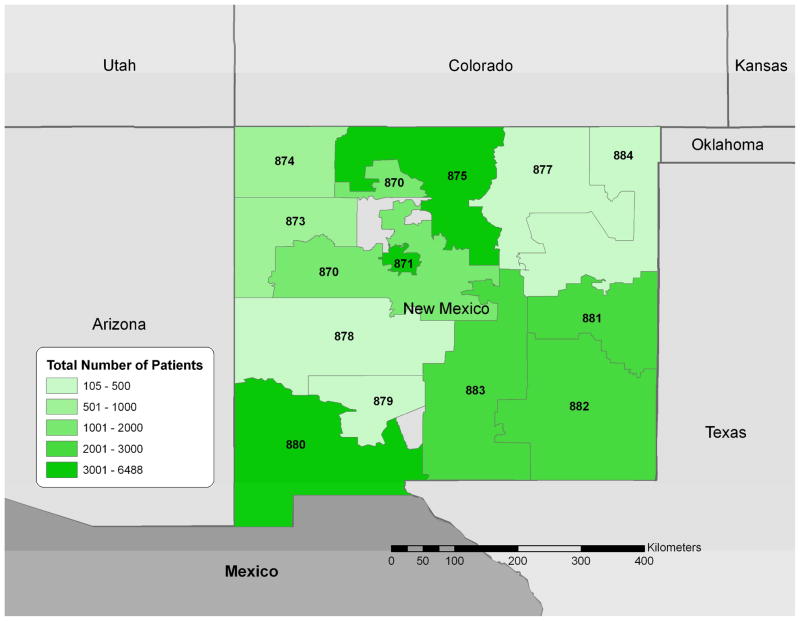
Figure 1.
Study region (New Mexico) indicating number of patients seen at treatment facilities by the 3-digit ZIP code of their home address (October 1, 2006-September 30, 2008). The 3 digit labels are the 3-digit zip codes for each area.
Analytic Approaches
Spatial Signal Detection
Cluster detection takes on two forms: (1) comparing whether cases occur in a non-random pattern relative to the pattern of non-cases and assuming a binary data distribution; and, (2) isolating areas with raised incidence rates which takes into account base population data and assumes the number of cases have a Poisson distribution 22. A variety of methods are available to assess case location and region count clustering in general, clustering at specific locations, and clustering in relation to putative point sources. An emerging set of cluster detection techniques are those that impose a moving window, usually circular, of variable size and location in order to precisely map potential clusters. An example is the spatial scan statistic that can be used to test for a random distribution over space and time 23, 24. Space-time scan statistics avoid assumptions of other methods and are useful to identify “suspect clusters” of case locations or region counts by using a window that moves in time and space.
We used a spatial scan statistic to test whether product specific opioid abuse was randomly distributed over space using SaTScan 25–27, which has been previously applied to alcohol and drug-related incidents 28, 29. A Poisson-based model was chosen, where the number of abuse cases in the area is Poisson distributed under the null hypothesis of spatial randomness 25. We aggregated patient data to the 3-digit ZIP code level, based on the patient’s home address. The 3-digit ZIP code resolution was selected as the spatial unit to preserve patient privacy. The total number of patients interviewed per 3-digit ZIP code was used as the background population data. Because this SaTScan requires point data to perform the statistical analysis, the geographic centroids for each of 3-digit ZIP code were calculated.
The spatial scan statistic centers a circular window around each 3-digit ZIP code centroid. This window varies continuously in size from 0 up to where it includes 50 percent of the total population at risk in the larger study area, in this case all of New Mexico 25, 26, 30. The circles will therefore contain different sets of neighboring 3-digit ZIP codes. For each iteration, this method tests the null hypothesis that the risk inside the window is the same as outside for each of the circles at each of the centroids. The SaTScan software calculates a likelihood ratio for each window with the assumption of a Poisson distribution to identify the most likely clusters 25, 30. Thus, the presence of prescription opioid abuse clusters can be assessed with the maximum likelihood ratio test statistic. The associated p value for the most likely cluster was calculated through a Monte Carlo simulation consisting of 999 random replications of the data set 31. For clusters found to be significant, we present basic demographics of the patients providing data and residing therein, including age, gender, race/ethnicity, other drugs abused in the past 30 days (heroin, cocaine, alcohol to intoxication >3 times per week, inhalants), insurance status, and the proportions reporting abuse of the prescription opioid at the class level (Table 1).
Table 1.
Characteristics of Patients in Substance Abuse Treatment by New Mexico 3-digit ZIP Code
| 870 | 871* | 873* | 874 | 875 | 877 | 878 | 879 | 880* | 881* | 882 | 883 | 884 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||||||
| 18 to 24 years | 23.5 | 18.7 | 21.8 | 23.6 | 19.0 | 21.2 | 21.9 | 24.6 | 23.3 | 19.3 | 21.0 | 22.6 | 13.8 |
| 25 to 44 years | 54.6 | 55.9 | 57.2 | 61.6 | 52.9 | 50.9 | 60.0 | 47.3 | 55.4 | 49.2 | 54.3 | 47.6 | 54.2 |
| 45+ years | 20.2 | 30.0 | 20.6 | 14.8 | 27.5 | 27.9 | 17.1 | 27.5 | 21.2 | 28.9 | 23.8 | 29.7 | 31.6 |
| Gender | |||||||||||||
| Male | 63.6 | 60.5 | 69.1 | 65.0 | 64.6 | 66.9 | 74.3 | 59.3 | 66.8 | 49.3 | 53.0 | 53.4 | 54.8 |
| Female | 36.4 | 39.5 | 30.9 | 34.7 | 35.4 | 33.1 | 25.7 | 40.7 | 33.2 | 50.7 | 47.0 | 46.6 | 45.0 |
| Race/Ethnicity | |||||||||||||
| White | 20.7 | 31.3 | 14.2 | 37.8 | 24.4 | 17.5 | 30.5 | 58.7 | 26.9 | 46.8 | 53.0 | 55.7 | 48.4 |
| Black | 1.1 | 5.2 | 1.2 | 1.7 | 1.5 | 0.8 | 0.0 | 1.2 | 3.7 | 8.5 | 3.5 | 5.2 | 1.3 |
| Mexican | 28.0 | 34.4 | 14.2 | 14.6 | 40.0 | 61.7 | 46.7 | 29.9 | 54.2 | 32.2 | 37.9 | 27.4 | 34.0 |
| Other Hispanic/Latino | 4.8 | 8.5 | 2.1 | 2.8 | 12.0 | 5.6 | 2.9 | 3.6 | 4.3 | 2.6 | 2.0 | 4.3 | 4.2 |
| American Indian | 33.2 | 8.3 | 62.6 | 41.4 | 6.3 | 1.3 | 14.3 | 0.6 | 1.2 | 2.2 | 1.7 | 4.1 | 1.5 |
| Other | 12.3 | 12.3 | 5.5 | 1.7 | 15.7 | 13.1 | 5.7 | 6.0 | 9.6 | 7.6 | 1.9 | 3.4 | 10.6 |
| Insurer | |||||||||||||
| Medicaid/Medicare | 8.3 | 35.7 | 12.1 | 5.8 | 12.9 | 21.9 | 63.6 | 12.2 | 8.2 | 22.0 | 24.5 | 20.0 | 32.9 |
| Private Insurance | 0.7 | 1.3 | 1.6 | 0.3 | 1.2 | 1.3 | 0.0 | 2.4 | 0.7 | 3.4 | 1.6 | 6.4 | 4.7 |
| Self-pay | 7.3 | 9.3 | 7.3 | 58.6 | 46.1 | 9.6 | 18.2 | 0.0 | 8.2 | 31.0 | 36.5 | 48.7 | 37.6 |
| Uninsured | 28.6 | 13.1 | 15.3 | 0.3 | 13.9 | 20.6 | 4.5 | 12.2 | 43.5 | 1.1 | 10.7 | 3.0 | 10.6 |
| Commercial Payer | 1.4 | 3.8 | 8.1 | 1.6 | 6.0 | 5.3 | 0.0 | 0.0 | 0.4 | 1.4 | 6.1 | 11.0 | 10.6 |
| Other | 53.7 | 36.8 | 55.6 | 33.3 | 19.9 | 41.2 | 13.6 | 73.2 | 39.0 | 41.0 | 20.6 | 11.0 | 3.5 |
| AdmissionsPrompted By Criminal Justice System | 62.0 | 43.1 | 54.4 | 51.1 | 48.9 | 45.8 | 72.4 | 58.7 | 58.1 | 25.2 | 35.1 | 223.0 | 39.2 |
| Heroin Use in Past 30 Days | 4.5 | 10.0 | 0.4 | 0.9 | 3.6 | 2.5 | 4.8 | 0.6 | 2.2 | 0.7 | 2.9 | 0.7 | 1.2 |
| Cocaine Use in Past 30 Days | 7.3 | 12.2 | 8.9 | 4.5 | 14.7 | 15.4 | 7.6 | 3.0 | 13.6 | 7.9 | 6.8 | 4.4 | 4.8 |
| Alcohol to Intoxication in Past 30 Days | 23.5 | 23.4 | 29.8 | 32.2 | 31.0 | 36.9 | 30.5 | 24.6 | 31.2 | 24.4 | 29.7 | 21.8 | 25.3 |
| Inhalant Use in Past 30 Days | 0.3 | 0.4 | 0.4 | 0.1 | 0.5 | 0.8 | 1.0 | 1.2 | 0.7 | 0.3 | 0.5 | 0.7 | 0.2 |
| Total No. Patients | 1499 | 6537 | 563 | 646 | 4214 | 520 | 105 | 167 | 3060 | 2203 | 2379 | 2077 | 482 |
Data presented as percent of all patients in treatment in specified New Mexico 3-digit ZIP code
= 3-digit ZIP codes where product-specific clusters of abuse observed after availability adjustment
Sensitivity Analysis
Because high rates of abuse may be a function of overall drug availability rather than underlying population distribution 32, we anticipated a relationship between the local drug availability and local abuse of specific drug classes. We accounted for this potential confounding by adjusting our denominator by prescription rates for the opioids under study in our cluster models. Data on retail drug distribution by 3-digit ZIP code for New Mexico were obtained from a commercial vendor, SDI Health (formerly Verispan, LLC) using the Vector One Market Pharmacy Sub-National Pain Market Prescription Tracking.
These data come from a variety of sources, including retail registrants, local-level distributors, and hospital pharmacies. Data are representative of the retail pharmacy universe, but do not include other potential channels of distribution including long-term care, hospital dispensing, mail order, etc. Total drug amounts (in milligrams) by opioid drug class volume and product were calculated for each 3-digit ZIP code for the period January 2007 through September 2008. For each drug class and product evaluated, we reran our scan statistic analysis to account for prescribed drug volume using total milligrams in a 3-digit ZIP code.
Results
Patient Population
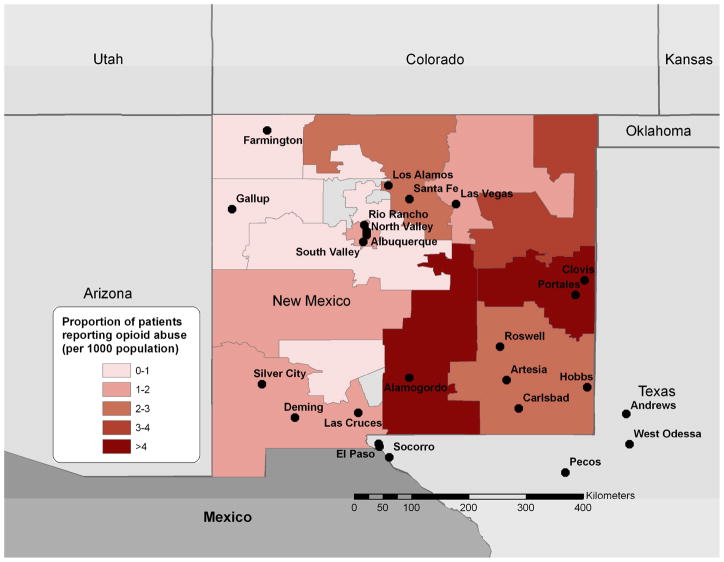
We collected data on 24,452 patients residing in New Mexico over the study period (Figure 1). Among those patients, 1779 (7.3%) reported abusing any prescription opioid in the previous 30 days. According to opioid type, we identified 979 patients (4.0%) who reported abuse of any hydrocodone in the past 30 days, 1007 (4.1%) for any oxycodone, and 108 (0.4%) for morphine. Of the 1,478 patients who abused any of these three compounds in the past 30 days, 62.7% used just one compound in the past 30 days, 32.9% used two of them, and 4.4% used all three. Product-specific abuse showed 507 patients (2.1%) reported abusing Vicodin® or a generic equivalent of Vicodin®, 390 patient (1.6%) reported abusing OxyContin®, and 63 (0.2%) reported abusing MS Contin® or a generic equivalent of MS Contin®. Highest rates of prescription opioid abuse were found in the Northern part of the state surrounding Albuquerque with a maximum of 8.6 patients indicating abuse per 100 interviewed patients (Figure 2). We also examined older age (over 55 years) as a possible confounder of the association between availability and abuse but found no significant correlations between age and abuse in the NM locations.
Figure 2.
Proportion of treatment facility patients reporting abuse of any prescription opioid by the 3-digit ZIP code of their home address per census population (October 1, 2006-September 30, 2008)
Cluster Detection Results
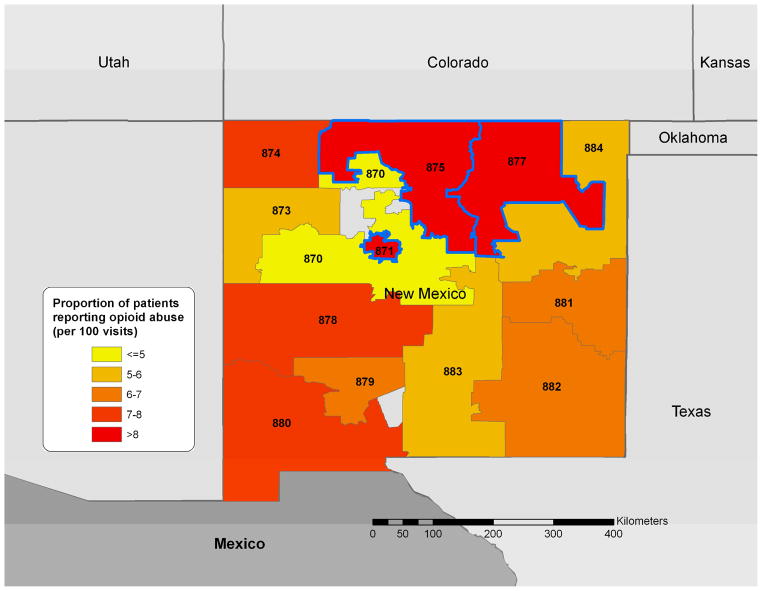
We found clustering of overall prescription opioid abuse. The cluster encompassed three 3-digit ZIP codes (871, 875 and 877) and included Albuquerque (P=0.001). This cluster (36.3 N, 106.2 W) had a relative risk of 1.35 and a radius of 146 km. Fifty-four percent (n=953) of the 1779 total patients reporting prescription opioid abuse resided this area.
Clustering by opioid class appeared to follow the same spatial pattern. Oxycodone abuse was significantly focused around Albuquerque (P=0.001). This cluster had a relative risk of 1.7 and a total of 609 patients reporting abuse. Increased risk of hydrocodone abuse was also identified in this cluster, with a relative risk of 1.25 (P=0.007) and 506 cases. Morphine clustering was only found in two 3-digit ZIP codes (875 and 871) with relative risk of 1.8 (P=0.031) and 63 cases.
We also found clustering by specific prescription opioid product. A significant cluster of OxyContin® and MS Contin® abuse was once again observed in northern New Mexico, including Albuquerque. These clusters had a relative risk of 1.9 (P=0.001) for OxyContin® and a relative risk of 2.4 (P=0.008) for MS Contin®. In contrast, Vicodin® abuse was significantly clustered in two areas. The primary cluster in 3-digit ZIP 880 was found to be unique to Vicodin®. This foci is located in the southern portion of the state near Las Cruces, NM and El Paso, Texas (RR=2.1; P=0.001) representing 119 cases out of the 570 total cases of Vicodin® abuse A secondary cluster 871 had a relative risk of 1.3 (P=0.04) and overlapped with the overall hydrocodone cluster. (Figure 3)
Figure 3.
Proportion of treatment facility patients reporting abuse of any prescription opioid by the 3-digit ZIP code of their home address per total number of visits from that 3-digit ZIP (October 1, 2006-September 30, 2008). Zip codes highlighted in blue represent the significant overall cluster identified. The 3 digit labels are the 3-digit zip codes for each area.
Sensitvity Analysis
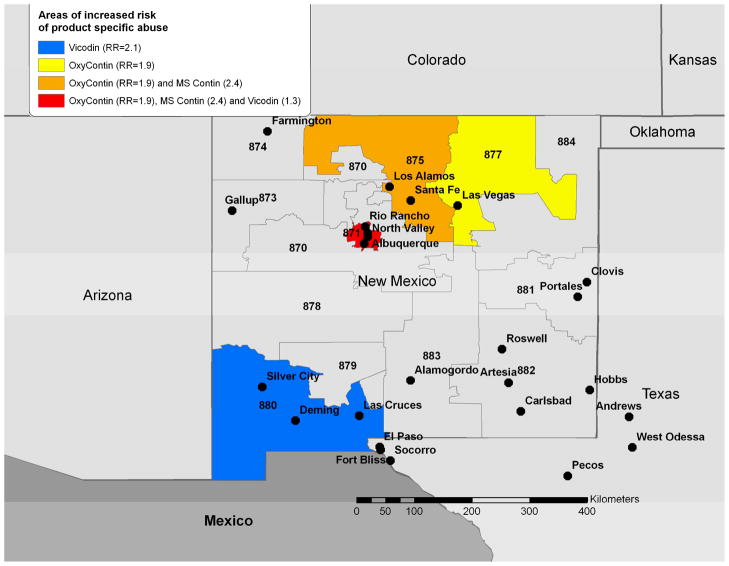
We analyzed whether clustering for oxycodone, morphine and hydrocodone occurs beyond would be expected given increased drug availability. We adjusted the denominator for retail prescription data by underlying population of the 3-digit ZIP code. Population data used were 2005 estimates based on 2000 population data for 3-digit ZIP code areas provided by the US Census and linked via GIS 33, 34. After including prescription data in our model, our observed primary clusters disappeared, indicating that these observed clusters may be directly related to increased product availability. After adjusting for prescription availability, we found new primary clusters for oxycodone (RR=10.4; P= 0.001), morphine (RR=11.7; P= 0.005), and hydrocodone (RR=5.7 ; P= 0.001). Interestingly, these clusters were located in the same single 3-digit ZIP code (881) near Clovis, NM.
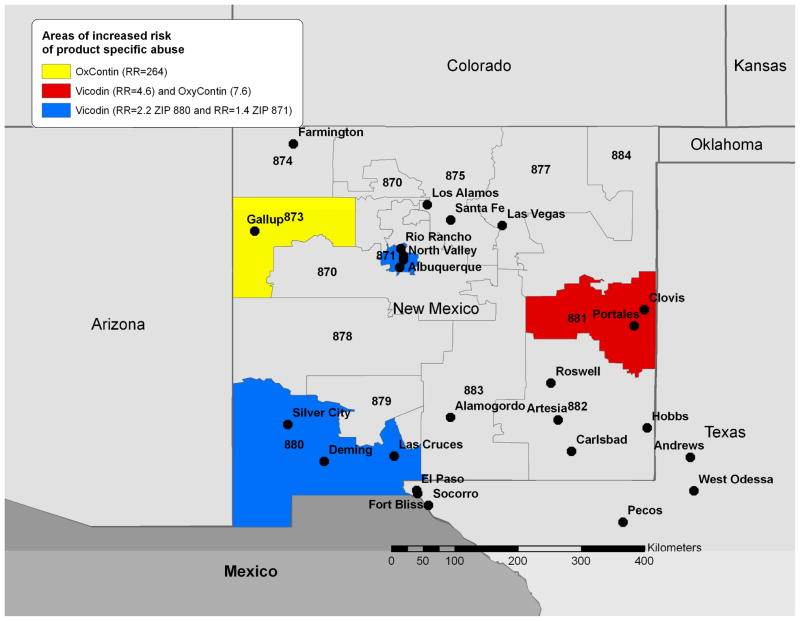
Similar geographic clustering was observed for specific drug products. Clusters for OxyContin® (RR=7.6; P= 0.001) and Vicodin® (RR=4.6; P= 0.001), were also observed at 3-digit ZIP 881. However, primary clustering was observed for OxyContin® in 3-digit ZIP 873 including Gallup, NM (RR=264; P= 0.001) located in Western New Mexico. This strikingly high relative risk arose from the extremely limited number of prescriptions of OxyContin® in the Gallup area. Prescriptions were ten times lower than the next lowest prescribed 3-digit ZIP and 587 times lower than the median prescription availability. Despite this, we still identified seven instances of OxyContin®-related abuse. Secondary clustering for Vicodin® remained in 3-digit ZIP 880 (RR=2.2; P= 0.001) in the Southwestern part of New Mexico near Las Cruces, NM and at 3-digit ZIP 871 (RR=1.4; P= 0.001) near Albuquerque. No clustering of MS Contin® was observed after adjustment for prescription data (Figure 4).
Figure 4.
Clustering of abuse for specific opioid products among treatment facility patients grouped by the 3-digit ZIP code of their home address (October 1, 2006-September 30, 2008). Relative risk estimates from the cluster detection analysis are included in the legend and identified statistically significant clusters are highlighted by color coding. The 3 digit labels are the 3-digit zip codes for each area.
Discussion
Our analysis provides insight into product-specific and compound-specific geographical areas of abuse. We find important product-specific clusters of high rates of prescription opioid abuse, particularly around the areas of Albuquerque, and Las Cruces, New Mexico. Our modeling highlights areas that are most likely to experience higher rates of abuse. However, we find that much of this clustering in New Mexico could be accounted for by drug availability, as these are among the areas with the greatest amount of prescribed oxycodone, hydrocodone, and morphine in New Mexico. When accounting for retail availability of the drug, we see the emergence of new clusters, which cannot be explained by drug availability. Furthermore, we do find a significant clustering in southern New Mexico around Las Cruces, which cannot be explained by retail drug availability.
The area of Las Cruces is situated along the El Paso/Juarez Corridor, bordering west Texas and Mexico, and is a known gateway for transporting illegal drugs Mexican black tar heroin, marijuana, cocaine, prescription drugs--many of which can be purchased legally over the counter in Mexico—and other substances are smuggled into the United States along this corridor 35. The proximity to the US/Mexico border may strongly influence supply and accessibility of abusable substances which were not completely captured in this analysis, thus enabling detection of a cluster of abuse rates in the Las Cruces area. The relationship between high rates of drug abuse and proximity to political borders with heavy drug supply and trafficking are well established (e.g., the Golden Triangle) 36, 37. Further, recent drug enforcement seizures of large quantities of Mexican black tar and brown heroin 38 may have reduced supply locally (creating a so-called, heroin “drought” 39–41). Such a situation may thereby have led to greater demand for the more readily accessible prescription opioids, consequently driving up and clustering prescription opioids abuse rates in the region. In New Mexico 3-digit ZIP codes where significant clustering of prescription opioids was identified (e.g., 880 and 881), the percentage of patients in treatment reporting recent heroin was lower than in ZIP codes where no clustering was identified. Among patients in substance abuse treatment in 3-digit ZIP code 880, located along the US/Mexico border, a greater percentage of patients were uninsured (43.5%) and their admissions were prompted by the criminal justice system (58.1%) as compared to other areas of New Mexico. Future studies should explore the dynamics of these micro drug economies and their effects on rates abuse and other drug-related harms.
Our analysis of clusters across 3-digit ZIP codes in New Mexico provides insight into the sensitivity of treatment facility data for the early detection of local clustering. The majority of clusters were identified based on relatively small differences in rates of abuse. Because this analysis accounts for product availability, the results indicate that such a system could be used to detect a signal or cluster that may result from other causes. For instance, we may see local increases in product-specific abuse after a pharmacy robbery or new supply chain. Future studies should focus on signal simulation to evaluate system performance as an alternative to the difficulties of examining naturally occurring outbreaks 42. While these simulations are limited as to how well they mimic the diversity and unpredictability of real-life events, they do allow a level of control that can help in the investigation of system performance across a range of common or expected scenarios.
Our analysis also more generally highlights the two-fold value of spatial surveillance: (1) cluster detection–identifying important clusters of high rates of abuse and high relative rates of product-specific abuse, and (2) risk mapping--developing robust surface maps of risk for overall and drug-specific opioid abuse. A rapid understanding of where prescription opioids are more likely to be abused compared to other geographic areas would aid greatly in the deployment of emergency surveillance and control efforts. Future evaluation should focus on whether local public health agencies may employ this model to rapidly describe human risk even when only sparse case reports are available or at the beginning of a drug release.
As with the aetiology of drug abuse more generally, a thorough understanding of prescription opioid abuse requires more than inquiring about individual history, correlating behaviors, and uncovering specific risk factors that give rise to abuse of prescription opioids. It also includes understanding contextual factors that contribute to and may predispose one to drug abuse problems, including economic, socio-cultural, endemic health, and local policy-relevant characteristics. Geographic analysis-to the extent that such data are available, time-matched, and specific-holds promise to help synthesize, associate, and place in proper context these multiple, extra-individual factors as they relate to abuse and other risk behaviors. We have begun to explore the correlation of contextual factors and observed patterns of prescription opioid abuse43; future research would benefit from further inquiry and from the application of qualitative as well as quantitative geographic analysis.
The current analyses have limited power, resulting from the short time series of historical background data across a small number of geographic areas. As more data become available from the ASI-MV® Connect in a wider range of geographic areas, there will be increased opportunities to understand the space-time distribution of prescription opioid abuse and evaluate methods for early detection and spatial localization of signals. These evaluations will help form the basis of automated surveillance systems that can be deployed prospectively and in real-time. Use of prescription data that are not exactly coinciding with the time frame of data collected at substance abuse treatment centers is a limitation of the study. This mismatch of time frame raises questions about how these data sources might vary over time. The present findings may reflect a robustness over time of the observed relationship. Nevertheless, definitive description of the relationship between these data sources will require further investigation. Our analyses also could not take into account drug availability from illegal sources such as cross-border smuggling, as SDI Health data are limited to legal drug distribution. Further, the lack of coverage from other drug distribution sources such as, mail order and hospitals is an inherent limitation to the SDI Health data. Although retail distribution channels still cover a large portion of the U.S. prescription market, reliance on data covering primarily retail distribution channels may have underestimated the medical availability of the drugs evaluated in some geographic areas studied, particularly areas where access to retail pharmacies is limited and other points of distribution such as mail order may be on the rise. This limitation introduces a level of uncertainty in the analyses conducted and may have resulted in some bias in the cluster areas detected, particularly if data for medical availability varies disproportionally at a local scale. The indirect approach to defining the association between abuse rates and drug availability may result in ecological fallacy, since we did not measure prescriptions among the treatment center populations.
It is important to note the problem of scale in this geographic analysis, namely, the crude 3-digit ZIP code used to reflect location of the respondent’s residence. Due to privacy and confidentiality concerns, this is the commonly accepted level of geographic specificity employed in drug abuse surveillance work (cf RADARS.org, navippro.com). As such, one must caution that aggregation bias or ecological fallacy, rather than true associations, may have been detected and that greater heterogeneity in abuse may be observed at finer geospatial levels. Future prospective epidemiologic studies could obtain more specific residence data with the informed consent of participants and test spatial hypothesis that have been generated in the present study.
Treatment admissions data are a convenient and widely-available source of information on drug abuse and dependency. However, treatment admissions for substance use are not necessarily representative to the overall population. For example, individual level considerations for entering a treatment program may be influenced by mandates from criminal justice proceedings, variations (both general and localized) in insurance and reimbursement, the availability of effective treatment paradigms, treatment program recidivism, transportation, employment, social and family support networks, stigma associated with seeking help, personal perceptions of risk from continued drug use and the benefits gained by entering treatment. As such, treatment program admissions are a highly selected subset of the broader drug using population and may be a significant source of bias in a surveillance dataset. In practice, however, fluctuations in specific drugs abused by those admitted to treatment programs are often detected as a surrogate for population-based surveys due to the availability of electronic medical data and because public agencies are often responsible for covering the financial cost of treatment programs. Regardless of the generalizability issue, studies on treatment program enrollees are a staple of substance use epidemiology pertaining to prescription opioid abuse and dependence 44, 45. In this paper, we present a methodology to utilize these data in a systematic and transparent manner that attempts to study drug use in context. Our model is useful in that it takes into account both supply (prescription data) and demand (treatment admissions) for prescription opioids.
Conclusions
This study illustrates a method and the potential value of a multi-disciplinary approach to rapidly identifying clusters of prescription opioid abuse among patients at treatment facilities and assessing risk in the surrounding community. Our analysis of the case distribution data demonstrates the application of these new technologies in facilitating a rapid response to drug introductions. The use of geographic information system technologies, in conjunction with spatial statistics, offers effective tools for real-time post-marketing surveillance of prescription pain medicine.
KEY POINTS
Consideration of the spatial distribution of opioid abuse at the local level may facilitate the application of public health interventions.
The use of geographic information system technologies, in conjunction with spatial statistics, offers effective tools for real-time post-marketing surveillance of prescription pain medicine.
The ASI-MVR® Connect, part of the NAVIPPRO™ pharmaceutical risk management system, provides product-specific data on scheduled pharmaceutical products from individuals entering treatment for substance abuse problems. These data, in combination with data on local prescribed availability and patient home 3 digit zip code, may be useful in characterizing abuse patterns in specific regions of the country.
Figure 5.
Clustering of abuse for specific opioid products among treatment facility patients grouped by the 3-digit ZIP code of their home address after adjusting for product availability (October 1, 2006-September 30, 2008). Relative risk estimates from the cluster detection analysis are included in the legend and identified statistically significant clusters are highlighted by color coding. The 3 digit labels are the 3-digit zip codes for each area.
Table 2.
Rates of Prescription Opioid Abuse per 10,000 patients in New Mexico 3-digit ZIP Codes
| 870 | 871* | 873* | 874 | 875 | 877 | 878 | 879 | 880* | 881* | 882 | 883 | 884 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxycodone | 253.5 | 576.7 | 230.9 | 433.4 | 488.8 | 500.0 | 476.2 | 239.5 | 362.7 | 331.4 | 256.4 | 288.9 | 103.7 |
| Hydrocodone | 293.5 | 414.6 | 213.1 | 588.2 | 479.4 | 634.6 | 381.0 | 179.6 | 470.6 | 286.0 | 382.5 | 279.2 | 332.0 |
| Morphine | 40.0 | 56.6 | 17.8 | 31.0 | 61.7 | 38.5 | 0.0 | 59.9 | 45.8 | 18.2 | 21.0 | 38.5 | 41.5 |
| OxyContin® | 126.8 | 232.5 | 124.3 | 201.2 | 182.7 | 211.5 | 381.0 | 59.9 | 147.1 | 99.9 | 79.9 | 77.0 | 83.0 |
| Vicodin® and generic equivalents | 140.1 | 253.9 | 106.6 | 170.3 | 123.4 | 192.3 | 190.5 | 119.8 | 388.9 | 122.6 | 227.0 | 144.4 | 145.2 |
| MS Contin® and generic equivalents | 26.7 | 36.7 | 0.0 | 15.5 | 40.3 | 19.2 | 0.0 | 59.9 | 26.1 | 9.1 | 4.2 | 14.4 | 20.7 |
| Total No. Patients | 1499 | 6537 | 563 | 646 | 4214 | 520 | 105 | 167 | 3060 | 2203 | 2379 | 2077 | 482 |
= 3-digit ZIP codes where product-specific clusters of abuse observed after availability adjustment
Acknowledgments
This research was supported in part by a grant to Author Butler from NIDA (DA024489) and grants to Inflexxion, Inc. from Endo Pharmaceuticals and King Pharmaceuticals, Inc.
References
- 1.Dasgupta N, Kramer ED, Zalman MA, et al. Association between non-medical and prescriptive usage of opioids. Drug Alcohol Depend. 2006;82:135–42. doi: 10.1016/j.drugalcdep.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Hopfer CJ, Mikulich SK, Crowley TJ. Heroin use among adolescents in treatment for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2000;39:1316–23. doi: 10.1097/00004583-200010000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15:618–27. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- 4.Butler SF, Budman SH, Licari A, et al. National addictions vigilance intervention and prevention program (NAVIPPRO): a real-time, product-specific, public health surveillance system for monitoring prescription drug abuse. Pharmacoepidemiol Drug Saf. 2008;17:1142–54. doi: 10.1002/pds.1659. [DOI] [PubMed] [Google Scholar]
- 5.Curtis LH, Stoddard J, Radeva JI, et al. Geographic variation in the prescription of schedule II opioid analgesics among outpatients in the United States. Health Serv Res. 2006;41:837–55. doi: 10.1111/j.1475-6773.2006.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulozzi LJ, Ryan GW. Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med. 2006;31:506–11. doi: 10.1016/j.amepre.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Dasgupta N, Jonsson Funk M, Brownstein JS. In: Geography and Drug Addiction. Richardson TD, Cheung I, editors. Springer Publishers (Collaboration between the National Institute on Drug Abuse and the Association of American Geographers); 2008. [Google Scholar]
- 8.Havens JR, Walker R, Leukefeld CG. Prevalence of opioid analgesic injection among rural nonmedical opioid analgesic users. Drug Alcohol Depend. 2006 doi: 10.1016/j.drugalcdep.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Leukefeld CG, Narevic E, Hiller ML, et al. Alcohol and drug use among rural and urban incarcerated substance abusers. Int J Offender Ther Comp Criminol. 2002;46:715–28. doi: 10.1177/0306624X02238164. [DOI] [PubMed] [Google Scholar]
- 10.Schoeneberger ML, Leukefeld CG, Hiller ML, et al. Substance abuse among rural and very rural drug users at treatment entry. Am J Drug Alcohol Abuse. 2006;32:87–110. doi: 10.1080/00952990500328687. [DOI] [PubMed] [Google Scholar]
- 11.Paulozzi LJ. Opioid analgesic involvement in drug abuse deaths in American metropolitan areas. Am J Public Health. 2006;96:1755–7. doi: 10.2105/AJPH.2005.071647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forrester MB. Oxycodone abuse in Texas, 1998–2004. J Toxicol Environ Health A. 2007;70:534–8. doi: 10.1080/15287390600870924. [DOI] [PubMed] [Google Scholar]
- 13.Havens JR, Talbert JC, Walker R, et al. Trends in controlled-release oxycodone (OxyContin) prescribing among Medicaid recipients in Kentucky, 1998–2002. J Rural Health. 2006;22:276–8. doi: 10.1111/j.1748-0361.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- 14.Brownstein JS, Rosen H, Purdy D, et al. Spatial analysis of West Nile virus: rapid risk assessment of an introduced vector-borne zoonosis. Vector Borne Zoonotic Dis. 2002;2:157–64. doi: 10.1089/15303660260613729. [DOI] [PubMed] [Google Scholar]
- 15.Glass GE. Update: spatial aspects of epidemiology: the interface with medical geography. Epidemiol Rev. 2000;22:136–9. doi: 10.1093/oxfordjournals.epirev.a018010. [DOI] [PubMed] [Google Scholar]
- 16.Ostfeld RS, Glass GE, Keesing F. Spatial epidemiology: an emerging (or re-emerging) discipline. Trends Ecol Evol. 2005;20:328–36. doi: 10.1016/j.tree.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Legendre P. Spatial autocorrelation: Trouble or new paradigm? Ecology. 1993;74:1659–1673. [Google Scholar]
- 18.Tobler WR. A computer model of simulating urban growth in the Detroit region. Economic Geography. 1970;46:234–240. [Google Scholar]
- 19.Mueller MR, Shah NG, Landen MG. Unintentional prescription drug overdose deaths in New Mexico, 1994–2003. Am J Prev Med. 2006;30:423–9. doi: 10.1016/j.amepre.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Butler SF, Budman SH, Goldman RJ, et al. Initial validation of a computer-administered Addiction Severity Index: the ASI-MV. Psychol Addict Behav. 2001;15:4–12. doi: 10.1037/0893-164x.15.1.4. [DOI] [PubMed] [Google Scholar]
- 21.Butler SF, Newman FL, Cacciola JS. Predicting ASI interviewer severity ratings for a computer-administered Addiction Severity Index. Psychological Assessment. 1998;10:399–407. [Google Scholar]
- 22.Wakefield JC, Kelsall JE, Morris SE. In: Spatial epidemiology. Elliot P, Wakefield JC, Best NG, Briggs DJ, editors. Oxford University Press; New York: 2000. [Google Scholar]
- 23.Naus JI. The distribution of the size of the maximum cluster of points on a line. Journal of the American Statistical Association. 1965;60:532–538. [Google Scholar]
- 24.Brownstein JS, Rosen H, Purdy D, et al. Spatial analysis of West Nile virus: rapid risk assessment of an introduced vector-borne zoonosis. Vector Borne & Zoonotic Diseases. 2002;2:157–64. doi: 10.1089/15303660260613729. [DOI] [PubMed] [Google Scholar]
- 25.Hjalmars U, Kulldorff M, Gustafsson G, et al. Childhood leukaemia in Sweden: using GIS and a spatial scan statistic for cluster detection. Stat Med. 1996;15:707–15. doi: 10.1002/(sici)1097-0258(19960415)15:7/9<707::aid-sim242>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Kulldorff M. A spatial scan statistic. Commun Stat-Theor M. 1997;26:1481–1496. [Google Scholar]
- 27.Kulldorff M, Feuer EJ, Miller BA, et al. Breast cancer clusters in the northeast United States: a geographic analysis. Am J Epidemiol. 1997;146:161–70. doi: 10.1093/oxfordjournals.aje.a009247. [DOI] [PubMed] [Google Scholar]
- 28.Sudakin DL, Power LE. Regional and temporal variation in methamphetamine-related incidents: applications of spatial and temporal scan statistics. Clinical Toxicology. 2009;47:243–46. doi: 10.1080/15563650802516160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanson CE, Wieczorek WF. Alcohol mortality: a comparison of spatial clustering methods. Social Science and Medicine. 2002;55:791–802. doi: 10.1016/s0277-9536(01)00203-9. [DOI] [PubMed] [Google Scholar]
- 30.Kulldorff M, Nargarwalla N. Spatial disease clusters: detection and inference. Stat Med. 1995;14:799–810. doi: 10.1002/sim.4780140809. [DOI] [PubMed] [Google Scholar]
- 31.Dwass M. Modified randomization tests for nonparametric hypotheses. Ann Math Stat. 1957;28:181–87. [Google Scholar]
- 32.Smith MY, Schneider MF, Wentz A, et al. Quantifying morbidity associated with the abuse and misuse of opioid analgesics: a comparison of two approaches. Clin Toxicol (Phila) 2007;45:23–30. doi: 10.1080/15563650600956170. [DOI] [PubMed] [Google Scholar]
- 33.ESRI. (ed. 9.2, A.) (2006).
- 34.US Census Bureau. US Dept Commerce, Economics and Statistics Administration. Washington, D.C: 2001. [Google Scholar]
- 35.U.S. Department of Justice. National Drug Intelligence Center. 2002. [Google Scholar]
- 36.Poshyachinda V. Drug injecting and HIV infection among the population of drug abusers in Asia. Bull Narc. 1993;45:77–90. [PubMed] [Google Scholar]
- 37.Kulsudjarit K. Drug problem in southeast and southwest Asia. Ann N Y Acad Sci. 2004;1025:446–57. doi: 10.1196/annals.1316.055. [DOI] [PubMed] [Google Scholar]
- 38.Drug Enforcement Agency. Briefs and Backgrounds, state fact sheet. New Mexico: 2007. Available at: http://www.dea.gov/pubs/states/newmexico.html. [Google Scholar]
- 39.Seidler R. Prescription Drug Abuse: Opioids. Current Therapeutics. 2002:33–35. Available at: http://www.nswrdn.com.au/client_images/6700.pdf.
- 40.Degenhardt L, Black E, Breen C, et al. Trends in morphine prescriptions, illicit morphine use and associated harms among regular injecting drug users in Australia. Drug Alcohol Rev. 2006;25:403–12. doi: 10.1080/09595230600868504. [DOI] [PubMed] [Google Scholar]
- 41.O’Brien S, Black E, Roxburgh A, et al. Australian Drug Trends 2006:Findings from the Illicit Drug Reporting System (IDRS) NDARC Monograph. 2007;60 [Google Scholar]
- 42.Buehler JW, Hopkins RS, Overhage JM. Framework for evaluating public health surveillance systems for early detection of outbreaks: Recommendations from the CDC working group. MMWR. 2004;53:1–11. [PubMed] [Google Scholar]
- 43.Green TC, Brownstein JS, Butler SF. College on Problems of Drug Dependence. Reno, NV: 2009. [Google Scholar]
- 44.Brands B, Blake J, Sproule B, et al. Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug Alcohol Depend. 2004;73:199–207. doi: 10.1016/j.drugalcdep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Rosenblum A, Parrino M, Schnoll SH, et al. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend. 2007;90:64–71. doi: 10.1016/j.drugalcdep.2007.02.012. [DOI] [PubMed] [Google Scholar]