Abstract
Objective
To quantify the concentration of superficial zone protein (SZP) in articular cartilage and synovial fluid of patients with advanced osteoarthritis (OA), and to further correlate the SZP content with the friction coefficient, OA severity, and levels of inflammatory cytokines.
Methods
Samples of articular cartilage and synovial fluid were obtained from patients undergoing elective total knee replacement surgery. Additional normal samples were obtained from donated body program and tissue bank sources. Regional SZP expression in cartilage obtained from the femoral condyles was quantified by enzyme-linked immunosorbant assay and visualized by immunohistochemistry. Friction coefficient measurements were obtained from cartilage plugs slid in the boundary lubrication regime. OA severity was graded using histochemical analyses. The concentration of SZP and inflammatory cytokines in synovial fluid were determined by enzyme-linked immunosorbant assays.
Results
A pattern of SZP localization in knee cartilage was identified, with load-bearing regions exhibiting high SZP expression. SZP patterns correlated to friction coefficient and OA severity; however SZP expression was observed in all samples at the articular surface, regardless of OA severity. SZP expression and aspirate volume of synovial fluid were higher in OA patients compared to normal controls. Expressions of cytokines were elevated in the synovial fluid of some patients.
Conclusion
The results reveal a mechano-chemical coupling in which physical forces regulate OA severity and joint lubrication. The findings of this study also suggest that SZP may be ineffective in reducing joint friction in the boundary lubrication regime at an advanced OA stage where other mechanisms may dominate the observed tribological behavior.
Keywords: articular cartilage lubrication, human osteoarthritis, knee joint synovial fluid, lubricin/PRG4/superficial zone protein (SZP)
INTRODUCTION
Superficial zone protein (SZP), also known as lubricin or PRG4, is a mucinous glycoprotein secreted by tissues lining the interior surfaces of animal joints (1). Down-regulation of SZP has been associated with the pathogenesis of osteoarthritis (OA) (2). SZP may act as a chondroprotective barrier against direct solid-to-solid contact in joints when the kinematic conditions are conducive to surface sliding in the boundary lubrication regime, characterized by the formation of an adsorbed molecular layer conformal with the articular tissue surface topography (3). In the absence of a strongly adsorbing, continuous, self-replenishing boundary lubricant layer, intermittent asperity-asperity interactions lead to rapid deterioration of the joint surface by various mechanical wear processes, such as adhesion, abrasion, surface fatigue, and delamination.
A striking pattern of SZP localization in bovine cartilage, with load-bearing regions exhibiting increased SZP expressions, was identified in a previous study (3). Regional SZP patterns were regulated by a mechanical shear force through transforming growth factor β receptor type I (TGFβRI) kinase activity and subsequent phospho-Smad2/3 activity, and were correlated to the tribological behavior. Direct relationships were observed between highest SZP expression, maximum contact pressure, and lowest friction coefficient. However, it is unknown whether regional SZP expression patterns also exist in human joints.
Osteoarthritis is a degenerative joint disease and the most common form of arthritis with tens of millions of affected people in the U.S. (4). The etiologies of this disease are largely unknown, but likely involve multiple factors including a biochemical imbalance between catabolic and anabolic factors and a progressive surface degradation caused by mechanical wear (3). In the most extreme cases, the cartilage may be completely worn, resulting in direct bone-to-bone contact that requires late-stage treatment strategies, including total knee arthroplasty. Although the decrease in synovial fluid lubricin concentration observed after anterior cruciate ligament injury may increase the risk of wear-induced joint damage due to the lack of effective boundary lubrication (5), the presence of SZP in cartilage and synovial fluid at advanced stages of OA is unknown. Thus the objective of this study was to investigate the localization and concentration of SZP in articular cartilage and synovial fluid of patients with advanced OA and correlate the SZP content with the articular surface friction coefficient, OA severity, and levels of inflammatory cytokines.
MATERIALS AND EXPERIMENTAL METHODS
Tissue Acquisition
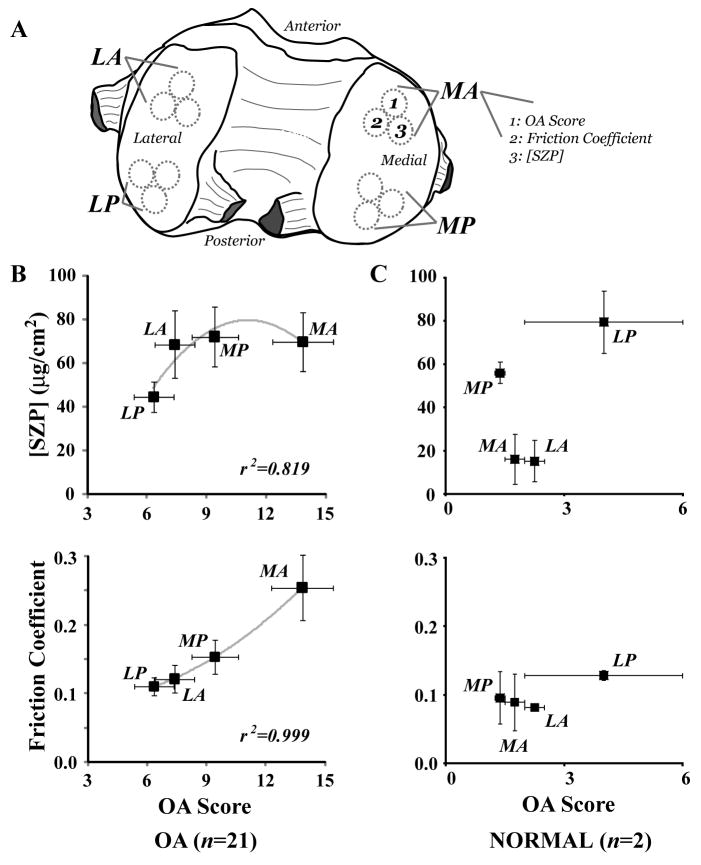
Multiple human tissue sources were used to study joint biochemistry and tribology. Patients (n = 21; males = 11; females = 10; average age = 66 years; age range = 44–79 years) undergoing elective surgery for joint replacement were studied according to Institutional Review Board approval and served as the primary tissue source. Both osteochondral tissue and synovial fluid were collected at the time of surgery. Cylindrical osteochondral samples (diameter = 5 mm; length = 5 mm) were harvested from standardized femur locations (Figure 1A; LA = lateral anterior, LP = lateral posterior, MA = medial anterior, and MP = medial posterior) using a coring reamer and custom cutting jig (3). LA and MA locations were from relatively high contact pressure joint regions, whereas locations LA and LP were from relatively low contact pressure joint regions. Major advantages with this approach are reduced (e.g., genetic) variation among multiple OA tissue donors and the possibility to sample from a continuum of (minimal to advanced) structural damage within a single subject’s joint. Thus, three samples from each location were used for protein quantification, histochemical analysis, and tribological testing (described subsequently). Samples were numerically labeled and stored at −80 °C for further study.
Figure 1.
Immunolocalization of SZP and friction coefficient showed a dependence on femoral condyle geometry and OA severity. A, Samples were harvested from standardized anterior and posterior locations of medial (M) and lateral (L) condyles. B, The surface expression of SZP (quantified relative to bovine standards) and friction coefficient of samples from OA subjects (n = 21) varied with OA score significantly. Anterior locations MA exhibited much higher OA scores and friction coefficients compared to posterior locations MP and LP and anterior location LA [P < 0.011 ([SZP]); P < 0.015 (friction coefficient)]. C, The strong correlation of SZP concentration and friction coefficient with OA score observed with samples from OA subjects was not encountered with samples from normal subjects (n = 2).
Additional samples were obtained from donated body program and tissue bank sources for a limited comparison with representative normal tissue. Tissue was harvested as described previously from subjects (n = 2; females = 2; average age = 54 years; age range = 52–56 years) obtained through the donated body program following radiographic screening for signs of cartilage degeneration. Additionally, allograft material was obtained from a single subject. Osteochondral samples (diameter = 8 mm; thickness = 5 mm) from this subject harvested from locations LA and LP were used for histochemical analysis.
SZP Localization
Protein was extracted from tissue samples using a tissue homogenizer and 1 mL o f 4 M Guanidine-HCl (pH 7.2) supplemented with 0.2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 10 mM EDTA, 0.05 M Tris, and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Homogenized tissue was centrifuged at 14,000 g for 10 min. The supernatant was buffer-exchanged with 6 M urea containing 0.05 M Tris (pH 7.4) using Centricon filter devices (Millipore, Bedford, MA).
Enzyme-linked immunosorbent assay (ELISA) was used to analyze the SZP content (μg/mL) in samples (compared to bovine SZP purified from explant culture systems and synovial fluid). Briefly, samples were analyzed by a sandwich ELISA using peanut lectin as a capture reagent and an anti-SZP monoclonal antibody (6). Black 96-well plates were coated with 50 μL of 1 μg/mL peanut lectin (PNA, E-Y laboratories) in 50 mM sodium carbonate buffer pH 9.5 overnight at 4 °C and then blocked with bovine serum albumin (BSA) in the same buffer for 1 h at room temperature. Two-fold serial dilutions of culture medium in phosphate buffered saline, 0.05% Tween, and 10 mM EDTA (PBSTE) were incubated with the lectin-coated plate for 1 h. The plate was washed with PBSTE, and incubated with 2 μg/mL of S6.79 mAb to SZP overnight (6). After washing with PBSTE, a secondary anti-mouse IgG HRP conjugate (Thermo, #PI31432) was incubated with the plate at a 1:3000 dilution for 1 hr at room temperature. The plate was washed and a chemiluminescent HRP Supersignal ELISA Femto substrate (Thermo, #PI37075) was added for 1 min. Chemiluminescence was measured in a multilabel plate reader. Concentration measurements were based on serial dilutions of purified SZP (1) using a BCA protein assay (Thermo, PI23235) with BSA as a protein standard. Importantly, confirmatory spiking experiments (through the addition of known amounts of purified SZP to samples) for this ELISA yielded recoveries of 109.0 ± 6.2% and 88.6 ± 10.6% for synovial fluid and cartilage extracts, respectively, and were considered sufficient for the experiments described herein. Finally, SZP concentrations were obtained in units of μg/cm2 using the final supernatant volume and the articular surface area of the sample, and computed as averages (± standard error of the mean) of all joints harvested from each location.
For immunohistochemistry, tissue sections from joints were fixed in Bouin’s solution for 24 h, followed by paraffin embedding and sectioning. Immunostaining was performed according to a standard method using S6.79 (1:5000) as the primary antibody (6) and ABC kit (Vector Laboratories, Burlingame, CA) with mouse IgG secondary antibody for signal detection.
Friction Experiments
To examine whether the SZP expression pattern was related to mechanical effects, friction tests were performed with plugs harvested from different cartilage locations. All of the friction tests were carried out under boundary lubrication sliding conditions using a pin-on-disk tribometer operated in reciprocating mode. Superficial sections of thickness equal to 4 mm were removed from plugs (harvested as described previously), immediately affixed to acrylic pins using ethyl cyanoacrylate, and then used in the friction experiments. The articular surface was brought into contact with a polished glass disk while being fully immersed in phosphate-buffered saline. The average contact pressure of 0.1 MPa applied in all the tests is within the physiological range during walking (7). Prior to the initiation of sliding, the sample was allowed to equilibrate for 2 min under the applied normal load to minimize fluid effects during testing. Thus, sliding occurred in the boundary lubrication regime using a standard set of load and speed conditions applied to all samples tested that enabled relative differences to be determined (3, 8, 9). Data processing was accomplished with a standard software package (Microsoft Excel). Data from the first 60 s of sliding were used to compute an average friction coefficient (± standard error of the mean) for each femoral condyle location.
OA Severity
OA severity was assessed based on histochemical analysis and a standard OA cartilage pathology scoring system (10), which includes six grades indicating the depth of the lesion and four stages reflecting the extent of OA over the joint surface. Tissue sections from fixed samples described previously were stained by hematoxylin and eosin (H&E) and toluidine blue. The OA score (= grade × stage) was assessed by two independent observers and averaged for correlation to immunolocalization and tribological data.
Analysis of Synovial Fluid
To investigate biochemical relationships between SZP expression and OA severity, synovial fluid was further analyzed for the concentration of OA-related cytokines. The SZP content in synovial fluid was serially diluted and analyzed by ELISA, as described previously. The total volume of synovial fluid aspirated from the joint was determined and the presence of blood in samples was visually detected. To investigate potential degradation resulting from OA as detected by S6.79 mAb, SZP was visualized by SDS-PAGE with subsequent immunoblotting, following standard procedures (3). Importantly, the same amount of SZP was loaded in each lane (based on SZP ELISA results), with OA patients having higher SZP content, therefore requiring less relative loads per lane, compared to normal patients. Concentrations of tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β) were obtained with Quantikine kits (R&D Systems, Minneapolis, MN). A quantitative sandwich enzyme immunoassay technique was used with monoclonal antibodies specific for TNF-α and IL-1β in addition to cytokine standards. Because the OA score varied within the joint, the maximum OA score for each joint was used as an independent variable.
Statistical Analysis
Differences in OA score, SZP expression, and friction coefficient at different femoral condyle locations were determined using a one-way ANOVA with Fisher’s PLSD post-hoc tests, a significance level of P < 0.05, and a standard software package (SAS Institute, Cary, NC). An unpaired t-test and significance level of P < 0.05 were used to determine differences in SZP expression for normal versus OA samples, OA samples with and without detectable blood, and aspirate volume of synovial fluid. The correlation coefficient (r2) was calculated using a second-order polynomial fit of the data.
RESULTS
Histology scores of cartilage damage severity in OA patients were highly correlated with with the friction coefficient (r2 = 0.999) and SZP expression (r2 = 0.819) from different regions of human OA cartilage (Figure 1B). The SZP expressions at MA, MP, and LA locations were consistently higher than that of posterior location LP; however, statistical differences between SZP expression levels in MA, MP, and LA locations were not observed (P > 0.125). Samples from anterior locations MA were characterized by significantly higher friction coefficient compared to samples from locations MP, LA, and LP (P < 0.015). In situ human SZP expression patterns and friction coefficients of OA samples demonstrated a dependence on articulating knee joint location and maximum OA score, in contrast to the SZP expression and friction coefficient of samples from normal subjects that did not show a dependence on maximum OA score but only variation with joint location (Figure 1C).
OA severity showed a dependence on knee joint location (Figure 1B). The maximum OA score at locations MA, MP, LA, and LP was found in 17/21 (81.0%), 3/21 (14.3%), 1/21 (4.8%), and 0/21 (0.0%) of all OA subjects, respectively. On average, the MA location demonstrated significantly higher OA scores compared to the MP, LA, and LP locations (P < 0.011). The OA scores of normal subjects were lower on average than those of similar locations of OA subjects (Figure 1C).
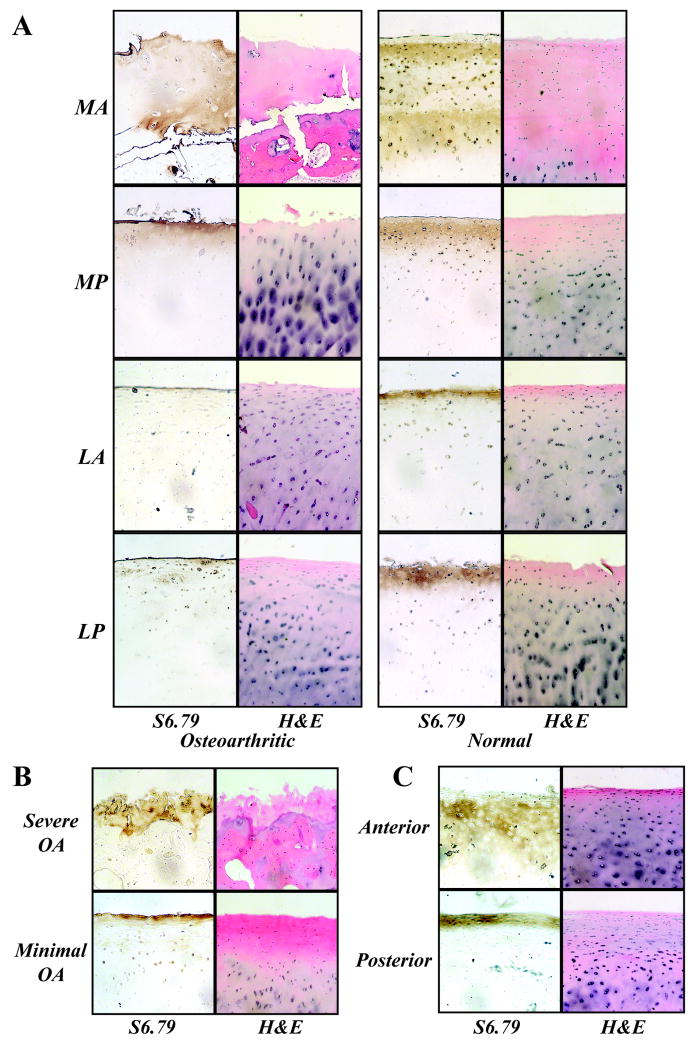
SZP expression was observed in all samples at the articular surface regardless of OA severity (Figure 2). Histochemistry (H&E staining) revealed cell and proteoglycan patterns through the cartilage thickness that depended on OA severity. For normal subjects, the depth of SZP expression was typically larger in the anterior region of the medial condyle MA (Figures 2A and 2C).
Figure 2.
SZP immunolocalization was observed in all samples at the articular surface regardless of OA severity. Histochemistry (H&E staining) revealed cell and proteoglycan patterns through the cartilage thickness that depended on OA severity. A, Samples from representative OA and normal subjects revealed the presence of SZP on exposed articular surfaces. SZP in OA samples was bound to the lamina splendens in locations LA and LP, and was observed in several cell layers into the tissue at MA and MP locations. SZP in normal samples was consistently observed in several cell layers into the tissue at MA location compared to other locations. B, From the OA study group, SZP was observed on the articular surface of samples representing the most severe OA (MA location) and minimal OA (LP location). C, In human allograft cartilage, SZP localized deeper into the tissue in the anterior location compared to the posterior location.
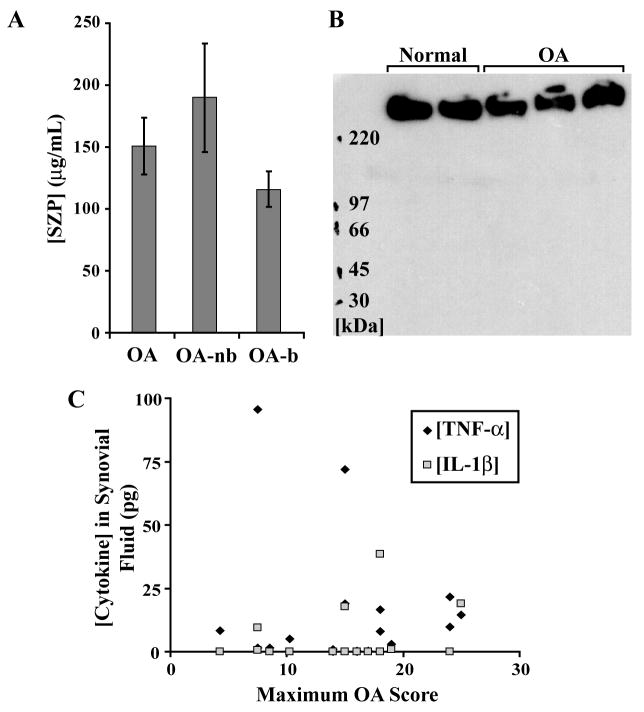
SZP expression was detected in synovial fluid of all subjects (Figure 3). Both the SZP expression (150.81 ± 23.08 μg/mL) and the aspirate volume of synovial fluid (3.82 ± 0.80 mL) were significantly elevated in OA subjects compared to normal subjects (34.82 ± 27.55 μg/mL and 0.98 ± 0.06 mL, respectively) (P = 0.048 and 0.002, respectively). Blood was visually observed in 52.6% of the OA samples collected. Although not significant (P = 0.110), a mean difference was noted between OA samples with and without a detectable presence of blood (Figure 3A). The monoclonal antibody S6.79 recognized the large (~345 kDa) PRG4 gene product (Figure 3B). The expression levels of cytokines TNF-α and IL-1β were elevated in the synovial fluid of some of the OA subjects (Figure 3C).
Figure 3.
The SZP concentration and aspirate volume of synovial fluid were elevated in OA samples (n = 21). Some OA samples showed higher cytokine expressions in synovial fluid. A, The total SZP content in OA samples (quantified relative to bovine standards) was elevated compared to normal samples (see text). The concentration of SZP may be indicative of the harvest procedure and could have been underestimated in OA samples with blood (OA-b) compared to those in which blood was not detected (OA-nb). B, Western blot of synovial fluids with the monoclonal antibody S6.79 recognized the large (~345 kDa) PRG4 gene product in both OA and normal patients. The same amount of SZP was loaded in each lane (based on SZP ELISA results), with OA patients having higher SZP content (and requiring less relative loads per lane) compared to normal patients. C, Some OA samples demonstrated elevated expressions of TNF-α and IL-1β cytokines in synovial fluid.
DISCUSSION
The objective of this study was to determine the localization and concentration of SZP in articular cartilage and synovial fluid of patients with advanced OA. The SZP content was further correlated with the articular surface friction coefficient, OA severity, and level of inflammatory cytokines. The main findings of this study can be summarized as follows: (1) OA severity at different regions of human cartilage correlated with the coefficient of friction, (2) SZP expression patterns and friction coefficient showed a dependence on articulating knee joint location and maximum OA score, (3) SZP expression was observed in all samples at the articular surface regardless of OA severity, and (4) high SZP expression was detected in synovial fluid of all subjects with advanced OA.
Regional SZP expression patterns revealed a coupling between biochemical and mechanical function in which physical forces regulate OA severity and joint lubrication through mechanically stimulated secretion of SZP. Particularly for the lateral condyle, for which the maximum OA score was relatively low compared to that of the medial condyle (Figure 1B), a higher SZP expression was observed in the load-bearing region (i.e., SZP expression levels in LA and LP locations; Figure 1B). Thus it appears that the physical forces from typical joint contact resulted in increased chondrocyte mechanotransduction, as reported previously (3) and as confirmed by the immunohistochemistry data for normal human subjects of this study (Figures 2A and 2C). It was not possible to confirm this result for the medial condyle of OA samples because the missing cells and tissue of the superficial zone due to the advanced OA precluded a complete analysis.
A surprising finding was the immunolocalization of SZP in the medial condyle despite the high OA score that reflected the loss of superficial zone cells producing the protein (Figure 2B). The fact that SZP was observed in all the samples can be explained by the increased SZP expression in the synovial fluid (with additionally increased intra-articular volumes; Figure 3), suggesting that SZP/lubricin in synovial fluid served as a boundary-lubricant reservoir for all surfaces lining the synovial joint. Further, it may be that there are increased binding sites in the damaged tissue structure due to the loss of proteoglycans during OA progression. These interpretations are supported by SZP immunolocalization results representative of severe and minimal OA (Figure 2B). Additionally, the presence of blood in some synovial fluid samples may have been indicative of the harvest procedure and may have underestimated the concentration of SZP overall (Figure 3C). Reduced concentrations of lubricin and markers of joint inflammation have been observed in the synovial fluid of patients within 32 to 364 days from anterior cruciate ligament injury (5), in contrast to the present study that showed increased SZP expression levels and markers of joint inflammation in some, but not all, late-stage OA patients (Figure 3). The obtained results suggest an effect of OA stage on SZP/lubricin expression, with a possible rebound and increased SZP expression encountered in the late stage of the disease. However, it is important to consider that the 345 kDa PRG4 gene product was consistently observed using monoclonal antibody S6.79 (Figure 3B), and thus it is unclear how other gene products varied as a function of location and degree of OA severity. Further, because the normal samples were obtained post-mortem, it is unknown if SZP levels precisely reflect concentrations in vivo.
SZP (or related biomolecules, such as lubricin) may be ineffective in reducing friction of boundary-lubricated human joints exhibiting advanced OA because other mechanisms may dominate the tribological response. The friction coefficient increased significantly with the maximum OA score, particularly in the case of medial condyles (MA), despite relatively similar levels of SZP expression with condyles from MP and LA joint locations (Figure 1B). It is thus possible that either cartilage damage causes an increase in the friction coefficient and/or changes in the friction coefficient lead to cartilage damage. This finding is attributed to other mechanisms that may dominate the tribological behavior (11, 12). It is well known that the friction coefficient shows a dependence on length scale and operating conditions (e.g., magnitude of contact stresses, apparent contact area, mechanical properties of interacting surfaces, and total sliding distance). For SZP to function properly in the boundary lubrication regime, the adsorbed molecular film must be conformal to the surface topography in a closed-packed arrangement. It is believed that the increased surface roughness and variable tissue stiffness in late-stage OA subjects (13) intensified the local stresses at asperity contacts, resulting in the rapid removal of the boundary lubricant and preventing its timely replenishment. The higher friction coefficients of OA samples (particularly for load-bearing MA location) compared to normal subjects indicate that the sliding conditions were not conducive to the maintenance and/or timely replenishment of a continuous and conformal boundary-lubricating layer. This may be attributed to the increased surface roughness of the OA samples (especially those characterized by a high maximum OA score) which promoted the dominance of other friction mechanisms, such as asperity deformation, adhesion, and plowing. These mechanisms are generally characterized by higher energy dissipation (friction) during sliding compared to shearing of a boundary-lubricant layer, which dominated the friction behavior of the smoother normal subjects. Moreover, it has been documented that although synovial fluid from patients with advanced OA lacks superficial zone chondrocytes (in some tissue regions), it maintains normal superficial zone lubricating ability in vitro, indicating that synovial fibroblasts contribute to joint lubrication through lubricin synthesis (14). Considering that the SZP/lubricin detected in the present study may possess normal lubricating ability (14), it is unclear to what extent recombinant lubricin will effectively treat OA (15), particularly when diagnosed in the late stage.
A strong correlation (r2 > 0.819) of SZP expression and friction coefficient with maximum OA score was found for OA samples (Figure 1B) despite the variability inherent to this disease patient population. Meaningful comparisons and tests were conducted because of a sufficiently large sample size (n = 21); however, such comparisons to normal samples were difficult because of the small sample size (n = 2). Although the principal objective of this study was to document SZP expression patterns in OA patients, it was also of interest to determine whether regional patterns in young bovine joints (3) were also present in normal human samples. Patterns similar to those observed in a previous study (3) were revealed by immunohistochemistry of normal human samples (Figures 2A and 2C), where a greater depth of SZP expression in tissue samples was observed in anterior (particularly medial) than posterior locations. Such patterns may be the result of an in vivo regulation effect of physical forces and joint contact typical of normal physiological activities, a concept reinforced by the OA severity at MA regions observed in 81% of the OA patients examined. However, ELISA data for normal SZP expression (Figure 1C) did not correspond to observed immunohistochemistry patterns. This disparity is attributed to the small sample size (n = 2) and that repeated testing of only these samples by the ELISA methods yielded highly variable values. Thus, the data of normal SZP expression shown in Figure 1C should be interpreted with caution because further studies are necessary to provide a more comprehensive understanding of regional SZP expression in normal human samples. Additionally, because a human SZP standard was not available, the use of a bovine standard may have altered the magnitude of SZP concentration overall.
Structure-function relationships abound in biological systems, such as Wolff’s law in bone (16), and the functional significance of single proteins are well documented in nature (17). The presented results reveal a coupling between biochemical and mechanical function in which physical forces regulate OA severity and joint lubrication through the development of SZP in articular cartilage by mechanotransduction. To our knowledge, this is the first demonstration that the mechanical measurement of the friction coefficient correlates so highly with the histological assessment of cartilage damage in OA patients. The findings of this study suggest that SZP (or similar biomolecules) may be ineffective in reducing friction under boundary lubrication conditions of human joints at advanced stages of OA where other mechanisms may dominate the tribological response. Although the SZP expression may be mediated by TGFβRI signaling (3), the precise mechanotransduction pathways by which mechanical signals regulate SZP expression and the mechanisms by which SZP lubricates human synovial joints over the lifetime of the organism require further investigation. Such regulatory pathways may provide insight into the progression of cartilage degeneration and regenerative therapies aimed at reconstitution and maintenance of effective boundary lubrication of articular cartilage prior to advanced OA.
Supplementary Material
Acknowledgments
The authors thank Larry Madsen, Laura Matsen, and Stephanie Chan for technical assistance. One of the authors (TMS) acknowledges funding from NIH through Grant No. R01 AR050457.
Footnotes
COMPETING INTERESTS STATEMENT The authors declare that they have no competing financial interests.
References
- 1.Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311(1):144–52. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- 2.Young AA, McLennan S, Smith MM, Smith SM, Cake MA, Read RA, et al. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Therapy. 2006;8(2):R41. doi: 10.1186/ar1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neu CP, Khalafi A, Komvopoulos K, Schmid T, Reddi AH. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor β signaling. Arthritis Rheum. 2007;56(11):3706–14. doi: 10.1002/art.23024. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778–99. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 5.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58(6):1707–15. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su JL, Schumacher BL, Lindley KM, Soloveychik V, Burkhart W, Triantafillou JA, et al. Detection of superficial zone protein in human and animal body fluids by cross-species monoclonal antibodies specific to superficial zone protein. Hybridoma. 2001;20(3):149–57. doi: 10.1089/027245701750293475. [DOI] [PubMed] [Google Scholar]
- 7.Donahue TL, Hull ML, Rashid MM, Jacobs CR. A finite element model of the human knee joint for the study of tibio-femoral contact. J Biomech Eng. 2002;124(3):273–80. doi: 10.1115/1.1470171. [DOI] [PubMed] [Google Scholar]
- 8.Dowson D, Higginson GR. Elastohydrodynamic Lubrication. New York, NY: Pergamon Press; 1977. [Google Scholar]
- 9.DuRaine G, Neu CP, Chan SMT, Komvopoulos K, June RK, Reddi AH. Regulation of the friction coefficient of articular cartilage by TGF-β1 and IL-β. J Orthop Res. 2009;27(2):249–56. doi: 10.1002/jor.20713. [DOI] [PubMed] [Google Scholar]
- 10.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Neu CP, Komvopoulos K, Reddi AH. The interface of functional biotribology and regenerative medicine in synovial joints. Tissue Eng Part B Rev. 2008;14(3):235–47. doi: 10.1089/ten.teb.2008.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones AR, Chen S, Chai DH, Stevens AL, Gleghorn JP, Bonassar LJ, et al. Modulation of lubricin biosynthesis and tissue surface properties following cartilage mechanical injury. Arthritis Rheum. 2009;60(1):133–42. doi: 10.1002/art.24143. [DOI] [PubMed] [Google Scholar]
- 13.Mow VC, Ratcliffe A, Poole RA. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13(2):67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 14.Jay GD, Elsaid KA, Zack J, Robinson K, Trespalacios F, Cha CJ, et al. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol. 2004;31(3):557–64. [PubMed] [Google Scholar]
- 15.Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermudez MA, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60(3):840–7. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 16.Wolff J. The Law of Bone Remodeling. Berlin, Germany: Springer-Verlag; 1892. [Google Scholar]
- 17.Krupinski J, Coussen F, Bakalyar HA, Tang WJ, Feinstein PG, Orth K, et al. Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science. 1989;244(4912):1558–64. doi: 10.1126/science.2472670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.