Abstract
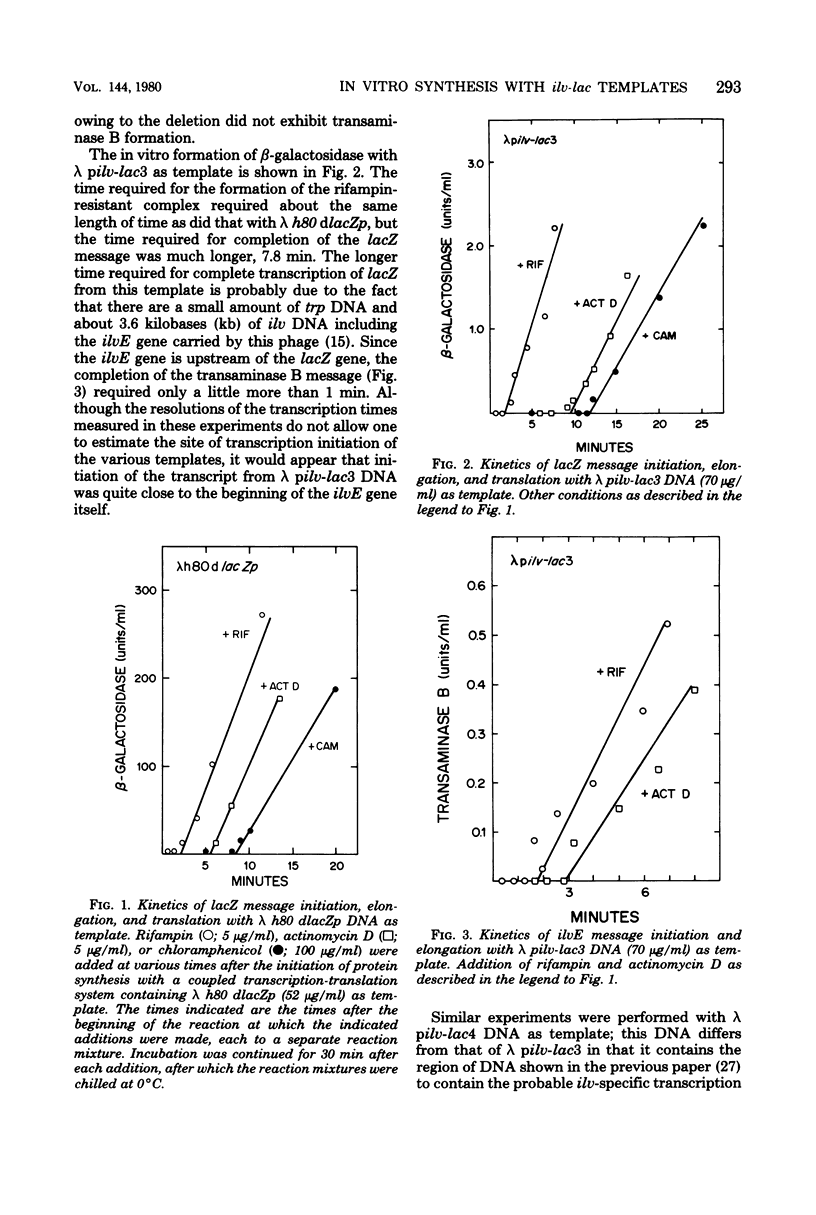
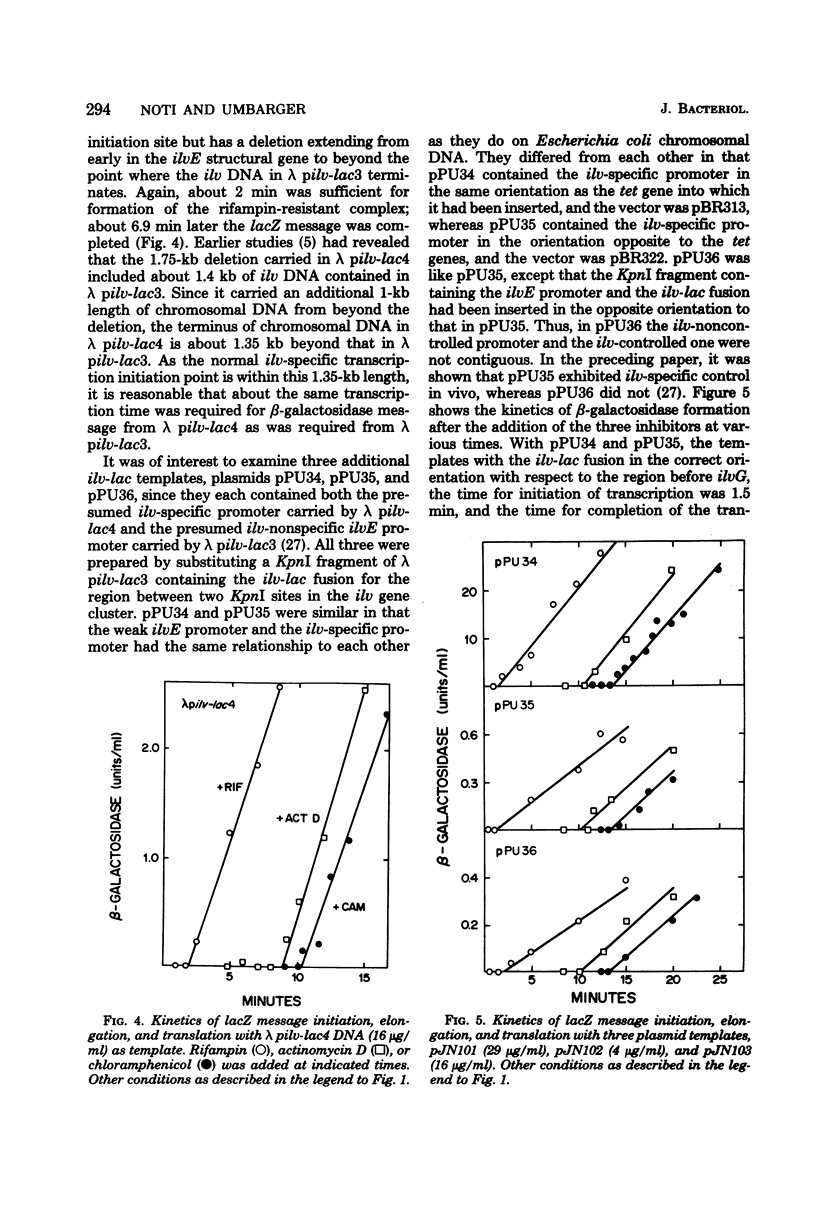
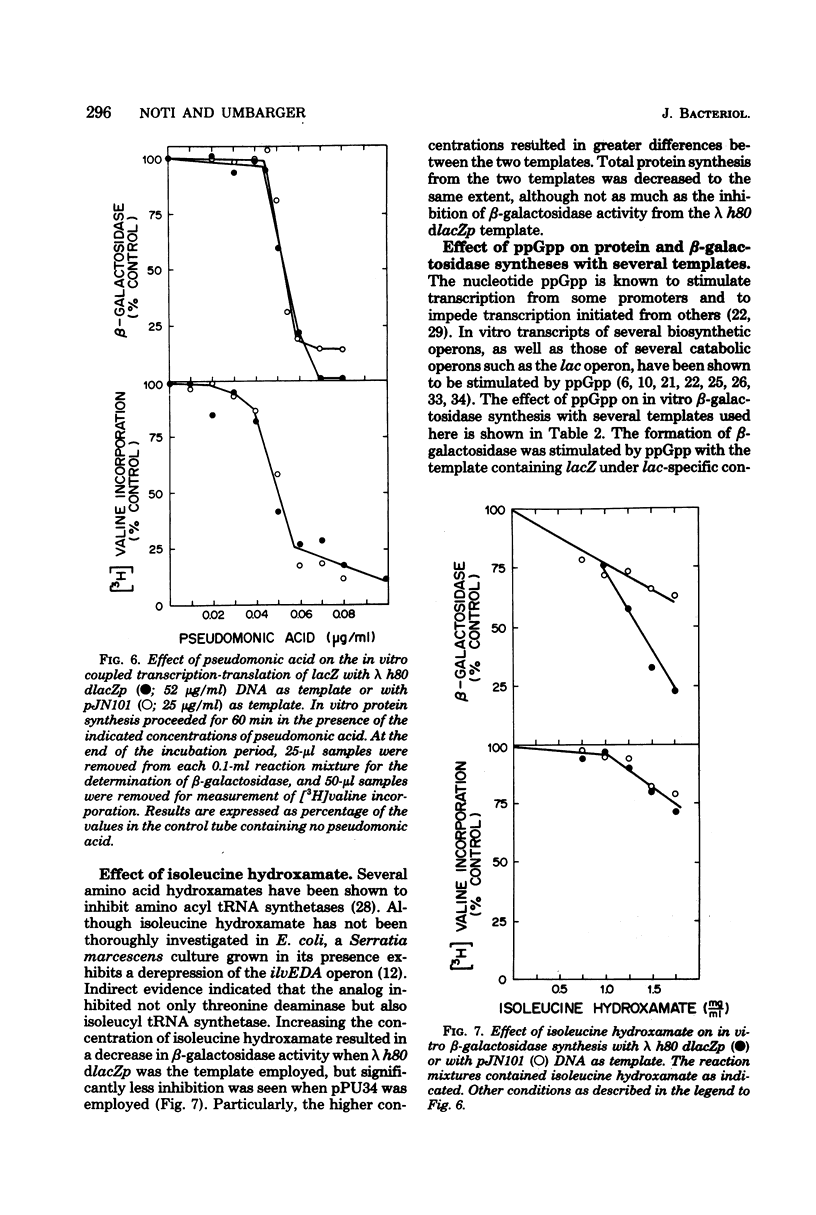
An in vitro coupled transcription-translation system was used to synthesize transaminase B and beta-galactosidase in the presence of a deoxyribonucleic acid template containing lac deoxyribonucleic acid under normal lac-specific control and in the presence of several deoxyribonucleic acid templates containing lac deoxyribonucleic acid fused to the ilvD gene. Time course experiments revealed that transcription of the lacZ gene from the fusion template required a longer time than did that initiated at the lac promoter. With a phage template containing an intact ilvE gene but lacking the normal ilv-specific promoter, synthesis of ilvE message was completed before synthesis of lacZ message. A phage template that contained the normal ilv-specific promoter but from which part of ilvE had been deleted also allowed formation of beta-galactosidase. Three plasmids containing the ilv-lac fusion were also used as templates. Two plasmids that contained both an intact ilvE gene and the normal ilv-specific promoter required longer times for lacZ transcription but were more efficient templates than was a plasmid in which the ilv-lac fusion, the ilvE gene, and the contiguous non-specific ilvE promoter were inverted with respect to the normal ilv-specific promoter. beta-Galactosidase synthesis was stimulated by guanosine 3'-pyrophosphate-5'-pyrophosphate with all templates tested except that in which the ilv-lac fusion had been inverted. Presumptive evidence was obtained for the generation of a limiting isoleucine signal by incorporating inhibitors of isoleucyl transfer ribonucleic acid synthetase into the coupled transcription-translation system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artz S. W., Broach J. R. Histidine regulation in Salmonella typhimurium: an activator attenuator model of gene regulation. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3453–3457. doi: 10.1073/pnas.72.9.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Gottesman M., Pastan I., Varmus H. E., Emmer M., Perlman R. L. Regulation of lac mRNA synthesis in a soluble cell-free system. Nat New Biol. 1971 Mar 10;230(10):37–40. doi: 10.1038/newbio230037a0. [DOI] [PubMed] [Google Scholar]
- Duggan D. E., Wechsler J. A. An assay for transaminase B enzyme activity in Escherichia coli K-12. Anal Biochem. 1973 Jan;51(1):67–79. doi: 10.1016/0003-2697(73)90453-3. [DOI] [PubMed] [Google Scholar]
- Gayda D. J., Leathers T. D., Noti J. D., Smith F. J., Smith J. M., Subrahmanyam C. S., Umbarger H. E. Location of the multivalent control site for the ilvEDA operon of Escherichia coli. J Bacteriol. 1980 May;142(2):556–567. doi: 10.1128/jb.142.2.556-567.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heincz M. C., McFall E. Role of small molecules in regulation of D-serine deaminase synthesis. J Bacteriol. 1978 Oct;136(1):104–110. doi: 10.1128/jb.136.1.104-110.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler E., Calvin M. Isoleucyl transfer ribonucleic acid synthetase of Escherichia coli B. A rapid kinetic investigation of the L-isoleucine-activating reaction. Biochemistry. 1972 Sep 26;11(20):3741–3752. doi: 10.1021/bi00770a012. [DOI] [PubMed] [Google Scholar]
- Hughes J., Mellows G. Inhibition of isoleucyl-transfer ribonucleic acid synthetase in Escherichia coli by pseudomonic acid. Biochem J. 1978 Oct 15;176(1):305–318. doi: 10.1042/bj1760305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H. M., Barnes W. M., Chumley F. G., Bossi L., Roth J. R. Model for regulation of the histidine operon of Salmonella. Proc Natl Acad Sci U S A. 1980 Jan;77(1):508–512. doi: 10.1073/pnas.77.1.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelker N. E., Maas W. K., Yang H. L., Zubay G. In vitro synthesis and repression of argininosuccinase in Escherichia coli K12; partial purification of the arginine repressor. Mol Gen Genet. 1976 Feb 27;144(1):17–20. doi: 10.1007/BF00277298. [DOI] [PubMed] [Google Scholar]
- Kelker N., Eckhardt T. Regulation of argA operon expression in Escherichia coli K-12: cell-free synthesis of beta-galactosidase under argA control. J Bacteriol. 1977 Oct;132(1):67–72. doi: 10.1128/jb.132.1.67-72.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Sugiura M., Chibata I. Isoleucine hydroxamate, an isoleucine antagonist. J Bacteriol. 1971 Sep;107(3):741–745. doi: 10.1128/jb.107.3.741-745.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. F., Brot N., Spears C., Chen B., Weissbach H. Studies on the in vitro transcription and translation of the lac operon. Arch Biochem Biophys. 1974 Jan;160(1):168–174. doi: 10.1016/s0003-9861(74)80023-8. [DOI] [PubMed] [Google Scholar]
- Kung H., Tainsky M., Weissbach H. Regulation of the in vitro synthesis of the alpha-peptide of beta-galactosidase directed by a restriction fragment of the lactose operon. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1000–1010. doi: 10.1016/0006-291x(78)91450-x. [DOI] [PubMed] [Google Scholar]
- Leathers T. D., Noti J., Umbarger H. E. Physical characterization of ilv-lac fusions. J Bacteriol. 1979 Oct;140(1):251–260. doi: 10.1128/jb.140.1.251-260.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinthal M., Nikaido H. Consequences of deletion mutations joining two operons of opposite polarity. J Mol Biol. 1969 Jun 28;42(3):511–520. doi: 10.1016/0022-2836(69)90239-3. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Post L., Nomura M. DNA-dependent in vitro synthesis of fibosomal proteins, protein elongation factors, and RNA polymerase subunit alpha: inhibition by ppGpp. Cell. 1976 Nov;9(3):439–448. doi: 10.1016/0092-8674(76)90089-1. [DOI] [PubMed] [Google Scholar]
- McCorkle G. M., Leathers T. D., Umbarger H. E. Physical organization of the ilvEDAC genes of Escherichia coli strain K-12. Proc Natl Acad Sci U S A. 1978 Jan;75(1):89–93. doi: 10.1073/pnas.75.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargang F. E., Subrahmanyam C. S., Umbarger H. E. Nucleotide sequence of ilvGEDA operon attenuator region of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1823–1827. doi: 10.1073/pnas.77.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks J. S., Gottesman M., Perlman R. L., Pastan I. Regulation of galactokinase synthesis by cyclic adenosine 3',5'-monophosphate in cell-free extracts of Escherichia coli. J Biol Chem. 1971 Apr 25;246(8):2419–2424. [PubMed] [Google Scholar]
- Primakoff P., Artz S. W. Positive control of lac operon expression in vitro by guanosine 5'-diphosphate 3'-diphosphate. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1726–1730. doi: 10.1073/pnas.76.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiness G., Yang H. L., Zubay G., Cashel M. Effects of guanosine tetraphosphate on cell-free synthesis of Escherichia coli ribosomal RNA and other gene products. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2881–2885. doi: 10.1073/pnas.72.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier A. A., Schimmel P. R. Transfer ribonucleic acid synthetase catalyzed deacylation of aminoacyl transfer ribonucleic acid in the absence of adenosine monophosphate and pyrophosphate. Biochemistry. 1972 Apr 25;11(9):1582–1589. doi: 10.1021/bi00759a006. [DOI] [PubMed] [Google Scholar]
- Sidikaro J., Masayasu N. In vitro synthesis of the E3 immunity protein directed by Col E3 plasmid deoxyribonucleic acid. J Biol Chem. 1975 Feb 10;250(3):1123–1131. [PubMed] [Google Scholar]
- Smolin D. E., Umbarger H. E. Specificity of the stimulation of in vitro ribonucleic acid synthesis by guanosine 5'-diphosphate 3'-diphosphate. Mol Gen Genet. 1975 Dec 9;141(4):277–284. doi: 10.1007/BF00331449. [DOI] [PubMed] [Google Scholar]
- Stephens J. C., Artz S. W., Ames B. N. Guanosine 5'-diphosphate 3'-diphosphate (ppGpp): positive effector for histidine operon transcription and general signal for amino-acid deficiency. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4389–4393. doi: 10.1073/pnas.72.11.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subrahmanyam C. S., Noti J. D., Umbarger H. E. Regulation of ilvEDA expression occurs upstream of ilvG in Escherichia coli: additional evidence for an ilvGEDA operon. J Bacteriol. 1980 Oct;144(1):279–290. doi: 10.1128/jb.144.1.279-290.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosa T., Pizer L. I. Biochemical bases for the antimetabolite action of L-serine hydroxamate. J Bacteriol. 1971 Jun;106(3):972–982. doi: 10.1128/jb.106.3.972-982.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. RNA polymerase specificity and the control of growth. Nature. 1976 Oct 21;263(5579):641–646. doi: 10.1038/263641a0. [DOI] [PubMed] [Google Scholar]
- Vonder Haar R. A., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli K-12: detection and measurement of ilv-specific messenger ribonucleic acid. J Bacteriol. 1974 Nov;120(2):687–696. doi: 10.1128/jb.120.2.687-696.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M. D., Wild J., Umbarger H. E. Positive control of ilvC expression in Escherichia coli K-12; identification and mapping of regulatory gene ilvY. J Bacteriol. 1979 Sep;139(3):1014–1020. doi: 10.1128/jb.139.3.1014-1020.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox G., Meuris P., Bass R., Englesberg E. Regulation of the L-arabinose operon BAD in vitro. J Biol Chem. 1974 May 10;249(9):2946–2952. [PubMed] [Google Scholar]
- Wild J., Smith J. M., Umbarger H. E. In vitro synthesis of beta-galactosidase with ilv-lac fusion deoxyribonucleic acid as template. J Bacteriol. 1977 Dec;132(3):876–883. doi: 10.1128/jb.132.3.876-883.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Urm E., Heiness G., Cashel M. Effects of guanosine tetraphosphate, guanosine pentaphosphate, and beta-gamma methylenyl-guanosine pentaphosphate on gene expression of Escherichia coli in vitro. Proc Natl Acad Sci U S A. 1974 Jan;71(1):63–67. doi: 10.1073/pnas.71.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalkin H., Yanofsky C., Squires C. L. Regulated in vitro synthesis of Escherichia coli tryptophan operon messenger ribonucleic acid and enzymes. J Biol Chem. 1974 Jan 25;249(2):465–475. [PubMed] [Google Scholar]
- Zubay G., Lederman M., DeVries J. K. DNA-directed peptide synthesis. 3. Repression of beta-galactosidase synthesis and inhibition of repressor by inducer in a cell-free system. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1669–1675. doi: 10.1073/pnas.58.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G., Morse D. E., Schrenk W. J., Miller J. H. Detection and isolation of the repressor protein for the tryptophan operon of Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1100–1103. doi: 10.1073/pnas.69.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]



