Abstract
As more women survive allogeneic stem cell transplantation (SCT), the development of genital human papilloma virus (HPV)-related squamous intraepithelial lesions (SIL) warrants study. Thirty-five of 38 females followed prospectively long-term after SCT for hematological malignancies (median: 7 years posttransplant) were adults and had cervical cytology testing. Acute graft-versus-host-disease (aGVHD) occurred in 9 and chronic (cGVHD) in 34 patients. Six (17%) continued receiving systemic immunosuppressive therapy (IST) for cGVHD >3 years after SCT. Of 15 (43%) with abnormal cytology, 12 (34%) patients had HPV-related SIL (median time to SIL 51 months, range: 22-108) including high-grade SIL in 7 (20%). Patients requiring continued IST had the highest risk (odds ratio [OR] 4.6, 95% confidence interval [CI] 1.1-16.4; P = .019). This high incidence of SIL in long-term SCT survivors underscores the importance of gynecologic assessment after transplantation, especially in those requiring IST. This may portend an increased risk of genital or other HPV-related malignancies.
Keywords: Cervical dysplasia, HPV, long-term survivors, stems cell transplantation
INTRODUCTION
As nearly 90% of patients alive 2 years after allogeneic stem cell transplantation (SCT) will become long-term survivors [1], the focus of care has shifted from cure of the original disease to the identification and treatment of delayed and long-term complications that may affect quality of life. Chronic GVHD (cGVHD) has attracted most attention, but large retrospective cohort analyses identify high frequencies of other problems such as hypothyroidism, second cancers, infertility, bone loss, and pulmonary complications [2-6]. Because systematic prospective studies of long-term survivors after SCT are lacking, we developed an observational protocol to follow long-term survivors of SCT. Patients were enrolled at their third annual visit after transplantation. Annual screening included evaluation for cGVHD, major organ dysfunction, second malignancies, hormonal profile, gynecologic examination, and quality of life. Here, we present the risk of genital human papilloma virus (HPV)-related squamous intraepithelial lesions (SIL) in an adult female cohort with a 7-year median survival posttransplant.
STUDY DESIGN, PATIENTS, AND METHODS
Four hundred seventeen patients with hematologic disorders received SCT from an HLA identical sibling between 1993 and 2003. We conducted a cross-sectional study of patients a minimum of 3 years posttransplantation between April 2005 and July 2007 in an institutional review board-approved protocol (NHLBI 05-H-0130;ClinicalTrials.gov Identifier NCT00106925). Of the 111 patients surviving 3 or more years at study initiation in 2005, 92 (83%) patients gave written informed consent, of whom, 38 were female. Of these, 35 were adults and 3 were children. Annual gynecologic examination was undertaken in all adult (n = 35) female patients over age 18 years at posttransplant follow-up. Medical records from their home gynecologist were also reviewed. In this cohort, transplant conditioning mainly consisted of total body irradiation (TBI) 12-13.6 Gy and cyclophosphamide (with or without additional fludarabine), followed by a allogeneic SCT (n = 29). Nine received reduced-intensity conditioning (RIC) of fludarabine 125 mg/m2 and cyclophosphamide 60 mg/kg × 2 days, followed by a peripheral blood stem cell transplant (PBSCT). All received cyclosporine as GVHD prophylaxis.
Annual gynecologic examination included assessment for vulvovaginal GVHD [7] and cervical cytology testing. Cytology abnormalities were classified according to the Bethesda 2001 classification system. Those classified as atypical cells of uncertain significance were accompanied by HPV subtyping (Digene Corporation, Gaithersburg, MD), and reported as low risk (HPV DNA 6, 11, 42, 43, 44) and high risk (HPV DNA 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 69). All women with at least low grade squamous intraepithelial lesions on cytology and those with vaginal or vulvar lesions underwent biopsy of affected areas (vulva, vagina, or cervix) to diagnose the grade of dysplasia. Any found to have moderate dysplasia or worse, and some with only genital wart disease underwent treatment. If abnormal test results were found, the earliest occurrence posttransplant of the most severe abnormal result was used in the analysis.
Statistical Analysis
Clinical and transplant characteristics were used to evaluate risk factors for SIL. Age (at transplantation as well as at time of diagnosis of cervical dysplasia) and duration of follow-up were included as continuous variables. Other clinical variables included disease risk: standard versus high (standard risk included patients in first complete remission with ALL and AML, and those with chronic phase CML) ; duration of follow-up <10 versus ≥10 years; systemic immunosuppressive therapy: <3 versus ≥3 years post-SCT; aGVHD (grade II-IV): present versus absent; cGVHD present versus absent; genital cGVHD [7] present versus absent. In univariate analyses, chi-squared or Fisher's exact tests were used for categoric variables and Mann-Whitney U-test for continuous variables. Logistic regression was used for multivariate analysis, which included factors found to be significant in univariate analyses. Statistical significance was accepted at P < .05. Data analysis was performed using SPSS 15 for Windows (SPSS Inc., Chicago, IL) software.
RESULTS
Female patients (n = 38) ranged from 9 to 60 years (median 33) at time of transplantation. All were alive at a median follow-up of 85 months (range: 45-163) post-SCT. In the 35 adult women, grade II-IV acute GVHD (aGVHD) occurred in 9 (27.5%) patients and cGVHD in 34 (97%), which was extensive in 10 (26%). Six (17%) continued on immunosuppressive treatment beyond 3 years from transplantation.
Cervical Dysplasia
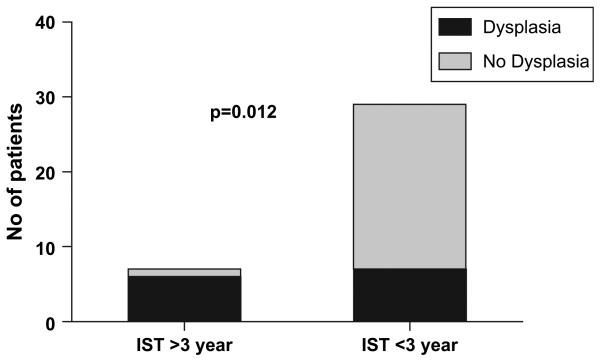
Most patients had normal cervical cytology testing prior to transplantation. One had undergone multiple cone biopsies and a hysterectomy pretransplant and another was noted to have genital warts within the first 90 days posttransplant. Of 35 patients with posttransplant cervical cytology testing, 15 (43%) were abnormal. Twelve (34%) samples with SIL all showed biopsy-proven HPV disease; 7 (20%) had high-grade lesions and low grade in 5 (14%). The other 3 patients with abnormal cytology had atypical cells of undetermined significance (ASCUS) negative for HPV subtyping. The median time to developing SIL was 51 months (range: 22-108) posttransplantation. Patients with an abnormal cytology had a median age of 42 years (range: 19-62). Eight of 29 (28%) patients had genital GVHD [7], there was no association between genital GHVD and HPV-related cervical dysplasia (3 of 7 with genital GVHD had cervical dysplasia versus 5 of 21 without genital GVHD; P = .37). Multivariate logistic regression analysis showed HPV-related cervical dysplasia was significantly associated only with cGVHD requiring prolonged systemic immunosuppressive therapy (odds ratio [OR] 4.6, 95% confidence interval [CI] 1.1-16.4; P = .019): 5 of 6 versus 7 of 29 patients (Figure 1).
Figure 1.
Duration of immunosuppressive therapy (IST) and cervical dysplasia.
DISCUSSION
Our study demonstrates that genital HPV disease is a significant late complication of allo-SCT in women; occurring in one-third of our long-term survivors. This high rate of HPV-related genital dysplasia in patients who are cured of their underlying disease is of concern. As anticipated, prolonged systemic immunosuppressive treatment for cGVHD was associated with a higher risk of developing HPV-related SIL. This may follow a loss of protective cell and antibody-mediated HPV immunity after SCT leading to reactivation of latent virus. We recently described a similar risk of hepatitis B reactivation following the loss of protective anti-HBs antibody posttransplantation [8].
Other immunocompromised populations are at increased risk of HPV-related SIL and cancers. Among solid organ transplant recipients, lower genital tract dysplasia is common [9-11]. Also, HPV was detected in 70% to 90% of cutaneous squamous cell cancers [12,13]. Similarly, HPV disease is common and difficult to treat in patients with HIV [14-17]. Although this is the first analysis of risk factors for HPV related cervical dysplasia, there are previous reports of increased incidence of abnormal cervical cytology and condyloma following allo-SCT [18-20]. Our study also details for the first time the prevalence of high-risk versus low-risk HPV serotypes. This is important in the light of the newly available HPV vaccine.
Second neoplasias are a significant long-term complication after allo-SCT [3,4]. In a large multicenter European study, the actuarial risk of second malignances at 15 years was 11.5 ± 2.3% [3]. The most frequent cancers occurred in skin, oral cavity, larynx, cervix, and uterus, many of which were squamous cell cancers. The factors associated with developing these cancers were older age and cGVHD. Rosenberg et al. [21] also reported a higher risk of squamous cell cancer (SCC) in Fanconi Anemia patients after transplant who had either acute or cGVHD [21]. Bhatia et al. [4] also reported an increased risk of squamous cell cancers, specifically oral cavity and cervical cancer, in long-term survivors of allo-SCT. Although squamous cell cancers involving these sites are associated with HPV infection, the relation between HPV infection and second cancers is not known in SCT survivors [12,13,22-24].
Our findings argue for regular gynecologic examination and cervical cytology testing after SCT, not only to identify abnormal cytology but also to diagnose and treat genital cGVHD. Studies are clearly needed to determine if squamous cell cancers, as the most common second malignancies after allo-SCT, are HPV-related. Consideration should be given to assessing pretransplant HPV antibodies to identify patients at risk for disease reactivation. If such a strong relationship between HPV and second malignancies after SCT exists, studies to evaluate the immunogenicity and efficacy of quadrivalent HPV vaccine (subtypes 6, 11, 16, 18) should be considered in both male and female patients.
Our study is limited by the fact that we did not have a control group but our data shows that patients receiving prolonged IST have a higher risk of developing HPV-related cervical dysplasia. This group could be targeted for preemptive strategies including aggressive screening and vaccinations to prevent HPV-related SCC in allo-SCT recipients.
ACKNOWLEDGMENTS
This work was supported by the intramural research program of the NHLBI. and NICHD B.N.S designed and wrote the study and wrote the manuscript. P.S. performed gyn examinations, treated patients, and wrote the manuscript. A.S. made a critical analysis of results and the manuscript, and treated patients. E.K. made data collection and patient recruitment. S.G. made critical analysis of the results and the manuscript. A.J.B., senior author, supervised the study, study design discussion, and wrote the manuscript.
Footnotes
Declaration of commercial interest: None.
REFERENCES
- 1.Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 2.Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol. 2005;23:6596–6606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- 3.Kolb HJ, Socie G, Duell T, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann Intern Med. 1999;131:738–744. doi: 10.7326/0003-4819-131-10-199911160-00004. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–471. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 5.Savani BN, Donohue T, Kozanas E, et al. Increased risk of bone loss without fracture risk in long-term survivors after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:517–520. doi: 10.1016/j.bbmt.2007.01.085. [DOI] [PubMed] [Google Scholar]
- 6.Savani BN, Montero A, Srinivasan R, et al. Chronic GVHD and pretransplantation abnormalities in pulmonary function are the main determinants predicting worsening pulmonary function in long-term survivors after stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:1261–1269. doi: 10.1016/j.bbmt.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stratton P, Turner ML, Childs R, et al. Vulvovaginal chronic graft-versus-host disease with allogeneic hematopoietic stem cell transplantation. Obstet Gynecol. 2007;110:1041–1049. doi: 10.1097/01.AOG.0000285998.75450.86. [DOI] [PubMed] [Google Scholar]
- 8.Le Q, Savani BN, Shenoy A, Kozanas E, Barrett AJ. Hepatitis B reverse seroconversion in long term survivors of allogeneic hematopoietic stem cell transplantation. Blood. 2007;110:1978. abstract. [Google Scholar]
- 9.Ozsaran AA, Ates T, Dikmen Y, et al. Evaluation of the risk of cervical intraepithelial neoplasia and human papilloma virus infection in renal transplant patients receiving immunosuppressive therapy. Eur J Gynaecol Oncol. 1999;20:127–130. [PubMed] [Google Scholar]
- 10.Seshadri L, George SS, Vasudevan B, Krishna S. Cervical intraepithelial neoplasia and human papilloma virus infection in renal transplant recipients. Indian J Cancer. 2001;38:92–95. [PubMed] [Google Scholar]
- 11.Bobrowska K, Kaminski P, Cyganek A, et al. Multiple neoplastic lesions of the lower genital tract in a human papilloma virus-infected kidney-pancreas allograft recipient: a case report. Transplant Proc. 2005;37:2093–2095. doi: 10.1016/j.transproceed.2005.03.098. [DOI] [PubMed] [Google Scholar]
- 12.Stockfleth E, Nindl I, Sterry W, et al. Human papillomaviruses in transplant-associated skin cancers. Dermatol Surg. 2004;30:604–609. doi: 10.1111/j.1524-4725.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 13.Berkhout RJ, Bouwes Bavinck JN, ter SJ. Persistence of human papillomavirus DNA in benign and (pre)malignant skin lesions from renal transplant recipients. J Clin Microbiol. 2000;38:2087–2096. doi: 10.1128/jcm.38.6.2087-2096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nappi L, Carriero C, Bettocchi S, et al. Cervical squamous intraepithelial lesions of low-grade in HIV-infected women: recurrence, persistence, and progression, in treated and untreated women. Eur J Obstet Gynecol Reprod Biol. 2005;121:226–232. doi: 10.1016/j.ejogrb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Cameron JE, Hagensee ME. Human papillomavirus infection and disease in the HIV1 individual. Cancer Treat Res. 2007;133:185–213. doi: 10.1007/978-0-387-46816-7_7. [DOI] [PubMed] [Google Scholar]
- 16.Cameron JE, Snowhite IV, Chaturvedi AK, Hagensee ME. Human papillomavirus-specific antibody status in oral fluids modestly reflects serum status in human immunodeficiency virus-positive individuals. Clin Diagn Lab Immunol. 2003;10:431–438. doi: 10.1128/CDLI.10.3.431-438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicol AF, Nuovo GJ, Salomao-Estevez A, et al. Immune factors involved in the cervical immune response in the HIV/HPV co-infection. J Clin Pathol. 2008;61:84–88. doi: 10.1136/jcp.2007.047290. [DOI] [PubMed] [Google Scholar]
- 18.Sasadeusz J, Kelly H, Szer J, et al. Abnormal cervical cytology in bone marrow transplant recipients. Bone Marrow Transplant. 2001;28:393–397. doi: 10.1038/sj.bmt.1703141. [DOI] [PubMed] [Google Scholar]
- 19.Daneshpouy M, Socie G, Clavel C, et al. Human papillomavirus infection and anogenital condyloma in bone marrow transplant recipients. Transplantation. 2001;71:167–169. doi: 10.1097/00007890-200101150-00030. [DOI] [PubMed] [Google Scholar]
- 20.Ganguly N, Waller S, Stasik CJ, Skikne BS, Ganguly S. Giant anal condylomatosis after allogeneic bone marrow transplantation: a rare complication of human papilloma virus infection. Transpl Infect Dis. 2008;10:56–58. doi: 10.1111/j.1399-3062.2007.00233.x. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg PS, Socie G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood. 2005;105:67–73. doi: 10.1182/blood-2004-04-1652. [DOI] [PubMed] [Google Scholar]
- 22.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 23.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 24.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]