Abstract
Neural circuits mediating repetition effect for semantically congruous words on functional MRI were investigated in seventeen normal elderly (mean age = 70). Participants determined if written words were semantically congruent (50% probability) with spoken statements. Subsequent cued-recall revealed robust explicit memory only for congruous items (83% versus 8% for incongruous). Event-related BOLD responses to New > Old congruous words were found in the left > right cingulate and fusiform gyri, left parahippocampal cortex, middle and inferior frontal gyri (IFG). A group with above-median subsequent recall had markedly more widespread BOLD responses than a Low-Recall subgroup, with larger responses in the left medial temporal lobe (LMTL), IFG, and bilateral cingulate gyri. The magnitude of LMTL activation (New–Old) correlated with subsequent cued-recall, while the spatial extent of LMTL activation (New > Old) correlated with recall and recognition. Both magnitude and spatial extent of left fusiform activation correlated with subsequent recall/recognition. A neural circuit of left-hemisphere brain regions, many identified as P600 generators by invasive electrophysiological studies, was activated by New > Old congruous words, likely mediating successful verbal encoding.
Keywords: Aging, Memory, Neuroimaging, Learning, Semantic, Language, Medial temporal lobe, Fusiform gyrus
1. Introduction
Many, but not all, elderly adults exhibit a decline in declarative memory abilities (Wilson et al., 2002), including the encoding and retrieval of events (Kral, 1962; Tulving, 1983), despite the relative stability of their implicit memory for events and semantic knowledge (Grady and Craik, 2000; Nessler et al., 2006). In addition, age-related decrements in processing speed, attentional capacity, and working memory are commonly found (Kail and Salthouse, 1994; Salthouse, 1992), which may be causally related to both encoding and retrieval declines/difficulties (Craik, 1994; Hedden and Gabrieli, 2004). Long-term longitudinal studies have demonstrated great variance in the rate of age-related decline on neuropsychological measures of memory. Furthermore, decline in memory retention is fairly highly correlated with perceptual speed and working memory (Wilson et al., 2002).
In young adults, several neuroanatomic correlates of successful memory encoding have been identified using functional magnetic resonance imaging (fMRI). Successful encoding of verbal material has been associated with greater blood-oxygen level dependent (BOLD) responses in the medial temporal lobe (MTL) (both parahippocampal gyrus; PHG; Daselaar et al., 2003; Wagner et al., 1998a,b) and hippocampus (Fernández et al., 1998; Grady et al., 2003; Otten et al., 2001; Otten and Rugg, 2001), the fusiform gyrus (Wagner et al., 1998a,b), and inferior prefrontal cortex (PFC; Cabeza et al., 2005; Wagner, 1999). Electrophysiological studies, using both intracranial (Fernández et al., 1999; Halgren et al., 1994a,b) and scalp-recordings (Friedman and Johnson, 2000; Nessler et al., 2006), have implicated some of the same brain regions (e.g., inferior frontal, deep MTL sources) as critical for encoding success (see Friedman and Johnson, 2000 for review).
Neuroimaging studies of aging and memory have shown that older and young adults activate similar brain regions during verbal encoding, with some subtle differences that may provide clues to the source of encoding deficits in normal aging. Relative to young adults, older adults often show reduced asymmetry (bilateral rather than left-hemisphere activation) in PFC during both encoding and retrieval (e.g., Cabeza, 2002; Morcom et al., 2003). This has been variously interpreted as compensatory recruitment of the right PFC to aid encoding or retrieval, or as “dedifferentiation due to age-related changes in anatomical or functional connectivity” (Li and Baltes, 2006). In contrast, relatively few imaging studies have found age-related differences in MTL activation during encoding. This is surprising given that both MTL atrophy and early-stage Alzheimer's disease (AD) pathology are often found in elderly persons with normal cognition for their age (Braak and Braak, 1997; Buckner, 2004). The relative paucity of imaging evidence for MTL dysfunction in older adults may be partly due to magnetic susceptibility artifacts, especially prominent in the anterior extent (Ojemann et al., 1997). However, fMRI evidence for MTL dysfunction in normal aging has been mounting in recent years. For example, using a recognition task and novel methods to model recollection vs. familiarity-related processes, Daselaar et al. (2006) found an age-related reduction of hippocampal activity related to recollection, but an age-related increase of the rhinal cortex sensitivity to familiarity (with decreased BOLD response for repeated words perceived as more familiar). During incidental verbal encoding, Daselaar et al. (2003) reported left anterior MTL (perirhinal/parahippocampal cortex and hippocampus) activation in both young adults and healthy older adults; however, they found significantly less activation in this region of otherwise-healthy older adults whose subsequent memory for stimuli was below the “normal range” (>1SD below the mean of young normals). This underscores the heterogeneity of the aging population, and suggests that the strategy of partitioning a healthy elderly sample into Low- and High-memory subgroups may improve the ability of functional neuroimaging measures to characterize and detect early-stage pathological brain aging. Also relevant is a report of slightly higher values for the BOLD response amplitude in MTL in elderly adults compared with young people, although the age group differences were not significant (Restom et al., 2007). It is, thus, possible that increases in BOLD response may reflect MTL “dysfunction” in a paradigm-dependent manner. Along these lines, Miller et al. (2008) recently reported that increased hippocampal activation during visual scene encoding predicted faster decline in patients with Mild Cognitive Impairment (MCI).
We have developed a cross-modal word-repetition paradigm that, in ERP studies, has shown sensitivity to memory impairment in early-stage AD (including MCI), and to verbal memory ability in healthy older adults (Olichney et al., 2000, 2006, 2008). In this paradigm, robust learning normally occurs for the semantically congruous stimuli, but not for the incongruous target words (Olichney et al., 2000). Repetition of semantically congruous words modulates (decreases) the ERP P600 component, and the magnitude of this congruous repetition effect correlates with declarative memory performance (Olichney et al., 2000, 2002). This is consistent with a large body of ERP literature linking the P600, or ‘late positive component’, to memory processes (e.g., Donchin and Coles, 1988; Duzel et al., 1997; Fernández et al., 1998, 2001; Paller and Kutas, 1992). For example, subsequently recalled or recognized words generally have larger late positivities than non-recalled words (Mangels et al., 1999; Paller et al., 1987) and the size of this difference (often called “Dm”) can be reduced by “directed forgetting” instructions (Paller, 1990). Very large P600-like potentials, which show a ‘Dm’ effect (Fernández et al., 1999), have been recorded in the human hippocampus (Halgren et al., 1994a), but it is unclear to what extent these contribute to the scalp-P600. The PHG and other limbic (e.g., temporal pole), paralimbic (e.g., cingulate, orbitofrontal), and association neocortical regions (e.g., IFG, parietal, and fusiform higher visual cortex), are also thought capable of producing P600s (Halgren et al., 1994a,b; Friedman and Johnson, 2000; Guillem et al., 1999).
In contrast, repetition of incongruous target words results in only minimal declarative learning/memory and primarily modulates (decreases) the ERP N400 component, and not the P600. The amplitude of this “incongruous ERP repetition effect” has generally not correlated with verbal memory ability in our experiments (Olichney et al., 2008). The fMRI incongruous word repetition effect results are beyond the scope of this report.
In order to define the spatio-temporal characteristics of congruous word repetition effects in normal healthy elderly and their relationship to successful encoding, we adapted this paradigm for fMRI. In the present study, fMRI data were obtained as participants performed semantic category membership judgments on cross-modal stimuli, i.e., auditory category statements, followed by semantically congruous or incongruous visual words. Incidental memory for the stimuli was assessed afterwards. Analyses were conducted on the entire group, as well as High- and Low-Recall subgroups defined by subsequent cued-recall performance.
Three hypotheses were tested. First, repetition of congruous words was expected to decrease BOLD signal (i.e., New > Old) in putative neural generators of the P600 (e.g., PHG, hippocampus, cingulate, ventrolateral PFC). Second, more robust New > Old congruous-trial responses were expected in the High-Recall (than Low-Recall) performance subgroup. Third, the spatial extent and magnitude of the New > Old congruous repetition effect in the left MTL was expected to correlate with subsequent recall, with greater repetition-related reduction in subjects with better recall abilities.
2. Methods
2.1. Subjects
Seventeen healthy, cognitively normal, right-handed elderly persons (10 male, 7 female; mean ± SD; age = 69.7 ± 11.7 years, education = 15.3 ± 2.4 years, MMSE range 28–30) were recruited from the Shiley-Marcos ADRC and the San Diego community. Exclusion criteria included history of neurological (CNS) or psychiatric disorders; cardiac, respiratory, renal, or hepatic failure; and severe loss of hearing (e.g., use of hearing aid or difficulty with hearing conversational speech) or vision (corrected distant visual acuity poorer than 20/50).
Based on performance on the post-scan cued-recall test (described below), participants were divided into Low-Recall (n = 8 scoring below the median; 3 males; age = 72.9 ± 9.3 years) and High-Recall (n = 8 above the median; 6 males; age = 70.0 ± 10.3 years) subgroups. Age, sex, and education levels did not differ significantly between the subgroups, although trends were present for more males (Chi-square, p = 0.13) and higher education (t-test, p = 0.11) in the High-Recall subgroup (see Table 1).
Table 1.
Demographic and behavioral data for the High (n = 8) and Low (n = 8) memory performance subgroups.
| High | Low | p | |
|---|---|---|---|
| Age (years) | 70.0 ± 10.3 | 72.9 ± 9.3 | 0.57 |
| Education (years) | 16.4 ± 2.4 | 15.3 ± 2.4 | 0.11 |
| Sex | 6M, 2F | 3M, 5F | 0.13 |
| %Correct—congruous | 97 ± 2 | 94 ± 5 | 0.13 |
| %Correct—incongruous | 99 ± 2 | 99 ± 1 | 0.83 |
| RT—Congruous New (ms) | 1018 ± 187 | 1330 ± 323 | 0.04* |
| RT—Congruous Old | 620 ± 136 | 907 ± 239 | 0.01* |
| ΔRT—congruous (New–Old) | 399 ± 168 | 423 ± 184 | 0.79 |
| RT—Incongruous New (ms) | 1136 ± 182 | 1436 ± 291 | 0.051 |
| RT—Incongruous Old | 877 ± 239 | 1195 ± 274 | 0.03* |
| ΔRT—incongruous (New–Old) | 260 ± 136 | 240 ± 169 | 0.80 |
| Free recall—all words (%) | 16 ± 6 | 11 ± 6 | 0.14 |
| Cued-recall—congruous (%) | 93 ± 5 | 71 ± 14 | 0.001* |
| Cued-recall—incongruous (%) | 14 ± 22 | 0 | 0.09 |
| Recognition—congruous (%) | 97 ± 4 | 85 ± 13 | 0.03* |
| Recognition—Incongruous (%) | 86 ± 17 | 47 ± 30 | 0.006* |
RT, Reaction time.
p < 0.05 (t-tests, except chi-square test for categorical variable ‘sex’).
2.2. Materials and procedure
2.2.1. Category decision task
A set of 72 trials was constructed, each consisting of a unique short auditory category statement followed by a visual target word (noun), half of which were semantically congruous (e.g., “Part of the face—CHEEKS”) and half of which were incongruous (e.g., “A citrus fruit—PORT”). The task was to indicate whether the target word belonged to the stated category. Congruous category exemplars were of medium typicality, usually between the 3rd to 6th most common exemplar produced, according to published norms (Battig and Montague, 1969; Shapiro and Palermo, 1970) or a locally administered questionnaire (Olichney et al., 2000). The mean frequency (Francis and Kucera, 1982) and length of the target words were comparable in the congruous (frequency mean = 38.7 ± 50.5; length = 5.9 ± 1.7 letters) and incongruous (frequency mean 37.4 ± 43.6; length = 5.7 ± 1.4 letters) conditions.
2.2.2. Experimental procedure
After informed consent was obtained, subjects were briefly trained on the category decision task outside of the scanner until reliable performance was demonstrated. The subject was instructed to remain silent and to minimize movements during all anatomical (~7 min) and functional image acquisition. Prior to each functional run, the experimenter repeated the category decision task instructions.
For the category decision task, auditory category statements (digitized audio files; 22 kHz, 16-bit stereo, volume adjusted to a subjectively loud, but comfortable volume) were presented via MRI-compatible noise-attenuating headphones (Gradient Muff Headset: Resonance Technology, Inc.) with earplugs also placed in both ear canals. Visual stimuli (instructions, fixation crosshairs, and target words) were projected onto a screen and viewed through a mirror (visual angle of words ~0.5°, which requires 20/100 visual acuity or better to read). When uncorrected far vision was poorer than 20/50, corrective lenses in plastic frames were used to improve visual acuity to 20/50 or better. Responses were made with an MRI-compatible two-button mouse placed in the dominant hand.
On each trial, fixation crosshairs (‘+’) and an auditory category statement were presented together (total duration of audio file = 3 s, including brief inter-stimulus interval), then followed by a visual target word (duration = 500 ms), followed by a blank screen for a variable inter-trial interval (ITI). The ITI was jittered to produce trials of variable duration (7.5, 10, 12.5 and 15 s), which facilitates event-related analyses and the estimation of hemodynamic responses (Aguirre and D'Esposito, 1999). Stimulus presentation was controlled with E-Prime software (v.1.1; Psychological Software Tools, Inc.). Reaction time (RT) and accuracy data were recorded. See Supplementary Fig. 1 for an illustration of a sample single trial.
Stimuli were presented in 6 Runs of 24 trials (12 new and 12 repeated items; all repetitions occurred within runs). The lag between the repetition of items was, on average, 93 s (range: 15–178 s). Runs 1, 3 and 5 consisted of 5/6 congruous items and 1/6 incongruous items. Runs 2, 4, and 6 consisted of 5/6 incongruous items and 1/6 congruous items. Therefore, across runs, 50% of the trials were congruous and 50% were incongruous. Stimulus presentation commenced 5 s after the onset of each functional-image acquisition run, and data from the first two TRs discarded. Each run lasted 265 s. In addition, two rest condition (visual fixation only) runs lasting 90 s each were collected, one at the beginning and one at the end of the MRI session. Approximately, 30 min of functional MRI data were collected for each subject.
2.2.3. Post-task recall and recognition tests
Immediately following the MRI session, participants were given unanticipated tests of free recall, cued-recall, and multiple-choice recognition, in that order (Olichney et al., 2000). In the cued-recall task, participants were given a list of category statements and asked to fill in the associated target words seen earlier (regardless of congruity). The multiple-choice recognition task consisted of category statements, each with six possible completions (four congruous, two incongruous). The cued-recall and multiple-choice questionnaires were weighted towards congruous trials (35 congruous and 8 incongruous items; maximum score = 43; one point for each correct answer regardless of congruity).
2.3. Imaging methods and analysis
2.3.1. Image acquisition
Imaging was performed on a 1.5 T Siemens (Erlangen, FRG) Magnetom MRI scanner. Anatomical images were acquired using a magnetization-prepared rapid acquisition 3D gradient-echo (MPRAGE) pulse sequence to obtain 180 sagittal slices, 1 mm thickness (TR = 11.4 ms, TE = 4.4 ms, flip angle = 10°, FOV = 256 mm). This sequence provided high-resolution (1 mm 1 mm × 1 mm) T1-weighted images of the entire brain. BOLD response was assessed with gradient-echo planar imaging (EPI) sequences (29 axial slices, 4 mm thickness, 4 mm × 4 mm in-plane resolution, TR = 2.5 s, TE = 32 ms, flip angle = 90°, FOV = 256 mm). For each functional run, 106 repetitions were performed which resulted in time series fMRI data for the entire bilateral cerebral hemispheres, most of the cerebellum and brainstem.
2.3.2. Individual subject data processing and analysis
The MRI analyses were performed primarily with the AFNI software package (Cox, 1996; specific AFNI programs indicated below in quotes). Individual anatomical volumes were processed to extract brain (white and gray matter) from surrounding CSF, meninges, skull, and other non-brain tissues using ‘3dSkullStrip’ and FSL's FAST tissue segmentation program (Smith et al., 2004; Zhang et al., 2001). Estimates of gray and white matter compartment volume were obtained from the resulting stripped and segmented brain volumes.
Individual subject functional data were processed as follows. For each functional run, small head movements were corrected using ‘3dVolreg’ to register all brain volumes to a reference volume, chosen to minimize the total correction. Timepoints with large head movements not correctable by ‘3dVolreg’ or containing other scanner artifacts were censored from further analyses. The average linear motion was estimated at 0.09 mm, relative to the reference volume, in the non-censored timepoints. Residual motion estimates in three planes were factored into the regression analysis (see below).
Functional image runs were analyzed in an event-related manner using ‘3dDeconvolve’. Multiple linear regression analysis was performed on the motion-corrected concatenated timeseries data, with BOLD signal intensity as the dependent variable, predicted by the independent effects of the four experimental conditions (Congruous-New (CN), Congruous-Old (CO), Incongruous-New (IN), Incongruous-Old (IO)) and motion estimates in three orthogonal planes. This analysis produced functional activation maps for all four conditions as well as for two contrasts (New vs. Old Congruous; New vs. Old Incongruous) for each timepoint. Analyses were conducted with stick-function (square wave with duration = 1 TR or 2.5 s) regressors at two timepoints, 3-TR (7.5 s) and 4-TR (10 s) after the onset of the trial. Because the target word was presented 3 s following trial onset, the 3- and 4-TR latencies captured the rising/peak (~4.5 s) and falling (~7 s) phases, respectively, of the BOLD response to the target word. See Supplementary Fig. 1 for an illustration of the time course of the stimuli and the expected hemodynamic response.
The ‘3dDeconvolve’ program also provided estimates of the Hemodynamic Response Function (HRF) for each voxel by stimulus condition. These analyses used stick-function references at timepoints 0–5 (0–12.5 s post-trial onset), with the 5-TR (12.5 s) timepoint based only on trials of duration 10 or 12.5 s to avoid contamination from overlapping early BOLD responses to subsequent trials. HRFs shown here are averaged across subjects within a region of interest (ROI). The use of stick-function regressors makes no assumptions about the shape of the HRF (Birn et al., 2002; Liu et al., 2001). Whereas gamma-function models are appropriate for estimating the magnitude of BOLD responses (Boynton et al., 1996; Cohen, 1997; Rajapakse et al., 1998), particularly in young subjects (Cohen et al., 2002), the HRF is often delayed and broader in older subjects (D'Esposito et al., 1999, 2003). This may particularly hold in our limbic and association cortex ROIs.
2.3.3. Group analyses
Individual subject maps were smoothed (isotropic Gaussian kernel, full-width half-maximum (FWHM) = 4 mm) and spatially transformed into standardized anatomical coordinates (Talairach and Tournoux, 1988). Data were analyzed for all subjects, and the Low- and High-Recall subgroups separately. Statistical maps (t-tests) were generated using ‘3dttest’ to evaluate the neural response to each condition relative to baseline, and to evaluate the repetition effects (New vs. Old contrasts). Statistical thresholding for all group maps was set at p < 0.025 for each voxel. For the whole brain analyses, clusters of 12 or more adjacent voxels were considered significant (whole brain α < 0.05; 4 mm FWHM Gaussian smoothing; connectivity radius = 5.66 mm; 23,660 voxels in whole brain mask). For voxels active at both 3-TR and 4-TR (shown as yellow or purple in Figs. 1 and 2) the probability that this is due to chance alone is 0.4 × 10−7 corresponding to p = 0.015 to find one such voxel in the entire brain. In addition, clusters of 6 or more adjacent voxels (volumes ≥ 384 mm3) were considered significant within the left MTL, our main hypothesized ROI. Monte Carlo Simulation (Forman et al., 1995) done within this ROI (4 mm FWHM Gaussian kernel; connectivity radius = 5.66) showed that a volume-corrected α < 0.05 would result in a minimum spatial threshold of 5 voxels (α = 0.018 for cluster volumes ≥ 320 mm3), and that α = 0.003 for our chosen spatial threshold of 6 voxels.
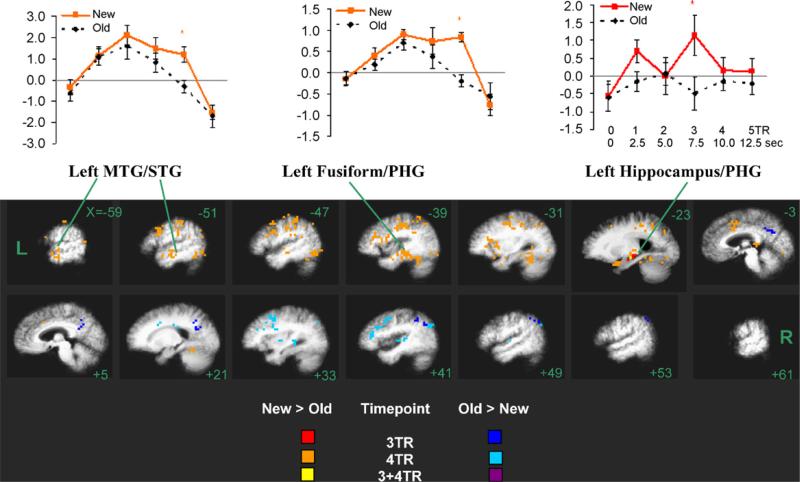
Fig. 1.
Maps of the congruous repetition effect on BOLD response and estimates of the hemodynamic response function (HRF) shown for New and Old congruous words. Clusters of voxels with significant New > Old BOLD (‘hot’ colors) and Old > New BOLD (‘cold’ colors) effects at 3- and 4-TR timepoints are shown (see key for color scheme) superimposed on the group-averaged (n = 17) anatomical image. Slices are labeled with Talairach x-coordinates. HRF estimates for selected clusters are shown above; error bars indicate standard error across subjects. *p < 0.05; PHG = parahippocampal gyrus, MTG = middle temporal gyri, STG = superior temporal gyri.
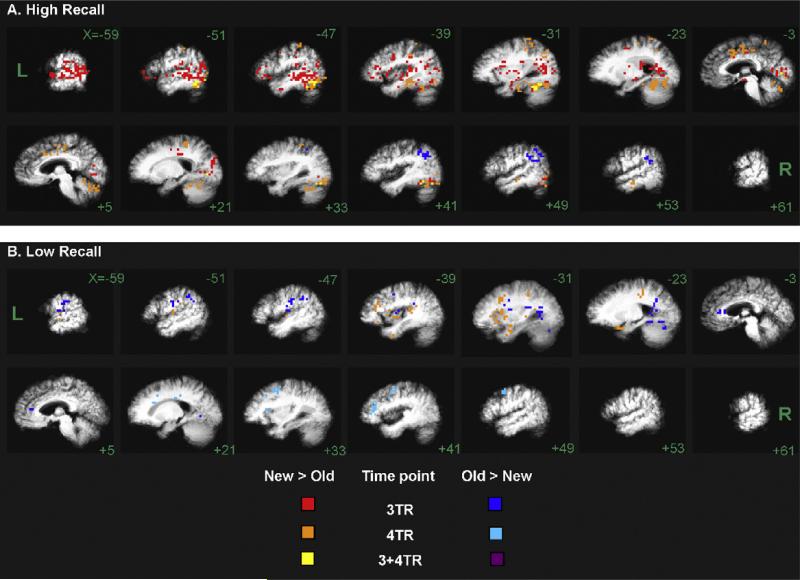
Fig. 2.
Maps of the congruous repetition effect on BOLD response for memory-performance subgroups. (A) High Recall (n = 8), and (B) Low Recall (n = 8), superimposed over their respective group-averaged anatomical images. New > Old and Old > New BOLD effects are colored as in Fig. 1 (see key and legend). Slices are labeled with Talairach x-coordinates.
To test the hypothesized relationship between MTL activity and subsequent memory, the spatial extent (number of significant voxels) and magnitude (New–Old BOLD response, averaged across 3-TR and 4-TR timepoints) of repetition effects in left and right MTL, were correlated with cued-recall and multiple-choice recognition scores. MTL was defined as the region encompassing the PHG and hippocampus (per AFNI's Talairach daemon), warped into the subject's native (non-Talairach transformed) brain space and resampled to 4-mm isotropic voxels. This automated masking process was monitored by a researcher (JRT) blinded to the subjects’ memory performance. In cases where the MTL mask did not cover the most inferior and medial portion of a subject's MTL, significant voxels laying within this region were manually counted; in cases where the mask extended into surrounding CSF, any significant voxels outside the brain parenchyma were not counted. Analogous methods were used to obtain active-voxel counts and magnitude of repetition effects for the left and right fusiform gyri. Exploratory analyses of the repetition effect magnitude were performed for the left cingulate cortex (divided into anterior, mid- and posterior cingulate per the AFNI daemon), and bilateral primary visual (BA 17) and auditory (BA 41) cortex, as defined by the AFNI Talairach daemon.
3. Results
3.1. Behavioral results
3.1.1. Category decision task
Performance accuracy on the category decision task approached ceiling (96.0 ± 4.0% on congruous trials; 98.8 ± 1.3% on incongruous trials), with the accuracy on incongruous trials being significantly higher than for congruous trials (t = 2.66, p < 0.05). For both congruous and incongruous trials, RTs were faster for Old than for New trials (M RTs: CN vs. CO: 1168 ms vs. 764 ms, t = 9.78, p < 0.0001; IN vs. IO: 1273 ms vs. 1025 ms, t = 6.98, p < 0.0001).
A two-way within-subjects ANOVA with factors of Congruity (congruous vs. incongruous) and Repetition (New vs. Old) revealed that both the main effects and the interaction were significant, with greater RT ‘priming’ for congruous than incongruous trials (Congruity: F(1,15) = 53.5, p < 0.0001; Repetition: F(1,15) = 96.8, p < 0.0001; Congruity × Repetition: F(1,15) = 15.79, p < 0.005).
3.1.2. Subsequent recall and recognition performance
Free recall was generally poor, and was significantly lower for target words presented in an incongruous than in a congruous context (4 ± 11% vs. 24 ± 12.5%, t = 5.04, p < 0.0001). When cued with category statements, recall of target words was higher, but with an even stronger dissociation between cued-recall for congruous vs. incongruous words (83% vs. 8%, t = 22.2, p < 0.00001). This likely reflects the very heavy demands upon episodic and source memory encumbered when the ‘invalid’ incongruous category cues were provided. Multiple-choice recognition was also better for congruous than incongruous words (91 ± 11% vs. 66 ± 31%, t = 28.8, p < 0.00001) despite the presence of more congruous than incongruous ‘foils’ on the questionnaire.
3.2. Imaging results: congruous trials
3.2.1. BOLD response to congruous trials
We examined separately the BOLD response to congruous trials for each repetition condition (i.e., New and Old), measuring each of these relative to the baseline BOLD signal. The resulting cluster-thresholded t-test brain maps (≥12 voxels, 768 mm3) are available as Supplementary Fig. 2.
3.2.1.1. New vs. baseline
Widespread and robust BOLD responses above baseline were seen for New Congruous trials throughout much of the bilateral cerebral hemispheres at both 3- and 4-TR timepoints (Supplementary Fig. 2A). Throughout the text, numbers in square brackets indicate the Talairach x-coordinate of the relevant sagittal slice in the figure.
3.2.1.2. Old vs. baseline
BOLD response to Old Congruous trials (>baseline) was less widespread in the left-hemisphere compared to New Congruous trials (red and orange voxels in Supplementary Fig. 2B). Unlike New trials, robust responses to Old trials were found in much of the right parietal cortex (BA 7, 39, and 40 [see x = +37, +49, +57 in Supplementary Fig. 2B]), and much of this activity was sustained over both timepoints (yellow voxels). Also unlike New words, at later 4-TR timepoint (light blue voxels), widespread deactivation (baseline > Old) was present in the left-hemisphere (full cluster reports available from authors on request).
3.2.2. BOLD congruous word repetition effects
Of particular interest is the identification of brain regions that show repetition-related decreases (i.e., New > Old) or increases (i.e., Old > New) on congruous trials. The corresponding clustered t-test maps (Fig. 1) are color-coded according to the direction of the effect and the timepoints at which a given voxel was significant (New > Old effects in ‘hot’ colors, Old > New effects in ‘cold’ colors; see key of Fig. 1).
3.2.2.1. New > Old
Several left-hemisphere regions showed a repetition-related reduction (New > Old) at both the 3- and 4-TR timepoints, with larger and more extensive clusters present at the 4-TR latency (Fig. 1; red, orange, and yellow voxels). At the 3-TR timepoint, the largest cluster was centered in the left PHG (640 mm3, center coordinates = −22, −20, −6) and and included hippocampus entorhinal cortex (BA 28 [−23]). Additional smaller foci of activation (eight clusters between 384 and 448 mm3) were found at 3-TR within the left and right fusiform gyri (BA 37), left IFG (BA 47), left thalamus, and lingual gyri (BA 18; right > left), but since these were below our spatial threshold for the whole brain analysis, these are not in Fig. 1. See Table 2A for more information about left PHG cluster and Fig. 1 for its estimated HDR.
Table 2A.
Clusters with response to congruous words (New > Old).
| Structure (Brodmann's area) | CM |
Volume (mm3) | Mean (SE) | MI |
Comments | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||||
| 3-TR | |||||||||
| LPHG(28), hippocampus | –22 | –20 | –6 | 640 | 1.41(0.07) | –22 | –25 | –4 | |
| 4-TR | |||||||||
| LIPL(40), LCG(31) | –35 | –34 | 43 | 10,688 | 1.05(0.02) | –50 | –29 | 48 | |
| LFG(20,37), LPHG(36) | –38 | –39 | –13 | 10,304 | 1.15(0.03) | –46 | –53 | –20 | |
| L Insula(13), LIFG(44) | –39 | 15 | 17 | 6,144 | 1.10(0.04) | –30 | 15 | –16 | |
| LLG(18), LFG(19), L Declive | –15 | –79 | –9 | 3,008 | 1.18(0.05) | –6 | –81 | –8 | |
| L Thalamus | –11 | –28 | 6 | 1,984 | 1.24(0.06) | –2 | –33 | 4 | |
| LCG(24,32), L medFG(6), LSFG(8) | –4 | 11 | 42 | 1,664 | 1.16(0.05) | –2 | 11 | 32 | |
| L PreCG(4), L PostCG(3) | –40 | –18 | 56 | 960 | 1.30(0.08) | –42 | –21 | 60 | |
| R Culmen, R Declive | 17 | –52 | –14 | 832 | 1.08(0.08) | 18 | –57 | –16 | |
| LMTG(21), LSTG(22) | –62 | –15 | –3 | 832 | 1.59(0.13) | –62 | –13 | –4 | |
| LIPL(13) | –42 | –42 | 26 | 832 | 1.09(0.07) | –42 | –45 | 32 | |
| LPHG(34)/IFG(47), L Uncus(34) | –24 | 5 | –18 | 768 | 1.88(0.17) | –22 | 3 | –16 | |
| LSTG(39), LMTG(39,19) | –56 | –61 | 18 | 768 | 0.95(0.11) | –58 | –57 | 12 | |
Clusters above list center first (CM = center of mass), then other surrounding structures with activations (MI = maximal intensity (coordinates)). L = Left, R = right, IPL = inferior parietal lobule, FG = fusiform gyrus, LG = lingual gyrus, PHG = parahippocampal gyrus, IFG = inferior frontal gyrus, SFG = superior frontal gyrus, CG = cingulate gyrus, STG = superior temporal gyrus, MTG = middle temporal gyrus, medFG = medial frontal gyrus, PreCG = precentral gyrus, PostCG = postcentral gyrus.
At 4-TR, several large clusters were present. These included one centered in the left fusiform gyrus (BA 37, 20 [−47]), extending medially into the left MTL (including hippocampus [−23] and posterior PHG/perirhinal cortex (BA 36 [−31])), laterally into inferior temporal gyrus (ITG, BA 20 [−59]), and posteriorly to the middle occipital gyrus (BA 19 [−39]). Other large clusters were centered in the left IPL (BA 40 [−51 to −31]), cingulate (BA 32, 24; left > right [−3, +5]), left insula (BA 13 [−31, −39]), left middle/superior temporal gyrus ([−51, −59]) and thalamus (not shown in Fig. 1). See Table 2A for all clusters with volume ≥ 768 mm3 at the 4-TR timepoint, and Fig. 1 for time course of HDR for left MTG/STG and left fusiform gyrus clusters.
3.2.2.2. Old > New
Clusters with significant repetition-related increases (Old > New) in BOLD response were also more extensive at the 4-TR timepoint, but in contrast to the left-hemisphere bias of the New > Old repetition effect described above, these clusters were found mostly in right-hemisphere regions (Fig. 1, dark and light blue voxels), particularly in right parietal and frontal cortex. At 3-TR, the largest cluster (volume = 2496 mm3) was found centered on right precuneus (BA 7 [+5 and +21]), which extended down into posterior cingulate (BA 31, 23 [+5]), and across midline to left precuneus/posterior cingulate [−3]. Another large Old > New cluster was found in the right IPL (BA 40), which included the angular gyrus (BA 39 [+41, +49]). At the 4-TR timepoint, the right MFG showed a large cluster of Old > New response (BA 6, 8 [+33, +41] and BA 9 [+41]), extending to a small portion of IFG (deep to BA 45 and 47 [+41]). Other significant large 4-TR clusters were found in the right precuneus (BA 19 [+33], extending laterally to angular gyrus (BA 39 [+41 to +53]), and posteriorly to include MTG (BA 39 [+41])), right posterior cingulate (BA 24 and 31 [+21]), and right insula [+41]. See Table 2B for all clusters (≥768 mm3) with Old > New BOLD response to congruous words.
Table 2B.
Clusters with response to congruous words (Old > New).
| Structure (Brodmann's area) | CM |
Volume (mm3) | Mean (SE) | MI |
Comments | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||||
| 3-TR | |||||||||
| R Precuneus/R Posterior CG(31,23), R | 8 | –57 | 31 | 2496 | 1.16(0.05) | 2 | –65 | 28 | |
| Cuneus(7), RSPL(7) | |||||||||
| RIPL(40, 39) | 45 | –58 | 38 | 1344 | 1.25(0.07) | 46 | –61 | 36 | |
| 4-TR | |||||||||
| RMFG, RSFG, R PreCG(6,8,9) | 36 | 18 | 32 | 5376 | 1.11(0.03) | 38 | 11 | 44 | RMFG > RSFG |
| R Insula(13), RSTG(22), RCT | 39 | –22 | 4 | 1408 | 1.23(0.11) | 42 | –17 | 4 | |
| RCG(31,24) | 19 | –19 | 29 | 896 | 1.05(0.08) | 14 | –17 | 28 | |
| R Precuneus(19,39), RMTG(39), RAG(39) | 40 | –72 | 35 | 896 | 0.92(0.05) | 42 | –73 | 28 | |
Clusters above list center first (CM = center of mass), then other surrounding structures with activations (MI = maximal intensity (coordinates)). L = Left, R = right, IPL = inferior parietal lobule, SPL = superior parietal lobule, MFG = middle frontal gyrus, SFG = superior frontal gyrus, CG = cingulate gyrus, STG = superior temporal gyrus, MTG = middle temporal gyrus, PreCG = precentral gyrus, CT = caudate tail, AG = angular gyrus.
3.2.3. High- and Low-Recall subgroups
3.2.3.1. High- and Low-Recall subgroups: behavior and anatomy
To evaluate the relationship between imaging measures and memory in this group of normal older subjects, Low- and High-Recall subgroups were formed using a deleted-median split of cued-recall scores. The two subgroups had comparable accuracy on the semantic category decision task (see Table 1). The High-Recall subgroup, however, had generally faster RTs than the Low-Recall subgroup (Table 1). The two groups showed equivalent repetition priming of RT (p's ≥ 0.79).
No systematic differences were evident in the anatomical brain images (see group-averaged MPRAGE images in Fig. 2A and B). There were no significant intergroup differences on voxel-based estimates of whole-brain volume (gray plus white matter; 5.96 × 105 mm3 vs. 6.13 × 105 mm3 for Low and High, respectively; t < 1) or the ratio×of gray matter to gray-plus-white matter volume (0.53 vs. 0.54 for Low and High, respectively; t < 1). In contrast to their similar brain anatomies, functional maps for the High- and Low-Recall subgroups showed a dramatic dissociation (discussed below).
3.2.3.2. High-Recall subgroup: BOLD congruous repetition effects
3.2.3.2.1. High-Recall subgroup: New > Old
The High-Recall subgroup map (Fig. 2A) showed an extensive repetition-related decrease (New > Old) in BOLD response that was both early (3-TR, red voxels) and sustained (4-TR, orange voxels; both timepoints: yellow voxels). Indeed, the High-Recall subgroup map was much stronger (especially at shift 3-TR) than the corresponding map of the entire group (n = 17) shown in Fig. 1, despite a large decrement in statistical power.
The map of New > Old clusters in the High-Recall subgroup at the 3-TR timepoint included a huge cluster (volume = 36,480 mm3) with maximal response (BOLD change = 0.9% [TT coordinates = −58, 3, 0]) centered in left STG, involving much of the left lateral temporal cortex (BA 21, 22 [−59 through −47]), extending to the left MTL (entorhinal cortex, BA 28 [−23]), fusiform (BA 37), middle occipital gyrus (BA 19) and Heschl's gyrus (BA 41). This cluster also included the bilateral lingual gyri (BA 17, 18) and cuneus (BA 18, 23, 30 [−3, +5]), and extended into right inferior occipito-temporal cortex ([+41]). Other 3-TR clusters included left and right cingulate, left IFG, left insula, midbrain/diencephalon (including bilateral mammilary bodies) and right fusiform (BA 37, 19). At the 4-TR timepoint, a large cluster of response was found in bilateral inferior occipito-temporal cortex [−51 through −23 and +49 through +21], extending bilaterally from inferior−occipital gyrus (BA 19) through fusiform gyrus (BA 19, 37) to inferior temporal gyrus (BA 20). Other large 4-TR clusters included left MTL (PHG/perirhinal cortex, BA 35 and 36, and hippocampus), left cingulate (BA 32), left IPL (BA 40), sensory and motor cortex [−51 through −31]. See Tables 3A and 3B for list of cluster locations.
Table 3A.
Clusters with response to congruous words, High-Recall (n = 8).
| Structure (Brodmann's area) | CM |
Volume (mm3) | Mean (SE) | MI |
Comments | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||||
| New > Old, 3-TR | |||||||||
| LMTG, L Temporal, Parietal, and Occipital areas | –38 | –45 | 4 | 36,352 | 1.77(0.02) | –58 | 3 | 0 | L>R |
| LCG | –11 | –6 | 39 | 2,368 | 1.36(0.06) | –2 | 7 | 32 | L>R |
| LIFG(47) | –44 | 31 | 0 | 2,048 | 1.70(0.08) | –46 | 19 | –4 | |
| L Insula(13) | –35 | 19 | 9 | 1,664 | 1.50(0.10) | –34 | 15 | 0 | |
| L PreCG(6), LIFG, LMFG | –35 | 4 | 26 | 1,536 | 1.40(0.09) | –46 | –1 | 40 | |
| R Cuneus(18) | 17 | –80 | 21 | 1,472 | 1.44(0.09) | 2 | –73 | 16 | |
| RCG(24) | 20 | –15 | 40 | 1,216 | 1.27(0.06) | 10 | –9 | 40 | |
| L Culmen, L Declive | –31 | –50 | –23 | 1,024 | 1.59(0.09) | –30 | –45 | –28 | |
| RFG(19,37) | 42 | –68 | –13 | 1,024 | 2.12(0.23) | 46 | –73 | –16 | |
| RFG(37) | 45 | –51 | –15 | 768 | 2.13(0.21) | 50 | –57 | –16 | |
| L Red nucleus, L Mammillary body, L Thalamus | –5 | –15 | –4 | 768 | 2.53(0.46) | –6 | –13 | –8 | |
| LMOG(19) | –37 | –80 | 10 | 768 | 1.57(0.13) | –30 | –85 | 8 | |
| New > Old, 4-TR | |||||||||
| L Declive, L Occipito-temporal and Cerebellar areas | –13 | –63 | –16 | 18,304 | 1.67(0.04) | 14 | –85 | –20 | L>R |
| LPCL | –2 | –18 | 47 | 3,520 | 1.53(0.06) | 2 | –9 | 36 | L=R |
| RFG(19,37) | 40 | –69 | –14 | 2,688 | 2.24(0.17) | 46 | –65 | –16 | |
| LCG(32,24), L medFG(6) | –1 | 9 | 39 | 1,856 | 1.46(0.07) | 2 | 3 | 36 | |
| LPHG | –35 | –26 | –17 | 1,600 | 1.51(0.09) | –26 | –17 | –24 | |
| LIPL | –40 | –40 | 55 | 1,600 | 1.75(0.11) | –54 | –37 | 52 | |
| R PostCG(40), RIPL(40) | 27 | –37 | 54 | 1,088 | 1.48(0.08) | 22 | –37 | 48 | |
| L Precuneus(7) | –7 | –51 | 57 | 960 | 1.59(0.14) | –2 | –49 | 56 | |
| L Precuneus(7), LSPG(7) | –26 | –63 | 39 | 832 | 1.17(0.08) | –26 | –57 | 40 | |
| RMTG | 51 | –20 | –10 | 768 | 1.45(0.07) | 46 | –25 | 0 | |
| L PostCG(3) | –31 | –31 | 46 | 768 | 1.20(0.08) | –30 | –37 | 48 | |
| Old > New, 3-TR | |||||||||
| RIPL, RSG, RAG | 47 | –52 | 34 | 3,008 | 1.57(0.05) | 50 | –41 | 32 | |
Clusters above list center first (CM = center of mass), then other surrounding structures with activations (MI = maximal intensity (coordinates)). L = Left, R = right, IPL = inferior parietal lobule, FG = fusiform gyrus, PHG = parahippocampal gyrus, IFG = inferior frontal gyrus, MFG = middle frontal gyrus, CG = cingulate gyrus, MTG = middle temporal gyrus, medFG = medial frontal gyrus, MFG = middle frontal gyrus, PreCG = precentral gyrus, PostCG = postcentral gyrus, AG = angular gyrus, PCL = paracentral lobule, MOG = middle occipital gyrus, SG = supramarginal gyrus, SPL = superior parietal lobule.
Table 3B.
Clusters with response to congruous words, Low-Recall (n = 8).
| Structure (Brodmann's area) | CM |
Volume (mm3) | Mean (SE) | MI |
Comments | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||||
| New > Old, 4-TR | |||||||||
| L Insula(13), LIFG(10), LMFG(9) | –35 | 16 | 14 | 3136 | 1.71(0.07) | –38 | –1 | 0 | |
| LSTG(41), LTTG(41), L Insula(13) | –37 | –34 | 16 | 1152 | 1.39(0.09) | –38 | –29 | 16 | |
| LCG(31), L PostCG(3) | –21 | –31 | 42 | 1024 | 1.27(0.07) | –26 | –29 | 40 | |
| LSTG(28), L Uncus(28,34), IFG(47), LMTG(38), PHG, Amygdala | –27 | 6 | –23 | 832 | 2.09(0.21) | –22 | 11 | –20 | |
| LIFG(47), L Insula(13) | –32 | 20 | –4 | 832 | 1.59(0.08) | –30 | 15 | –8 | |
| LSTG(21,22), LMTG(21), L PreCG(22), L Insula(13) | –59 | –13 | 4 | 768 | 1.77(0.14) | –66 | –17 | 0 | |
| LPHG(28,34), L Uncus | –31 | –6 | –18 | 704 | 1.45(0.10) | –26 | –9 | –20 | |
| Old > New, 3-TR | |||||||||
| L Posterior CG(30), LLG(19), L Precuneus(31) | –20 | –52 | 19 | 3072 | 1.66(0.07) | –14 | –53 | 28 | |
| L PostCG(2), PreCG(6), LIPL(40) | –52 | –20 | 26 | 1856 | 1.72(0.09) | –46 | –25 | 32 | |
| L Declive, LFG(19), LLG(18) | –20 | –70 | –13 | 1344 | 1.74(0.10) | –10 | –69 | –4 | |
| L Insula(13), PreCG | –33 | –1 | 22 | 1280 | 1.59(0.07) | –38 | –5 | 8 | |
| LSG, LIPL(40) | –43 | –40 | 30 | 1152 | 1.82(0.11) | –50 | –37 | 36 | |
| LPHG(19,30), LLG(19), LFG(37), L Culmen | –19 | –47 | –1 | 896 | 1.86(0.15) | –18 | –49 | 0 | |
| R Anterior CG(24) | 1 | 30 | 10 | 832 | 2.65(0.18) | 2 | 31 | 12 | R>L |
| RLG(19,18), R Posterior CG(30), R Cuneus | 15 | –64 | 2 | 768 | 1.79(0.16) | 10 | –61 | 4 | |
| LSTG(42), LTTG(42,41) | –62 | –21 | 9 | 768 | 1.88(0.15) | –62 | –25 | 4 | |
| Old > New, 4-TR | |||||||||
| RMFG(8,6) | 30 | 13 | 38 | 1984 | 1.64(0.07) | 30 | 11 | 40 | |
| RMFG(46), RIFG(46), R Insula(13) | 41 | 32 | 14 | 1408 | 1.83(0.10) | 42 | 31 | 16 | |
| RMFG(6,9), PreCG(6) | 46 | 2 | 42 | 1152 | 1.62(0.10) | 46 | 7 | 36 | |
| RCG(31) | 18 | –20 | 30 | 768 | 1.64(0.11) | 14 | –17 | 28 | |
Clusters above list center first (CM = center of mass), then other surrounding structures with activations (MI = maximal intensity (coordinates)). L = Left, R = right, IPL = inferior parietal lobule, FG = fusiform gyrus, LG = lingual gyrus, PHG = parahippocampal gyrus, IFG = inferior frontal gyrus, MFG = middle frontal gyrus, CG = cingulate gyrus, STG = superior temporal gyrus, MTG = middle temporal gyrus, MFG = middle frontal gyrus, PreCG = precentral gyrus, PostCG = postcentral gyrus, SG = supramarginal gyrus, TTG = transverse temporal gyrus.
3.2.3.2.2. High-Recall subgroup: Old > New
Few voxels showed a repetition-related increase (Old > New; dark and light blue voxels), and these were mainly right parietal lobe. At 3-TR, only one significant cluster with Old > New response was found, which was centered in right IPL (BA 39, 40 [+33 through +53]). At 4-TR, no Old > New clusters reached our spatial threshold (the 3 largest clusters had volumes in 384–448 mm3 range, which included the right IFG and right IPL).
3.2.3.3. Low-Recall subgroup: congruous repetition effects
3.2.3.3.1. Low-Recall subgroup: New > Old
Unlike the results for the High-Recall subgroup, the Low-Recall subgroup maps showed no significant New > Old clusters at 3-TR (i.e., no red voxels). However, a moderate response was present in the left-hemisphere at 4-TR (orange voxels): significant clusters were found in superior and middle temporal gyri (BA 21, 22 [−59 through −47]; BA 38, 21 [−39]), posterior STG (BA 41 [−51 through −31]) with lateral extent deep to IPL (BA40), cingulate gyrus (BA 31 [not shown in figure]), postcentral gyrus (BA 3 [−23]), uncus and left IFG extending to insula (BA 47, 13 [−39, −31]; BA 13 [−39, −31]). A small cluster was also found in the left anterior MTL (PHG, BA 28, 34 [−39, −31], volume = 704 mm3).
3.2.3.3.2. Low-Recall subgroup: Old > New
Clusters showing a repetition-related increase (Old > New; blue voxels in Fig. 2B) were found throughout the left-hemisphere at 3-TR (dark blue), including superior temporal gyrus (STG, BA 42 [−59], 41, and 22 [not shown]), IPL with supramarginal gyrus (BA 40 [−51, −47]) extending to insula [−31], primary sensory and motor cortex (BA 2, 6 [−59 through −47]), lingual gyrus (BA 18, 19 [not shown]), − posterior PHG (BA 36, 37, 30 [−23]), fusiform gyrus (BA 19 [−23]) and anterior cingulate (BA 24 [−3]). Some right-hemisphere clusters were also found at 3-TR, including lingual gyrus (BA 18, 19 [+21]) and cuneus (BA 30 [not shown]).
At 4-TR, the Low-Recall subgroup had 4 significant Old > New clusters (light blue), all were present in the right-hemisphere. Two large clusters were centered in right MFG (BA 8, 6, 9 [+33 through +49]). Other clusters of right frontal Old > New response were found in right inferior and middle frontal gyri (BA 46 [+41]), pre-central gyrus (BA 6 [+41]) and insula (BA 13 [+33]). Medially, an additional Old > New cluster was centered deep to cingulate (BA 24 [+21 shows lateral extent]).
In summary, both the High- and Low-Recall subgroups displayed a “HERA” pattern (New > Old activation in left-hemisphere, but Old > New in right-hemisphere) of hemispheric encoding-retrieval asymmetry (Tulving et al., 1994) but with a different time course. Unlike the results for the High-Recall subgroup, the Low-Recall subgroup maps showed no significant New > Old clusters at 3-TR. It was not until the 4-TR timepoint that the Low-Recall subgroup showed more strongly activated left-hemisphere structures in response to New trials (orange voxels in Fig. 2B), and more right-hemisphere activation (light blue voxels) to Old trials, presumably reflecting retrieval processes elicited by recalled or familiar items (Haxby et al., 1996).
3.2.3.4. Subgroup differences: congruous repetition effect
Between-group t-tests were conducted to compare the congruous repetition effects (New > Old) between the High- and Low-Recall subgroups. At the 3-TR timepoint, 9 very large clusters (total volume = 62,592 mm3) were found, particularly extensive throughout the left-hemisphere, in which the congruous repetition effect was greater in the High-Recall group. Some notably large clusters were found in anterior cingulate, left insula, left STG, left (and some right) MTL, cuneus, lingual gyrus, and posterior PHG, posterior cingulate, and middle cingulate (full list of cluster locations and sizes available from author on request). At the 4-TR timepoint, there were 11 significant clusters (total volume = 20,544 mm3) showing larger congruous repetition effects in the High-Recall subgroup. Clusters were found in: medial frontal gyrus, middle cingulate, thalamus, right IFG (BA 44 and 47), bilateral fusiform gyrus, right MTL, right IPL, left posterior middle temporal gyrus and lingual gyrus. There were no significant clusters at either timepoint in which the New > Old effect was larger in the Low-Recall group.
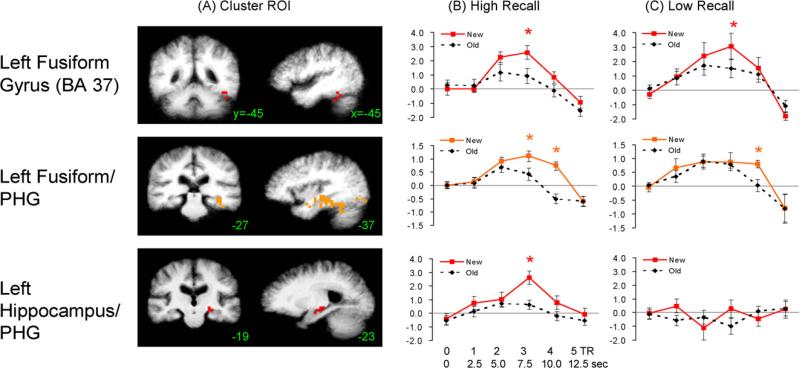
This pattern of results shows that the differential hemodynamic response for New > Old congruous trials was both earlier and more robust in the High-Recall than the Low-Recall subgroup. HRF estimates showed a qualitatively similar BOLD response in a left mid-fusiform (BA 37) cluster for the two subgroups (top of Fig. 3, ROI defined on basis of whole group map, with a volume (448 mm3) below our spatial threshold). A more anterior fusiform cluster, which extends into the perirhinal cortex and PHG, however, showed an earlier and somewhat larger New > Old response in the High Recall group (although both subgroups had significant repetition effects by 4-TR). More strikingly, the left MTL cluster showed a robust New > Old effect in the High-Recall group, but a scant event-related response in the Low-Recall group. This dysfunctional HRF was not limited to this particular cluster; an HRF estimate (not shown) extracted from the entire left MTL (hippocampus and PHG) showed a similar shape and lack of New vs. Old effect in the Low-Recall group. This dissociation between posterior fusiform and more anterior MTL responses suggests that elderly individuals with poorer recall may have intact neural priming effects in higher visual areas (BA 37) but have absent or reduced effects in regions implicated in declarative memory, i.e., within the MTL, perirhinal, and more anterior fusiform cortex.
Fig. 3.
HRF estimates for High- and Low-Recall subgroups selected clusters are shown separately. The chosen clusters showed a significant New > Old BOLD effect over the entire subject group (see Fig. 1). Cluster locations are indicated on representative coronal and sagittal slices (y- and x-coordinates given) in (A). HRF estimates are shown for (B) High-Recall (n = 8) and (C) Low-Recall (n = 8) subgroups; error bars represent standard error over subjects. *p < 0.05; ROI = region of interest; BA = Brodmann area; PHG = parahippocampal gyrus.
3.2.4. Correlations between subsequent memory and congruous repetition effects
Correlations between subsequent memory and the spatial extent of congruous repetition effects were computed for left and right MTL ROIs. Significant correlations were found between the number of voxels in left MTL with a significant New > Old BOLD response and subsequent memory (e.g., r = 0.62 with cued-recall and r = 0.60 with recognition for the congruous target words, Table 4). The number of voxels in the left MTL with significant Old > New response, however, showed negative non-significant correlations with subsequent memory (r range = −0.32 to −0.21). A ‘selectivity index’ (number of New > Old voxels minus the number of Old > New voxels), reflecting the predominant direction of differential BOLD responses to New vs. Old words, also correlated strongly with cued-recall performance (r's ≥ 0.67, p's < 0.005). The same correlations using right-MTL voxel-counts were not significant (all r's ≤ 0.23). The magnitude of left MTL activation (New–Old, BOLD response averaged across 3-TR and 4-TR timepoints) also correlated significantly with cued-recall, but not with recognition scores (Table 4). Neither cued-recall nor recognition correlated with the magnitude of right MTL activation (r's ≤ 0.34).
Table 4.
Correlations between verbal memory and BOLD response (shifts 3 + 4-TR) in the left medial temporal lobe and fusiform gyrus.
| Left MTL |
Left fusiform |
|||||||
|---|---|---|---|---|---|---|---|---|
| Spatial extent |
Magnitude | Spatial extent |
Magnitude | |||||
| New > Old (‘Red’ voxels) | Old>New (‘Blue’ voxels) | Selectivity index (‘Red–Blue’) | New–Old | New > Old (‘Red’ voxels) | Old>New (‘Blue’ voxels) | Selectivity index (‘Red–Blue’) | New–Old | |
| Cued-recall (congruous) | 0.62* | –0.30 | 0.67** | 0.60* | 0.76** | –0.15 | 0.66* | 0.74** |
| Cued-recall (total) | 0.61* | –0.32 | 0.68** | 0.60* | 0.77** | –0.18 | 0.67** | 0.77** |
| Multiple-choice (congruous) | 0.60* | –0.21 | 0.60* | 0.45 | 0.70** | –0.04 | 0.57* | 0.65* |
| Multiple-choice (total) | 0.48 | –0.27 | 0.55* | 0.35 | 0.73** | –0.19 | 0.65* | 0.69** |
p < 0.05.
p < 0.005.
Parallel analyses of the extent of significant left fusiform responses showed very similar results, and there were moderately high intercorrelations between left MTL and left fusiform BOLD responses (r range = 0.45–0.73). The extent of New > Old response correlated with all the memory measures in Table 4 (r's ≥ 0.70; p's < 0.005), and the extent of Old > New response had only negative, non-significant correlations (r range = −0.04 to −0.19). The extent of right fusiform BOLD response did not correlate significantly with subsequent recall measures (r's in 0.10–0.48 range). Unlike the left, right-hemisphere intercorrelations between extent of MTL and fusiform BOLD responses were all non-significant (|r's| < 0.30). The magnitude of the left, but not right, fusiform congruous repetition effect (New–Old) also correlated strongly with subsequent cued-recall and recognition (Table 4). We also conducted exploratory analyses for the magnitude of the repetition effects in the cingulate gyrus (anterior, mid-, posterior cingulate), primary visual (BA 17) and primary auditory (BA 41) cortex, as defined by the AFNI Talairach daemon. This revealed that the magnitude of BOLD response at 3-TR and 4-TR in the posterior cingulate (0.63 ≤ r's ≤ 0.70), and mid-cingulate (0.51 ≤ r's 0.56), but not anterior cingulate (0.25 ≤ r's ≤ 0.45), correlated significantly with cued-recall and recognition memory. There was no significant correlation between the size of the repetition effects in left and right primary visual (at 3-TR and 4-TR) or left and right auditory cortex (at 2-TR and 3-TR) and subsequent memory (all r's ≤ 0.49).
3.2.5. Correlations between subsequent memory and incongruous repetition effects
The fMRI incongruous repetition effect results are beyond the scope of this report; however, it is of interest to test if the incongruous repetition effects in the ROIs were correlated with subsequent memory. These results showed that neither the extent of left MTL nor left fusiform New > Old BOLD response had significant correlations with subsequent memory (all r's ≤ 0.41, p's ≥ 0.10 for memory measures in Table 1).
4. Discussion
Our study examined the neural basis of word repetition effects to semantically congruous words, and their variability in normal older persons. Our ERP studies have shown this paradigm to be sensitive to memory disorders and to the variability in verbal memory abilities across normal individuals. Importantly, ERP repetition effects to the congruous words were due to modulations of the P600 component (in contrast to the modulations in the N400 component characterizing the incongruous word repetition effects). Normal participants typically show robust declarative memory only for the congruous target words, as replicated in this report. In the present fMRI study, New congruous words were found to activate a widely distributed network of brain regions during this cross-modal semantic category decision task (Supplementary Fig. 2A). Repetition of congruous items resulted in lower activity relative to initial presentation (i.e., New > Old BOLD response) in many left-hemisphere regions, and relatively higher activity (i.e., Old > New BOLD response) in a few regions, mainly in the right-hemisphere (Fig. 1). This pattern of results is consistent with the Hemispheric Encoding/Retrieval Asymmetry (HERA) model (Haxby et al., 1996; Tulving et al., 1994). Congruous, but not incongruous, repetition effects in the left MTL were strongly related to subsequent memory performance.
This fMRI word repetition paradigm involves attention, perceptual, conceptual and episodic memory processes and requires a motor response. As such, it produced widespread activation of the cerebral cortex (sensory, association, paralimbic, limbic and motor cortex), which was generally more pronounced for the novel stimuli. Our BOLD response maps to congruous new words vs. baseline (Supplementary Fig. 2A) resemble magneto-encephalography (MEG) studies of visual word repetition. Like Dhond et al. (2001), we found widespread bilateral occipitotemporal activation along the ventral stream of higher visual processing. Analyses of earlier timepoints (beyond the scope of this report) showed activation in the primary auditory cortex (BA 41), with a peak BOLD response at shift 2-TR. The size of the congruous repetition effect in primary auditory or visual (BA 17) cortex, however, was not correlated with declarative memory of the stimuli. Clearly, one disadvantage of using a complex cognitive task such as category judgment, along with cross-modal stimuli, is that it is difficult to isolate the specific cognitive processes performed by a given anatomical structure. However, when New vs. Old congruous words contrasts were made at timepoints chosen to model the BOLD response to the visual target words, a well-circumscribed neural circuit of interconnected structures thought important for verbal memory emerged, as discussed in detail below.
4.1. Congruous repetition effects
As hypothesized, congruous repetition effects were found in putative generators of the P600 ERP component (Halgren et al., 1994a,b; Guillem et al., 1999). These regions included the left PHG, hippocampus, prefrontal (MFG and IFG), lateral temporal (MTG and STG), IPL, insula, bilateral fusiform (L > R), and cingulate cortex (Fig. 1). Our analysis, based on time-shifted reference functions, found earlier clusters of New > Old BOLD regions in the left hippocampus and PHG, followed by later differential responses in the fusiform, cingulate and neocortical regions listed above. Interestingly, the PHG, a ‘convergence zone’ like the hippocampus, has afferent or efferent connections to many of these same association neocortical (e.g., BAs 20, 21, 22, 46, 9) and paralimbic (e.g., BA 23) structures (Van Hoesen, 1982). Thus, it is ideally suited for memory ‘binding’ and a critical structure for successful memory encoding. Prior fMRI studies have also shown left IFG to be related to successful encoding (Wagner et al., 1998a,b) and successful verbal memory retrieval (Konishi et al., 2000). Similarly, left lateral parietal cortex activation has also been previously related to successful verbal memory retrieval (Konishi et al., 2000) and is greater in ‘remember’ vs. ‘know’ judgments (Henson et al., 1999).
The congruous repetition effects were dramatically different for High- and Low-Recall subgroups formed on the basis of subsequent cued-recall performance (Fig. 2). As hypothesized, fMRI congruous word repetition effects were greater in the High-Recall elderly subgroup (who also had superior delayed recognition abilities). The High-Recall group map showed a robust New > Old congruous repetition effect that was both early (3-TR timepoint) and sustained (4-TR timepoint) throughout much of the left-hemisphere; some early Old > New clusters were also found in right inferior parietal cortex. The right IPL activity observed during Old trials may be due to either resumption of ‘default mode’ activity (due to decreased attentional demands), or less likely to recognition processes per se. The High-Recall subgroup analysis showed both faster and more extensive differential BOLD responses to New > Old congruous items than the whole group analysis, despite a large decrement in statistical power. This highlights the importance of accounting for the heterogeneity in the BOLD response time course prominent even in ‘healthy elderly’ samples (D'Esposito et al., 1999). While reiterative bootstrapping approaches can be tried to model the HDR in each subject, the extent of deviation from young healthy subjects may itself be a useful index of brain aging. In contrast to the High-Recall subgroup, the Low-Recall subgroup had no clusters of significant New > Old responses in the left-hemisphere until a later timepoint (shift 4-TR; orange voxels in Fig. 2B). However, their group cluster map did show several left-hemisphere (mainly frontal and parietal) regions with greater Old > New responses (note opposite polarity). This may reflect elaborative semantic processing, and/or the engagement of additional attentional resources by repeated stimuli, perhaps associated with more effortful encoding. The Low-Recall subgroup, especially at the 4-TR timepoint, showed a ‘HERA’ pattern in which the left-hemisphere responded more during initial encoding, but the right-hemisphere responded greater to repeated congruous stimuli, such has been attributed to recognition processes in past studies (Haxby et al., 1996). Some investigators have shown right prefrontal activity to reflect retrieval effort more than retrieval success (Kapur et al., 1995; Konishi et al., 2000; Wagner et al., 1998b), which is consistent with our Low-Recall group showing much more extensive right frontal Old > New effects than the High-Recall subgroup, who mainly had right parietal Old > New effects.
As in our prior ERP studies, congruous repetition effects on BOLD response were strongly related to subsequent memory (both recall and recognition abilities). The spatial extent of the congruous repetition effect in left, but not right, MTL correlated with subsequent memory for stimuli. This finding is as we predicted, and consistent with several (Dickerson et al., 2004; Wagner et al., 1998a), but not all (Johnson et al., 2001) past fMRI studies, relating left MTL activation to successful verbal memory encoding. The magnitude of the left MTL repetition effect (New–Old) correlated significantly with cued-recall, but not recognition. In the fusiform gyrus, similar correlations were found for the left-hemisphere, in which larger repetition effects (magnitude or spatial extent) correlated with superior recall and recognition for the stimuli, but not for the right-hemisphere. Exploratory analysis also showed that the magnitude of New–Old response in the left posterior and mid-cingulate was highly correlated with subsequent memory.
We were not as confident that fusiform activation would correlate with memory. The fusiform gyrus has been found to generate both N400 and P600-like activity (Halgren et al., 1994a) in response to visual words. While our ERP studies have consistently found strong correlations between verbal memory and the P600 word repetition effect (elicited by congruous words), the N400 word repetition effect (elicited by semantically incongruous stimuli) has generally not correlated with subsequent memory performance (Olichney et al., 2000, 2002, 2006). Our finding that the spatial extent of left fusiform New > Old BOLD response did correlate strongly with both subsequent recall and recognition is, however, consistent with Wagner et al. (1998a) prior fMRI “Dm” study which found activation in lateral and medial fusiform regions associated with successful verbal encoding (i.e., greater BOLD responses to subsequently recalled words). While Schott et al. (2006) related HDR decreases in a left lateral fusiform cluster to “priming” and implicit memory for word stem completions, their results also showed larger responses to subsequently remembered than forgotten items (see Fig. 4, p. 798 in Schott et al., 2006) suggesting a role in explicit memory, as well. Several animal studies have implicated the anterior fusiform and perirhinal cortex in visual recognition memory (Murray and Bussey, 1999; Winters and Bussey, 2005; Winters et al., 2008). Our fine-grained analyses showed that the more posterior areas in the fusiform gyrus (e.g., BA 37, cluster shown in Fig. 3) had relatively similar HDR's in High and Low recall subgroups. However, the more anterior fusiform regions, similar to the neighboring perirhinal and parahippocampal cortex, showed smaller, later New > Old effects in the Low-Recall group.
The most impressive intergroup difference was the dissociation between the High vs. Low-Recall subgroups in a cluster of the hippocampus and PHG, driven by the differential response in the High-Recall subjects only. It will be of great interest to follow the outcome of the normal elderly participants with relatively Low-Recall to determine if this apparent hippocampal dysfunction could represent pre-clinical AD or other age-related hippocampal pathology (Braak et al., 1996). Alternatively, the robust intergroup differences observed could reflect the use of an incidental learning paradigm or differential sensitivities to the distractions inherent to the fMRI environment (Gutchess and Park, 2006), which may interact with differential sensory (i.e., auditory and visual) acuity. However, none of the participants included in these analyses reported significant difficulty hearing or seeing the stimuli. Further, both the High- and Low-Recall groups performed the category decision task near ceiling. It is therefore unlikely that a difference in sensory acuity between groups accounts for the robust differences in BOLD response. In addition, our experience with the ERP version of this paradigm leads us to believe that the magnitude of the congruous word repetition effect closely parallels learning efficiency. Normal younger persons almost always show large P600 word repetition effects, regardless of strategy or intentionality.
It also should be noted that the High- and Low-Recall subgroups’ behavioral data showed equivalent reaction time ‘priming’ (for both congruous and incongruous trials) and similar performance accuracy, but the Low-Recall group had generally slower RTs regardless of stimulus type. We take this as additional evidence that age-related decrements in processing speed are commonly associated with a decline in learning efficiency. However, it should also be noted that the amount of mean RT slowing (~300 ms) is quite discordant with the delayed “neural priming” as measured by fMRI congruous word repetition effects. New > Old effects were less widespread in the Low-Recall subgroup, even shifting the reference function an additional 2.5 s (1-TR) later. This could be due to neurovascular “uncoupling,” which has been observed in normal elderly performing simple RT tasks (D'Esposito et al., 1999). However, simultaneous ERP recordings would be required to prove this and alternative explanations could include an absence of earlier neural processes mediating the repetition effects of the High-Recall group together with replacement by later processes (e.g., attentional, monitoring, or memory related) in the Low-Recall group. However, examination of the New-baseline and Old-baseline maps in each of these subgroups (data not shown) does not support this hypothesis. Instead, relatively similar maps are produced by new words in both subgroups, but only the High-Recall group showed widespread left-hemisphere (especially in higher visual and lateral temporal regions) “overshoot” at 4-TR to Old congruous words. We interpret this as indicating that the High-Recall group benefits more from the repetition of congruous items, having very rapid memory updating processes which greatly lessen the requisite amount of memory updating (i.e., in working memory) and semantic processing after congruent word presentation. We have previously interpreted the P600 elicited in this paradigm as an index of the updating of working memory with long-term (semantic memory) stores (Olichney et al., 2000; Van Petten et al., 1991). Neuroanatomically, this updating could be achieved by communication between the MTL, paralimbic, and association cortex (i.e., a circuit of distributed P600 generators). This model also accords well with the proposal that working memory maintenance is accomplished by activation of long-term memory representations (Fuster, 1995; Kimberg and Farah, 1993).
4.2. Summary/conclusions
In summary, we have found striking dissociations between fMRI congruous word repetition effects in older persons depending on memory ability. Repeating semantically congruous verbal material produced an fMRI word repetition effect closely related to subsequent memory or “successful encoding”. The repetition effects produced by repeating semantically incongruous material (full results beyond the scope of this report) generally did not relate to memory performance, even when measured within the left MTL or fusiform. These semantically incongruous stimuli, known to produce robust N400 potentials, are extremely hard to learn presumably because of a lack of pre-existing semantic associations between the auditory ‘prime’ and subsequent target word. Therefore, the incongruous repetition effects apparent on fMRI likely represent implicit memory processes such as semantic priming (Olichney et al., 2000; Van Petten and Luka, 2006). The congruous word repetition effects, which involve many putative P600 generators, were much more robust in elderly participants with superior memory abilities. The spatial extent and magnitude of this effect in the left MTL and fusiform were both correlated with subsequent declarative memory performance.
We are not aware of any other prior fMRI studies in the elderly that have examined similar category decision repetition effects, using cross-modal stimuli. We believe this paradigm could be particularly useful for detecting pathological aging, because the earliest stages of ‘pre-clinical’ AD generally start in the anterior PHG (i.e., entorhinal cortex) and surrounding structures, which (1) show activation in this paradigm and (2) are connected to distant paralimbic and associative neocortical areas also activated by this paradigm. One limitation of the present study is the absence of a parallel young control group. Our laboratory's primary interest is in the development of paradigms sensitive to age-related memory disorders such as AD or amnestic MCI. Accordingly, this report focuses on normal healthy older persons typical of those used to control for aging in clinical AD research. Another possible study limitation is that, due to our modeling the brain's BOLD response at multiple timepoints for a given stimulus type or contrast, there is an associated risk of Type I error. On the other hand, the advantages of not applying overly rigorous statistical thresholding have been recognized in the brain mapping literature (Jernigan et al., 2003) and can be useful to illuminate the extent of a neural circuit or the extent of brain regions involved in a given task. In this regard, our event-related fMRI analysis resemble the word repetition effects found by methods with superior temporal resolution such as MEG. Dhond et al. (2001) found New–Old differences to be strongly left-lateralized and to include inferior ventral temporal regions, inferior prefrontal cortex and the superior temporal sulcus, resembling the word repetition effects to congruous stimuli in the present report. We have provided evidence above (correlational and subgroup analyses) that links these MTL and fusiform word repetition effects to declarative memory processes, perhaps to effective memory “binding”, i.e., encoding the various features (e.g., semantic, orthographic, phonemic) into a unified memory trace, thereby greatly facilitating retrieval upon stimulus repetition.
It is of great importance to determine the clinical significance of slower and smaller BOLD responses in apparent healthy elderly persons. We have demonstrated the power of event-related fMRI to detect minor variability in learning efficiency among normal elderly, but the clinical relevance and prognosis associated with these changes remain undetermined.
Supplementary Material
Acknowledgements
Supported by NIH grants #R01-AG18442, R01-AG08313 & P50-AG05131. Special thanks to the Center for Mind and Brain, California Department of Public Health Laboratory of Cognitive Imaging (LOCI), VA San Diego Healthcare System, Gregory Brown, Terry Jernigan, Eric Wong, Ron Mangun, Jeremy Smith, Lauren Keats, Alexander Bressler, Shaunna Morris, Leon Thal, and the Shiley-Marcos Alzheimer's Disease Research Center (ADRC).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.neurobiolaging.2008.10.010.
Footnotes
Disclosure statement: The authors have no conflicts of interest to disclose. All subjects gave informed consent to participate in the MRI and other experimental procedures as approved by the UCSD Human Research Protection Program.
References
- Aguirre GK, D'Esposito M. Experimental design for brain fMRI. Functional MRI. 1999:369–380. [Google Scholar]
- Battig WF, Montague WE. Category norms for verbal items in 56 categories: a replication and extension of the Connecticut category norms. J. Exp. Psychol. Monogr. 1969;80(3):1–44. [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Detection versus estimation in event-related fMRI: choosing the optimal stimulus timing. Neuroimage. 2002;15(1):252–264. doi: 10.1006/nimg.2001.0964. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J. Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J, Reintjes R. Age, neurofibrillary changes, Aβ-amyloid and the onset of Alzheimer's disease. Neurosci. Lett. 1996;210:87–90. doi: 10.1016/0304-3940(96)12668-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol. Aging. 1997;18(4):351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol. Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM. Neural correlates of relational memory: successful encoding and retrieval of semantic and perceptual associations. J Neurosci. 2005;25(5):1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J. Cereb. Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craik FIM. Memory changes in normal aging. Curr. Dir. Psychol. Sci. 1994;3:155–158. [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126(Pt 1):43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb. Cortex. 2006;16(12):1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat. Rev. Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage. 1999;10(1):6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- Dhond R, Buckner R, Dale AM, Marinkovic K, Halgren E. Spatiotemporal maps of brain activity underlying word generation and their modification during repetition priming. J. Neurosci. 2001;21(10):3564–3571. doi: 10.1523/JNEUROSCI.21-10-03564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Ann. Neurol. 2004;56(1):27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behav. Brain Sci. 1988;11:355–372. [Google Scholar]
- Duzel E, Yonelinas AP, Mangun GR, Heinze HJ, Tulving E. Event-related brain potential correlates of two states of conscious awareness in memory. Proc. Natl. Acad. Sci. U.S.A. 1997;94(11):5973–5978. doi: 10.1073/pnas.94.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández G, Weyerts H, Schrader-Bölsche M, Tendolkar I, Smid HG, Tempelmann C, Hinrichs H, Scheich H, Elger CE, Mangun GR, Heinze HJ. Successful verbal encoding into episodic memory engages the posterior hippocampus: a parametrically analyzed functional magnetic resonance imaging study. J. Neurosci. 1998;18(5):1841–1847. doi: 10.1523/JNEUROSCI.18-05-01841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández G, Effern A, Grunwald T, Pezer N, Lehnertz K, Dümpelmann M, Van Roost D, Elger CE. Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science. 1999;285(5433):1582–1585. doi: 10.1126/science.285.5433.1582. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Heitkemper P, Grunwald T, Van Roost D, Urbach H, Pezer N. Inferior temporal stream for word processing with integrated mnemonic function. Hum. Brain Mapp. 2001;14:251–260. doi: 10.1002/hbm.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in Functional Magnetic Resonance Imaging (FMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency Analysis of English Usage: Lexicon and Grammar. Houghton Mifflin; Boston: 1982. [Google Scholar]
- Friedman D, Johnson R., Jr. Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microsc. Res. Tech. 2000;51(1):6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Memory in the Cerebral Cortex. The MIT Press; Cambridge: 1995. [Google Scholar]
- Grady CL, Craik FI. Changes in memory processing with age. Curr. Opin. Neurobiol. 2000;10(2):224–231. doi: 10.1016/s0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13(5):572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Guillem F, Rougier A, Claverie B. Short- and long-delay intracranial ERP repetition effects dissociate memory systems in the human brain. J. Cogn. Neurosci. 1999;11:437–458. doi: 10.1162/089892999563526. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Park DC. fMRI environment can impair memory performance in young and elderly adults. Brain Res. 2006;1099(1):133–140. doi: 10.1016/j.brainres.2006.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Clarke M. Spatio-temporal stages in face and word processing. 1. Depth recorded potentials in the human occipital, temporal and parietal lobes. J. Physiol. 1994a;88(1):1–50. doi: 10.1016/0928-4257(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Chauvel P, Clarke M. Spatio-temporal stages in face and word processing. 2. Depth-recorded potentials in the human frontal and Rolandic cortices. J. Physiol. 1994b;88:51–80. doi: 10.1016/0928-4257(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Maisog JM, Rapoport SI, Grady CL. Face encoding and recognition in the human brain. Proc. Natl. Acad. Sci. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat. Rev. Neurosci. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recognition and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J. Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Fennema-Notestine C, Ostergaard AL. More “mapping” in brain mapping: statistical comparison of effects. Hum. Brain Mapp. 2003;19(2):90–95. doi: 10.1002/hbm.10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Saykin A, Flashman LA, McAllister TW, Sparling MB. Brain activation of fMRI and verbal memory ability: functional neuroanatomic correlates of CVLT performance. J. Int. Neuropsychol. Soc. 2001;7(1):55–62. doi: 10.1017/s135561770171106x. [DOI] [PubMed] [Google Scholar]
- Kail R, Salthouse TA. Processing speed as a mental capacity. Acta Psychol. 1994;86(2–3):199–225. doi: 10.1016/0001-6918(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Kapur S, Craik FIM, Jones C, Brown GM, Houle S, Tulving E. Functional role of the prefrontal cortex in retrieval of memories: a PET study. Neuroreport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Farah MJ. A unified account of cognitive impairments following frontal lobe damage: the role of working memory in complex, organized behavior. J. Exp. Psychol. Gen. 1993;122(4):411–428. doi: 10.1037//0096-3445.122.4.411. [DOI] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. NeuroImage. 2000;12(3):276–286. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- Kral VA. Senescent forgetfulness: benign and malignant. Can. Med. Assoc. J. 1962;86:257–260. [PMC free article] [PubMed] [Google Scholar]
- Li S-C, Baltes PB. Cognitive developmental research from lifespan perspectives: the challenge of integration. In: Bialystok E, Craik FIM, editors. Lifespan Cognition: Mechanisms of Change. Oxford University Press; New York, NY: 2006. pp. 344–363. [Google Scholar]
- Liu TT, Frank LR, Wong EC, Buxton RB. Detection power, estimation efficiency, and predictability in event-related fMRI. Neuroimage. 2001;13(4):759–773. doi: 10.1006/nimg.2000.0728. [DOI] [PubMed] [Google Scholar]
- Mangels JA, Picton TW, Craik FIM. Neurophysiological (ERP) correlates of encoding and retrieval from verbal episodic memory. Soc. Neurosci. Abs. 1999:1450. (abstract) [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J. Neurol. Neurosurg. Psychiatry. 2008;79(6):630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ. Perceptual–mnemonic functions of the perirhinal cortex. Trends Cogn. Sci. 1999;3:142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Nessler D, Johnson R, Jr., Bersick M, Friedman D. On why the elderly have normal semantic retrieval but deficient episodic encoding: a study of left inferior frontal ERP activity. Neuroimage. 2006;30(1):299–312. doi: 10.1016/j.neuroimage.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akubudak E, Synder A, McKinstry R, Raichle M, Conturo T. Anatomic localization and quantitative analysis of gradient focused echo-planar fMRI susceptibility artifices. Neuroimage. 1997;6:203–215. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Van Petten C, Paller K, Salmon DP, Iragui VJ, Kutas M. Word repetition in amnesia: electrophysiological measures of impaired and spared memory. Brain. 2000;123:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Morris SK, Ochoa C, Salmon DP, Thal LJ, Kutas M, Iragui VJ. Abnormal verbal event-related potentials in mild cognitive impairment and incipient AD. J. Neurol. Neurosurg. Psychiatry. 2002;73:377–384.45. doi: 10.1136/jnnp.73.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Iragui VJ, Salmon DP, Riggins BR, Morris SK, Kutas M. Absent event-related potential (ERP) word repetition effects in mild Alzheimer's disease. Clin. Neurophysiol. 2006;117:1319–1330. doi: 10.1016/j.clinph.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Gatherwright J, Taylor JR, Salmon DP, Kutas M, Iragui VJ. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology. 2008;70:1763–1770. doi: 10.1212/01.wnl.0000281689.28759.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001;124(Pt 2):399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. Task-dependency of the neural correlates of episodic encoding as measured by fMRI. Cereb. Cortex. 2001;11(12):1150–1160. doi: 10.1093/cercor/11.12.1150. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M, Mayes AR. Neural correlates of encoding in an incidental learning paradigm. Electroencephalogr. Clin. Neurophysiol. 1987;67:360–371. doi: 10.1016/0013-4694(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Paller KA. Recall and stem-completion priming have different electrophysiological correlates and are modified differentially by directed forgetting. J. Exp. Psychol. Learn. Mem. Cogn. 1990;16(6):1021–1032. doi: 10.1037//0278-7393.16.6.1021. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M. Brain potentials during memory retrieval provide neurophysiological support for the distinction between conscious recollection and priming. J. Cog. Neurosci. 1992;4:375–391. doi: 10.1162/jocn.1992.4.4.375. [DOI] [PubMed] [Google Scholar]
- Rajapakse JC, Kruggel F, Maisong JM, Cramon DY. Modeling hemodynamic response for analysis of functional MRI time-series. Hum. Brain Mapp. 1998;6:19–28. doi: 10.1002/(SICI)1097-0193(1998)6:4<283::AID-HBM7>3.0.CO;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restom K, Bangen KJ, Bondi MW, Perthen JE, Liu TT. Cerebral blood flow and BOLD responses to a memory encoding task: a comparison between healthy young and elderly adults. Neuroimage. 2007;37(2):430–439. doi: 10.1016/j.neuroimage.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Influence of processing speed on adult age differences in working memory. Acta Psychol. (Amst.) 1992;79(2):155–170. doi: 10.1016/0001-6918(92)90030-h. [DOI] [PubMed] [Google Scholar]
- Schott BH, Richardson-Klavehn A, Henson RN, Becker C, Heinze HJ, Duzel E. Neuroanatomical dissociation of encoding processes related to priming and explicit memory. J. Neurosci. 2006;26(3):792–800. doi: 10.1523/JNEUROSCI.2402-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SI, Palermo DS. Conceptual organization and class membership: normative data for representatives of 100 categories. Psychon. Monogr. Suppl. 1970;3(11):107–127. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. Review. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Steretotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Tulving E. Elements of Episodic Memory. Oxford UP; London: 1983. [Google Scholar]
- Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc. Natl. Acad. Sci. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoesen GW. The parahippocampal gyrus. Trends Neurosci. 1982;5:345–350. [Google Scholar]
- Van Petten C, Kutas M, Kluender R, Mitchiner M, McIsaac H. Fractionating the word repetition effect with event-related potentials. J. Cog. Neurosci. 1991;3:131–150. doi: 10.1162/jocn.1991.3.2.131. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Luka BJ. Neural localization of semantic context effects in electromagnetic and hemodynamic studies. Brain Lang. 2006;97(3):279–293. doi: 10.1016/j.bandl.2005.11.003. (Epub 2005 Dec 15) [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998a;281(5380):1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Desmond JE, Glover GH, Gabrieli JD. Prefrontal cortex and recognition memory. Functional-MRI evidence for context-dependent retrieval processes. Brain. 1998b;121(Pt 10):1985–2002. doi: 10.1093/brain/121.10.1985. [DOI] [PubMed] [Google Scholar]
- Wagner AD. Working memory contributions to human learning and remembering. Neuron. 1999;22(1):19–22. doi: 10.1016/s0896-6273(00)80674-1. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol. Aging. 2002;17(2):179–193. [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Glutamate receptors in perirhinal cortex mediate encoding, retrieval, and consolidation of object recognition memory. J. Neurosci. 2005;25(17):4243–4251. doi: 10.1523/JNEUROSCI.0480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci. Biobehav. Rev. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans. Med. Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.