Abstract
Background
The non-O alleles of the ABO genotype have been associated with an increased risk of thrombosis. Risk associated with the specific A1, A2, or B alleles is not well defined.
Objectives
To examine the association of ABO genotype with myocardial infarction (MI), ischemic stroke, hemorrhagic stroke, and venous thrombosis (VT).
Patients/Methods
We used data from 2 ongoing population-based case-control studies of MI, stroke, and VT. Cases included hypertensive adults and post-menopausal women with incident non-fatal MI (n=1063), ischemic stroke (n=469), and hemorrhagic stroke (n=91), and postmenopausal women with incident non-fatal VT (n=504). Controls were frequency matched to cases on age, sex, hypertension status, and year of identification. ABO genotypes were determined using single nucleotide polymorphisms and subjects were grouped by diplotype according to the presence of O1, O2, A11, A2, and B alleles. Logistic regression was used to test the association of diplotypes with risk of each outcome.
Results
Compared with the O1O1 group, the A11 allele was associated with an increased risk of VT (odds ratio [OR] 1.79, 95% confidence interval: 1.41–2.26) and MI (OR 1.23 [1.05–1.44]). The B allele was associated with an increased risk of VT (OR 1.82 [1.29–2.57]) and ischemic stroke (OR 1.59 [1.17–2.17]). The AB diplotype category was associated with a 2.7-fold risk of VT (OR 2.70 [1.73–4.21]). No other associations reached significance.
Conclusions
The VT and MI findings are confirmatory and the ischemic stroke finding with the B allele is a novel and needs replication.
Keywords: ABO genotype, hemorrhagic stroke, ischemic stroke, genetic polymorphism, myocardial infarction, venous thrombosis
The presence of the A and B blood group antigens, expressed on red blood cells and other cells and molecules within the body, has been associated with risk of both arterial and venous thrombosis. Most studies indicate an increased risk of thrombosis associated with the non-O blood group.[1–7] Early investigations relied on blood-group classifications, but with the discovery of the ABO gene and recent advances in genotyping techniques, the effect of blood group genotype on the risk of thrombosis can be more finely investigated.
The ABO gene codes for several glycosyl transferases that add sugar residues to the H(O) antigen thus forming the A and B antigens. These antigens reside on the surface of vWF, a carrier protein for coagulation factor VIII (FVIII).[8, 9] The clearance of vWF is associated with the ABO antigen type, and in particular the presence of the A or B antigens.[8, 10, 11] Risk of thrombosis has been shown to be increased with higher levels of vWF and FVIII, [12–17] and it is through the effect of ABO antigens on vWF clearance that ABO genotypes are hypothesized to affect thrombotic risk.[8, 10, 18–21] Although most studies of ABO genotype and risk of arterial and venous thrombosis have reported an increased risk of thrombosis with the non-O alleles,[15, 22–28] results vary among studies examining risks associated with the specific A1, A2, or B alleles. Investigations of ABO alleles and the risk of hemorrhagic stroke have not been reported.
We hypothesized that the A1 and B alleles would be associated with an increased risk of arterial and venous thrombosis compared with the O allele. We also explored the association of ABO alleles and hemorrhagic stroke. Our analytic approach involved genotype, haplotype, and diplotype associations with risks of thrombotic events and hemorrhagic stroke.
Methods
Setting and study design
The setting for this study was Group Health (GH), a large integrated health care system in western Washington State. Data were utilized from 2 ongoing population-based, case-control studies: 1 including incident myocardial infarction (MI) and stroke cases and a second including incident venous thrombosis (VT) cases. The 2 studies shared a common control group. Methods for the 2 studies have been described previously and are briefly summarized below.[29, 30] Both studies were approved by the human subjects committee at GH, and written informed consent was provided by all study participants.
Population
All study participants were GH members. MI and stroke cases and controls were aged 30–79 years and included men pharmaceutically treated for hypertension and women who were either pharmaceutically treated for hypertension or were peri- or postmenopausal. VT cases and controls were peri- or postmenopausal women aged 30–89 years. Case subjects experienced a non-fatal incident event occurring between January 1, 1995 and December 31, 2004, and were identified from several sources. Myocardial infarction and stroke cases were hospitalized and identified from computerized hospital discharge abstracts and billing records.[29, 30] VT cases, incident deep vein thrombosis and pulmonary embolism, were identified from both inpatient and outpatient computerized sources.[31] Controls were a random sample of GH members frequency matched to MI cases on age, sex, treated hypertension, and calendar year of identification.[29, 30] The index date for controls was a computer-generated random date within the calendar year for which they had been selected. For MI and stroke cases, the index date was the date of admission for the first acute MI or stroke, and for VT cases, the index date was the date of VT diagnosis.
Data collection
Eligibility and risk factor information were collected by trained medical record abstractors from a review of the GH medical record using only data available prior to the index date. Data on race were obtained from a telephone interview, and a venous blood sample was collected from all consenting subjects. DNA was extracted from white blood cells using standard procedures.
Genotyping
ABO single-nucleotide polymorphisms (SNPs) were selected for genotyping to allow discrimination among the common ABO alleles: O1 (including subtypes O11 and O12), O2, A1 (including subtypes A11 and A12), A2, and B.[32] Subtypes O12 and O11 contain the same G deletion at nucleotide 261 that renders the transferase enzyme inactive, but O12 differs from O11 by an additional 9 nucleotide mutations.[9] Subtype A12 differs from A11 by a C467T mutation that causes an amino acid substitution but does not appear to affect enzyme activity. [32] Genotyping of the ABO SNPs was performed using a GoldenGate custom panel using BeadArray® technology. Of the 14 SNPs selected, 11 were successfully genotyped on the Illumina platform, and 99.8% of the nucleotide pairs from the genotyped SNPs were successfully called. Four of the 11 SNPs were in high linkage disequilibrium with other SNPs and were dropped from further analyses. A total of 7 SNPs were included in the analyses.
Haplotype and Diplotype Construction
Haplotype data were inferred from SNPs using PHASE 2.1 software (University of Washington, Seattle; http://www.stat.washington.edu/stephens/software.html), which computes probabilities for each haplotype pair consistent with the observed data.[33, 34] When uncertainty in the haplotype estimation occurred, subjects were assigned multiple haplotype pairs, each with a probability. Six haplotypes with a frequency of 2% or greater were inferred from PHASE. These haplotypes from PHASE were the same as the ABO haplotypes that code for the specific ABO alleles previously described[32], and the frequencies were consistent with those reported for Caucasians.[35] Haplotypes with a frequency less than 2% in the study population were combined into a single “other” haplotype. Due to genotyping failures, we were not able to distinguish haplotype A12 from A2. Since the A2 allele is believed to be more common in Caucasians than the A12 allele, [9, 36] we refer to the allele coded by the haplotype for A12/A2 as A2 throughout this paper.
Using the haplotype pairs inferred by PHASE, diplotypes were constructed for each individual as indicator variables and probability weights applied in regression analyses for subjects with multiple possible haplotype pairs.
Data analysis
For the MI and stroke analyses, MI cases with a prior history of stroke, stroke cases with a prior history of MI, and controls with a prior history of either MI or stroke were excluded from the study. Controls with a prior VT were excluded for the VT analyses.
Odds ratios (ORs) and 95% confidence intervals (CIs) were assessed for the associations between each SNP and each of the outcomes using separate logistic regression models and adjusting for race and the matching criteria of age, sex, hypertensive status, and index year. In all analyses, an additive model was assumed with each SNP modeled as 0, 1, or 2 copies of the minor allele.
For haplotype analyses, OR and CI estimates were obtained using weighted logistic regression with sandwich variance estimators and haplotype probability weights. The number of haplotypes (0, 1, or 2) was used as the predictor, and the most common haplotype was used as the reference group in adjusted analyses.
Haplotypes produced 27 observed diplotypes. Because many of the diplotypes were rare, we grouped diplotypes into 6 hierarchical and mutually exclusive diplotype categories according to allelic presence: both A and B, any B, any A11, any A2, and two O alleles, with diplotypes containing one O1 allele and one O2 allele (diplotype category O1O2) separated from diplotypes containing two O1 alleles (diplotype category O1O1). Using the O1O1 diplotype category as reference, weighted logistic regression with sandwich variance estimators was employed to estimate OR and CI for each of the diplotype categories. To investigate the possibility that the association between ABO genotype and thrombosis is due to H antigen expression, we grouped the diplotypes by the amount of H antigen expressed. We also report on the comparison of O vs non-O genotypes.
Results
There were 1063 MI, 469 ischemic stroke, and 91 hemorrhagic stroke cases and 3462 frequency-matched controls included in the MI and stroke analyses. For VT analyses, 504 VT cases and 2172 controls were included. A summary of the subject characteristics is presented in Table 1. Mean ages of cases and controls ranged between 65 and 68 years, and most subjects were Caucasian. As would be expected, event-associated risk factors were more common in case subjects than in their controls.
Table 1.
Characteristics of Study Participants
| MI cases | Isch Stroke Cases |
Hem Stroke Cases |
MI/Stroke Controls |
VT Cases | VT Controls |
|
|---|---|---|---|---|---|---|
| Characteristic* | n=1063 | n=469 | n=91 | n=3462 | n=504 | n=2172 |
| Age, years | 66 | 68 | 65 | 65 | 67 | 68 |
| Female, % | 57 | 70 | 71 | 58 | 100 | 100 |
| Caucasian, % | 95 | 91 | 88 | 91 | 94 | 93 |
| Treated hypertension, % | 73 | 73 | 64 | 73 | 44 | 53 |
| Treated hyperlipidemia, % | 19 | 16 | 8 | 15 | 12 | 12 |
| Diabetes, % | 24 | 25 | 8 | 12 | 8 | 9 |
| Current smoker, % | 19 | 12 | 14 | 10 | 8 | 9 |
| Most recent systolic blood pressure, mmHg | 142 | 146 | 145 | 137 | 135 | 137 |
| Most recent diastolic blood pressure, mmHg | 80 | 81 | 83 | 80 | 78 | 78 |
| Untreated systolic blood pressure, mmHg | 164 | 169 | 166 | 161 | 164 | 165 |
| Untreated diastolic blood pressure, mmHg | 99 | 98 | 99 | 99 | 97 | 97 |
| Total cholesterol, mg/dl | 229 | 226 | 223 | 218 | 225 | 227 |
| High density lipoprotein, mg/dl | 49 | 52 | 58 | 54 | 59 | 60 |
| Glucose, mg/dl | 124 | 132 | 106 | 110 | 111 | 107 |
| Body mass index, kg/m2 | 30 | 30 | 28 | 30 | 31 | 29 |
| History of cardiovascular disease†, % | 22 | 15 | 6 | 11 | 21 | 14 |
| History of venous thrombosis, % | 1 | 4 | 6 | 2 | 0 | 0 |
| History of arterial thrombosis, % | 0 | 0 | 0 | 0 | 13 | 7 |
| Menopausal status: | ||||||
| peri- or postmenopausal, % (women only) | 97 | 99 | 100 | 97 | 100 | 100 |
| premenopausal, % (women only) | 3 | 2 | 0 | 3 | 0 | 0 |
| Current estrogen use, % (women only) | 35 | 33 | 40 | 33 | 38 | 34 |
| # visits | 7 | 7 | 5 | 6 | 10 | 6 |
| Years at Group Health | 19 | 20 | 23 | 22 | 22 | 23 |
Values are expressed as means unless otherwise noted
Includes history of any of the following: angina, stroke, claudication, coronary artery bypass grafting, angioplasty,
SNPs and Haplotypes
The associations of ABO SNPs and haplotypes with risk of MI, ischemic stroke, hemorrhagic stroke, and VT are summarized in Table 2. Table columns show 7 SNPs used in the analyses with OR, 95% CI, and p-values for the association of each SNP with each of the outcomes of interest. Minor alleles for the SNPs are given in bold. Rows in the table present the 6 common haplotypes and the combined group of rare haplotypes, the frequency of the haplotypes among controls, and the alleles defining the haplotypes along with OR, 95% CI, and p-values for the association of each haplotype with each of the 4 outcomes.
Table 2.
SNPs and Haplotypes
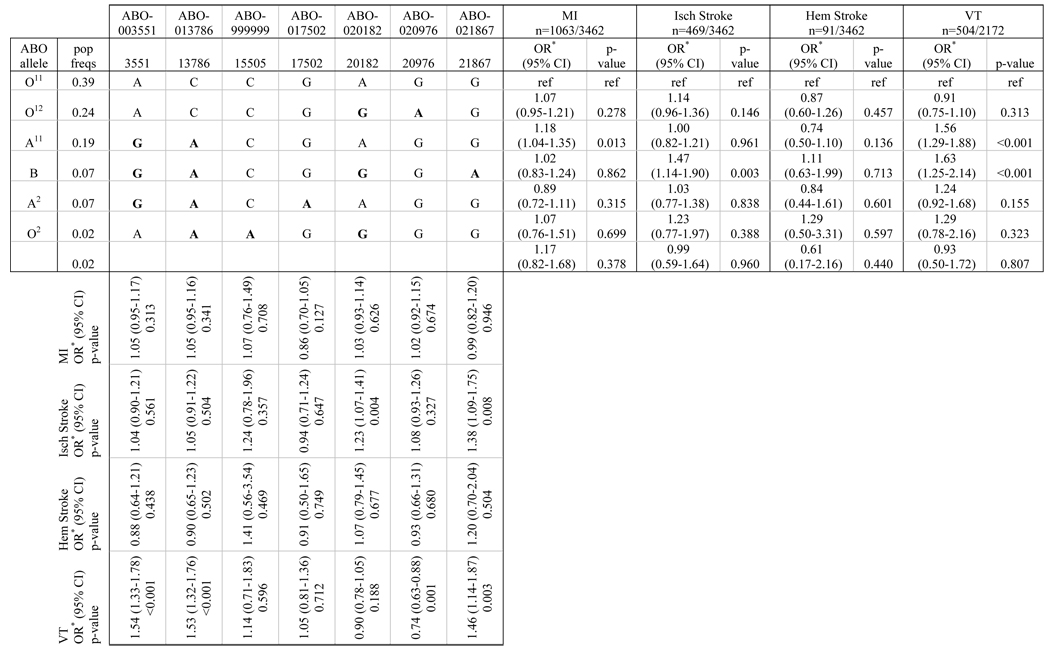 |
Adjusted for age, sex, treated hypertension, index year, and race
We found 2 haplotypes associated with an increased risk of VT compared with the reference haplotype O11, and these same haplotypes were either associated with an increased risk of MI or an increased risk of ischemic stroke. Compared with the O11 allele, risk of VT increased by 56% with the A11 allele (OR 1.56 [1.29–1.88]), and risk of MI increased by 18% with the A11 allele (OR 1.18 [1.04–1.35]). Presence of the B allele was associated with a 63% increased risk of VT (OR 1.63 [1.25–2.14]), and a 47% increased risk of ischemic stroke (OR 1.47 [1.14–1.90]). No associations were noted between haplotypes or SNPs and risk of hemorrhagic stroke.
Results from the SNP analyses correspond to the haplotype results. SNPs ABO-003551 and ABO-013786 distinguish the A11 haplotype from the reference haplotype, and both are associated with an increased risk of VT. Risk of ischemic stroke is increased with SNPs ABO-020182 and ABO-021867, which both distinguish the B haplotype from the other haplotypes. ABO-021867 also shows an association with VT, and risk of VT is decreased with the SNP that distinguishes O12 from A11 (ABO-020976).
Diplotypes
Table 3 presents the primary diplotype analyses. As with the haplotypes, the associations observed between diplotype category and MI and ischemic stroke outcomes were also observed with the outcome of VT. The A11 diplotype category was associated with a 79% increased risk of VT (OR 1.79 [1.41–2.26]) and a 23% increased risk of MI compared with the O1O1 diplotype category (OR 1.23 [1.05–1.44]). The B diplotype category was associated with an 82% increase in risk of VT (OR 1.82 [1.29–2.57]) and a 59% increase in risk of ischemic stroke compared with the O1O1 diplotype category (OR 1.59 [1.17–2.17]). Additionally, the AB diplotype category was associated with a 2.7-fold risk of VT (OR 2.70 [1.73–4.21]). This association was not observed among MI or ischemic stroke cases. No significant associations between diplotype categories and risk of hemorrhagic stroke were noted in our analyses. We attempted to analyze diplotypes grouped to reflect H antigen expression and to report explicitly on the effect of A11A11, A11B and BB diplotypes alone, however this categorization of diplotypes created groupings with too few subjects in several categories and risk estimates were not reliable. In our comparison of O vs non-O genotypes, the non-O category was not associated with risk of MI, ischemic stroke, or hemorrhagic stroke but was associated with a 77% increased risk of VT (OR 1.77, 95% CI 1.43–2.18).
Table 3.
Diplotypes
| MI n=1063/3462 |
Isch Stroke n=469/3462 |
Hem Stroke n=91/3462 |
VT n=504/2172 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Diplotype Category† |
pop freqs |
OR* (95% CI) | p- value |
OR* (95% CI) | p- value |
OR* (95% CI) | p- value |
OR* (95% CI) | p- value |
| O1O1 | 0.42 | ref | ref | ref | ref | ref | ref | ref | ref |
| O1O2 | 0.03 | 1.10 (0.73–1.65) | 0.660 | 1.11 (0.61–2.01) | 0.737 | 1.65 (0.57–4.76) | 0.351 | 0.64 (0.28–1.48) | 0.295 |
| A11 | 0.32 | 1.23 (1.05–1.44) | 0.012 | 1.01 (0.80–1.28) | 0.906 | 0.91 (0.55–1.51) | 0.722 | 1.79 (1.41–2.26) | <0.001 |
| A2 | 0.10 | 0.81 (0.63–1.06) | 0.125 | 1.07 (0.76–1.51) | 0.711 | 0.82 (0.38–1.78) | 0.616 | 1.28 (0.89–1.85) | 0.182 |
| B | 0.10 | 1.17 (0.92–1.49) | 0.191 | 1.59 (1.17–2.17) | 0.003 | 1.43 (0.73–2.79) | 0.295 | 1.82 (1.29–2.57) | 0.001 |
| AB | 0.04 | 0.80 (0.52–1.23) | 0.312 | 0.93 (0.52–1.65) | 0.793 | 0.60 (0.14–2.56) | 0.488 | 2.70 (1.73–4.21) | <0.001 |
Adjusted for age, sex, treated hypertension, index year, and race
- O1O1 = O11O11, O11O12, or O12O12
- O1O2 = O11O2 or O12O2
- A11 = A11A11, A11A2, A11O11, A11O12, or A11O2
- A2 = A2A2, A2O11, A2O12, or A2O2
- B = BB, BO11, BO12, or BO2
- AB = A11B or A2B
Discussion
In this population-based case-control study, we found that risk of arterial and venous thrombosis increased with the presence of the A1 and B alleles. Haplotype results were consistent with the diplotype results for MI, ischemic stroke, and VT. No association between risk of hemorrhagic stroke and ABO haplotype or diplotype categories was detected.
This study has several limitations. The case-control design of the study did not allow us to collect DNA information on fatal events or from those who did not survive until study contact. Findings may be biased if a genotype is associated with increased mortality. Because of population characteristics, MI and stroke findings may not apply to nonhypertensive men and VT findings may not apply to men. We were unable to investigate the association of the A2 or O1O2 diplotype categories due to the small number of subjects within each category. The method of genotyping employed did not allow for distinction between the A2 allele and the rare A12 allele and it was not be possible to explore independent associations. Finally, the number of cases of hemorrhagic stroke was small, limiting our statistical power for this outcome.
The increased risk of ischemic stroke with the B haplotype or B diplotype is a new finding. This finding is consistent with intermediate phenotype findings which have shown that plasma levels of vWF and FVIII are positively associated with the number of A1 or B alleles present.[10, 11, 21, 26, 27, 37] Only a few studies have examined the association between ABO blood group or genotype and risk of ischemic stroke, with most results non-significant.[38–40] In contrast to our results, an analysis of ABO genotype and cerebral ischemia of arterial origin showed a doubling of risk in those carrying at least one A allele (either A1 or A2) compared with all other genotypes.[22] No associations were present for the A1, A2, or B alleles individually.[22]
Our findings of an increased risk of MI with the presence of the A11 allele or A11 diplotype category are consistent with past studies of blood group phenotype and MI and also with the relationship between ABO genotype and levels of vWF and FVIII.[1, 2, 4, 41] Difficulty arises when comparing our results with those from studies examining the association between ABO genotype and risk of MI, however. Each study differed in its approach and in the reference groups utilized, and none examined the risk associated with the A1 allele independently. A case-control study of individuals examined with coronary angiography found a 39% decreased risk of MI with the presence of at least one O1 allele compared to no O1 alleles,[28] and a second smaller case-control study showed a three-fold risk of MI with the presence of the B allele compared to no B allele.[24] A prospective cohort study of postmenopausal women combined diplotypes for blood groups A and B in analyses and found that those with the A or B blood group genotype had almost a two-fold incidence of acute ischemic heart disease compared with those who were homozygous for the O allele.[25]
Studies of the association of ABO genotype and risk of VT have been more consistent than those for arterial risk. Analyses from a case-control study showed that those with the A1 allele had a three-fold risk of VT compared to those homozygous for the O allele.[27] In a second case-control study conducted in the Netherlands, the A1A1, A1O, B, and AB diplotype categories each were associated with a doubling of VT risk compared with the O1O1/O1O2 diplotype category.[23] Results similar to those observed with ABO genotypes also have been reported by studies investigating the relationship between ABO blood group phenotype and risk of VT.[3, 5, 7, 41] In addition, the findings of increased vWF and FVIII plasma levels in those with the A1 and B alleles support our results of increased risk of VT with the A11, B, and AB diplotype categories.[10, 11, 26, 27]
We found no association between ABO genotype and hemorrhagic stroke, possibly due in part to the limited power for this analysis. One study has reported results from an investigation of ABO blood groups and risk of hemorrhagic stroke, finding a excess of O and B blood groups among hemorrhagic stroke cases compared with controls.[39]
The relationship of ABO genotype with arterial and venous thrombosis appears to be mediated in part through vWF, which carries FVIII and protects it from degradation.[8] Current evidence suggests that the effect of ABO genotype on vWF and FVIII is due to the presence of the antigens A, B, and H(O) on vWF.[8, 10, 20, 21, 42] These antigens are believed to have an effect on the clearance of vWF, but the mechanism behind this effect is still unknown.[8, 10, 18–21] Circulating levels of vWF or FVIII were not measured in this study, so we could not explore the relationship between these levels and ABO genotype.
This study examined the association of ABO genotype and the risk of arterial and venous thrombosis. Our results showed an increased risk of MI and VT with the A11 allele, an increased risk of ischemic stroke and VT with the B allele, and an increased risk of VT with the AB diplotype. The ischemic stroke and B allele finding is novel and needs replication. Other findings are consistent with the findings from several other studies of arterial and venous thrombosis. We were unable to detect significant associations between the A11 allele and ischemic stroke and the B allele and MI, but the upper limits of the confidence intervals do not rule out possible risk. The exact mechanism through which ABO antigens affect vWF clearance has not been elucidated, and further research in this area might provide insight into ways in which ABO genotype affects both arterial and venous thrombosis and aide in better predicting thrombotic risk.
Supplementary Material
Acknowledgements
This study was supported by grants HL073410, HL068639, HL043201, HL060739, HL074745, and HL068986 from the National Heart, Lung, and Blood Institute; AG00956 from the National Institutes of Health; and The Leducq Foundation, Paris, France, for the development of Transatlantic Networks of Excellence in Cardiovascular Research.
References
- 1.Allan TM, Dawson AA. ABO blood groups and ischaemic heart disease in men. Br Heart J. 1968;30:377–382. doi: 10.1136/hrt.30.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bronte-Stewart B, Botha MC, Krut LH. ABO blood groups in relation to ischaemic heart disease. Br Med J. 1962;1:1646–1650. doi: 10.1136/bmj.1.5293.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jick H, Slone D, Westerholm B, Inman WH, Vessey MP, Shapiro S, Lewis GP, Worcester J. Venous thromboembolic disease and ABO blood type. A cooperative study. Lancet. 1969;1:539–542. doi: 10.1016/s0140-6736(69)91955-2. [DOI] [PubMed] [Google Scholar]
- 4.Medalie JH, Levene C, Papier C, Goldbourt U, Dreyfuss F, Oron D, Neufeld H, Riss E. Blood groups, myocardial infarction and angina pectoris among 10,000 adult males. N Engl J Med. 1971;285:1348–1353. doi: 10.1056/NEJM197112092852404. [DOI] [PubMed] [Google Scholar]
- 5.Robinson WM, Roisenberg I. Venous thromboembolism and ABO blood groups in a Brazilian population. Hum Genet. 1980;55:129–131. doi: 10.1007/BF00329140. [DOI] [PubMed] [Google Scholar]
- 6.Talbot S, Wakley EJ, Langman MJ. A19 A29 B, and O blood-groups, Lewis blood-groups, and serum triglyceride and cholesterol concentrations in patients with venous thromboembolic disease. Lancet. 1972;1:1152–1154. doi: 10.1016/s0140-6736(72)91375-x. [DOI] [PubMed] [Google Scholar]
- 7.Wautrecht JC, Galle C, Motte S, Dereume JP, Dramaix M. The role of ABO blood groups in the incidence of deep vein thrombosis. Thromb Haemost. 1998;79:688–689. [PubMed] [Google Scholar]
- 8.Jenkins PV, O'Donnell JS. ABO blood group determines plasma von Willebrand factor levels: a biologic function after all? Transfusion. 2006;46:1836–1844. doi: 10.1111/j.1537-2995.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- 9.Yip SP. Sequence variation at the human ABO locus. Ann Hum Genet. 2002;66:1–27. doi: 10.1017/S0003480001008995. [DOI] [PubMed] [Google Scholar]
- 10.Morelli VM, de Visser MC, van Tilburg NH, Vos HL, Eikenboom JC, Rosendaal FR, Bertina RM. ABO blood group genotypes, plasma von Willebrand factor levels and loading of von Willebrand factor with A and B antigens. Thromb Haemost. 2007;97:534–541. [PubMed] [Google Scholar]
- 11.Shima M, Fujimura Y, Nishiyama T, Tsujiuchi T, Narita N, Matsui T, Titani K, Katayama M, Yamamoto F, Yoshioka A. BO blood group genotype and plasma von Willebrand factor in normal individuals. Vox Sang. 1995;68:236–240. doi: 10.1111/j.1423-0410.1995.tb02579.x. [DOI] [PubMed] [Google Scholar]
- 12.Crawley JT, Lane DA, Woodward M, Rumley A, Lowe GD. Evidence that high von Willebrand factor and low ADAMTS-13 levels independently increase the risk of a non-fatal heart attack. J Thromb Haemost. 2008;6:583–588. doi: 10.1111/j.1538-7836.2008.02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folsom AR, Rosamond WD, Shahar E, Cooper LS, Aleksic N, Nieto FJ, Rasmussen ML, Wu KK. Prospective study of markers of hemostatic function with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Circulation. 1999;100:736–742. doi: 10.1161/01.cir.100.7.736. [DOI] [PubMed] [Google Scholar]
- 14.Kraaijenhagen RA, in't Anker PS, Koopman MM, Reitsma PH, Prins MH, van den Ende A, Buller HR. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost. 2000;83:5–9. [PubMed] [Google Scholar]
- 15.Ohira T, Cushman M, Tsai MY, Zhang Y, Heckbert SR, Zakai NA, Rosamond WD, Folsom AR. ABO blood group, other risk factors and incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE) J Thromb Haemost. 2007;5:1455–1461. doi: 10.1111/j.1538-7836.2007.02579.x. [DOI] [PubMed] [Google Scholar]
- 16.Tanis B, Algra A, van der Graaf Y, Helmerhorst F, Rosendaal F. Procoagulant factors and the risk of myocardial infarction in young women. Eur J Haematol. 2006;77:67–73. doi: 10.1111/j.1600-0609.2006.00656.x. [DOI] [PubMed] [Google Scholar]
- 17.Tracy RP, Arnold AM, Ettinger W, Fried L, Meilahn E, Savage P. The relationship of fibrinogen and factors VII and VIII to incident cardiovascular disease and death in the elderly: results from the cardiovascular health study. Arterioscler Thromb Vasc Biol. 1999;19:1776–1783. doi: 10.1161/01.atv.19.7.1776. [DOI] [PubMed] [Google Scholar]
- 18.Davies JA, Collins PW, Hathaway LS, Bowen DJ. von Willebrand factor: evidence for variable clearance in vivo according to Y/C1584 phenotype and ABO blood group. J Thromb Haemost. 2008;6:97–103. doi: 10.1111/j.1538-7836.2007.02809.x. [DOI] [PubMed] [Google Scholar]
- 19.Gallinaro L, Cattini MG, Sztukowska M, Padrini R, Sartorello F, Pontara E, Bertomoro A, Daidone V, Pagnan A, Casonato A. A shorter von Willebrand factor survival in O blood group subjects explains how ABO determinants influence plasma von Willebrand factor. Blood. 2008;111:3540–3545. doi: 10.1182/blood-2007-11-122945. [DOI] [PubMed] [Google Scholar]
- 20.Nossent AY, van Marion V, vanTilburg NH, Rosendaal FR, Bertina RM, van Mourik JA, Eikenboom HC. von Willebrand factor and its propeptide: the influence of secretion and clearance on protein levels and the risk of venous thrombosis. J Thromb Haemost. 2006;4:2556–2562. doi: 10.1111/j.1538-7836.2006.02273.x. [DOI] [PubMed] [Google Scholar]
- 21.O'Donnell J, Boulton FE, Manning RA, Laffan MA. Amount of H antigen expressed on circulating von Willebrand factor is modified by ABO blood group genotype and is a major determinant of plasma von Willebrand factor antigen levels. Arterioscler Thromb Vasc Biol. 2002;22:335–341. doi: 10.1161/hq0202.103997. [DOI] [PubMed] [Google Scholar]
- 22.Clark P, Meiklejohn DJ, O'Sullivan A, Vickers MA, Greaves M. The relationships of ABO, Lewis and Secretor blood groups with cerebral ischaemia of arterial origin. J Thromb Haemost. 2005;3:2105–2108. doi: 10.1111/j.1538-7836.2005.01535.x. [DOI] [PubMed] [Google Scholar]
- 23.Morelli VM, De Visser MC, Vos HL, Bertina RM, Rosendaal FR. ABO blood group genotypes and the risk of venous thrombosis: effect of factor V Leiden. J Thromb Haemost. 2005;3:183–185. doi: 10.1111/j.1538-7836.2004.01071.x. [DOI] [PubMed] [Google Scholar]
- 24.Nydegger UE, Wuillemin WA, Julmy F, Meyer BJ, Carrel TP. Association of ABO histo-blood group B allele with myocardial infarction. Eur J Immunogenet. 2003;30:201–206. doi: 10.1046/j.1365-2370.2003.00390.x. 390. [DOI] [PubMed] [Google Scholar]
- 25.Roest M, Voorbij HA, Barendrecht AD, Peeters PH, van der Schouw YT. Risk of acute ischemic heart disease in postmenopausal women depends on von Willebrand factor and fibrinogen concentrations, and blood group genotype. J Thromb Haemost. 2007;5:189–191. doi: 10.1111/j.1538-7836.2006.02285.x. [DOI] [PubMed] [Google Scholar]
- 26.Schleef M, Strobel E, Dick A, Frank J, Schramm W, Spannagl M. Relationship between ABO and Secretor genotype with plasma levels of factor VIII and von Willebrand factor in thrombosis patients and control individuals. Br J Haematol. 2005;128:100–107. doi: 10.1111/j.1365-2141.2004.05249.x. [DOI] [PubMed] [Google Scholar]
- 27.Tirado I, Mateo J, Soria JM, Oliver A, Martinez-Sanchez E, Vallve C, Borrell M, Urrutia T, Fontcuberta J. The ABO blood group genotype and factor VIII levels as independent risk factors for venous thromboembolism. Thromb Haemost. 2005;93:468–474. doi: 10.1160/TH04-04-0251. [DOI] [PubMed] [Google Scholar]
- 28.von Beckerath N, Koch W, Mehilli J, Gorchakova O, Braun S, Schomig A, Kastrati A. ABO locus O1 allele and risk of myocardial infarction. Blood Coagul Fibrinolysis. 2004;15:61–67. doi: 10.1097/00001721-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Psaty BM, Heckbert SR, Atkins D, Lemaitre R, Koepsell TD, Wahl PW, Siscovick DS, Wagner EH. The risk of myocardial infarction associated with the combined use of estrogens and progestins in postmenopausal women. Arch Intern Med. 1994;154:1333–1339. [PubMed] [Google Scholar]
- 30.Psaty BM, Heckbert SR, Koepsell TD, Siscovick DS, Raghunathan TE, Weiss NS, Rosendaal FR, Lemaitre RN, Smith NL, Wahl PW, et al. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274:620–625. [PubMed] [Google Scholar]
- 31.Smith NL, Heckbert SR, Lemaitre RN, Reiner AP, Lumley T, Weiss NS, Larson EB, Rosendaal FR, Psaty BM. Esterified estrogens and conjugated equine estrogens and the risk of venous thrombosis. Jama. 2004;292:1581–1587. doi: 10.1001/jama.292.13.1581. [DOI] [PubMed] [Google Scholar]
- 32.Olsson ML, Chester MA. Polymorphism and recombination events at the ABO locus: a major challenge for genomic ABO blood grouping strategies. Transfus Med. 2001;11:295–313. doi: 10.1046/j.1365-3148.2001.00320.x. [DOI] [PubMed] [Google Scholar]
- 33.Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gassner C, Schmarda A, Nussbaumer W, Schonitzer D. ABO glycosyltransferase genotyping by polymerase chain reaction using sequence-specific primers. Blood. 1996;88:1852–1856. [PubMed] [Google Scholar]
- 36.Yip SP. Single-tube multiplex PCR-SSCP analysis distinguishes 7 common ABO alleles and readily identifies new alleles. Blood. 2000;95:1487–1492. [PubMed] [Google Scholar]
- 37.Souto JC, Almasy L, Muniz-Diaz E, Soria JM, Borrell M, Bayen L, Mateo J, Madoz P, Stone W, Blangero J, Fontcuberta J. Functional effects of the ABO locus polymorphism on plasma levels of von Willebrand factor, factor VIII, and activated partial thromboplastin time. Arterioscler Thromb Vasc Biol. 2000;20:2024–2028. doi: 10.1161/01.atv.20.8.2024. [DOI] [PubMed] [Google Scholar]
- 38.Herman B, Schmitz PI, Leyten AC, Van Luijk JH, Frenken CW, Op De Coul AA, Schulte BP. Multivariate logistic analysis of risk factors for stroke in Tilburg, The Netherlands. Am J Epidemiol. 1983;118:514–525. doi: 10.1093/oxfordjournals.aje.a113657. [DOI] [PubMed] [Google Scholar]
- 39.Ionescu DA, Marcu I, Bicescu E. Cerebral thrombosis, cerebral haemorrhage, and ABO blood-groups. Lancet. 1976;1:278–280. doi: 10.1016/s0140-6736(76)91405-7. [DOI] [PubMed] [Google Scholar]
- 40.Larsen S, Anthonisen B, Marquardsen J. Cerebral infarction, cerebral haemorrhage, and ABO blood-groups. Lancet. 1977;1:1064. doi: 10.1016/s0140-6736(77)91310-1. [DOI] [PubMed] [Google Scholar]
- 41.Wu O, Bayoumi N, Vickers MA, Clark P. ABO(H) blood groups and vascular disease: a systematic review and meta-analysis. J Thromb Haemost. 2008;6:62–69. doi: 10.1111/j.1538-7836.2007.02818.x. [DOI] [PubMed] [Google Scholar]
- 42.Kamphuisen PW, Eikenboom JC, Bertina RM. Elevated factor VIII levels and the risk of thrombosis. Arterioscler Thromb Vasc Biol. 2001;21:731–738. doi: 10.1161/01.atv.21.5.731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


