Abstract
The luteinizing hormone receptor (LHR), one of the three glycoprotein hormone receptors, is necessary for critical reproductive processes, including gonadal steroidogenesis, oocyte maturation and ovulation, and male sex differentiation. Moreover, it has been postulated to contribute to certain neoplasms, particularly ovarian cancer. A member of the G protein-coupled receptor family, LHR contains a relatively large extracellular domain responsible for high affinity hormone binding; transmembrane activation then leads to G protein coupling and subsequent second messenger production. This review deals with recent advances in our understanding of LHR structure and structure-function relationships, as well as hormone-mediated changes in gene expression in ovarian cancer cells expressing LHR. Suggestions are also made for critical gaps that need to be filled as the field advances, including determination of the three-dimensional structure of inactive and active receptor, elucidation of the mechanism by which hormone binding to the extracellular domain triggers the activation of Gs, clarification of the putative roles of LHR in non-gonadal tissues, and the role, if any, of activated receptor in the development or progression of ovarian cancer.
Keywords: LH receptor, Gonadotropin, Human chorionic gonadotropin, Luteinizing hormone, Ovarian cancer, SKOV3 cells
1. Introduction
The glycoprotein hormone receptors (GpHRs) constitute a sub-family of the large G protein-coupled receptor (GPCR) family. Three receptors form the GpHRs, the luteinizing hormone (LH) receptor (LHR), the follicle-stimulating hormone (FSH) receptor (FSHR), and the thyroid-stimulating hormone (TSH) receptor (TSHR) (Ascoli et al., 2002; Caltabiano et al., 2008; Ascoli and Puett, 2009; Kleinau and Krause, 2009). FSHR and TSHR bind single glycoprotein hormones, FSH and TSH, respectively; LHR, on the other hand, binds two glycoprotein hormones, LH and human chorionic gonadotropin (hCG). Each of the four heterodimeric glycoprotein hormones contains a common α subunit and a hormone-specific β subunit. While there are single genes encoding LHβ, FSHβ, and TSHβ, there are six hcgβ genes, these being clustered with lhβ on chromosome 19 in the human genome (Ascoli and Puett, 2009).
The glycoprotein hormones and their cognate receptors regulate reproductive and metabolic processes; moreover, LHR activation and subsequent androgen production is required for male sex differentiation. The gonadotropins and their receptors are responsible for gonadal steroidogenesis and ovulation, while TSH and TSHR regulate thyroid hormone production. In addition to their established roles in the regulation of normal reproduction, development, and metabolism, the GpHRs are also implicated in various pathophysiological conditions. For example, mutations in lhr are known to be responsible for certain reproductive disorders (Themmen, 2005, Segaloff, 2009), and LH-mediated activation of LHR has been suggested to contribute to the etiology and/or progression of ovarian cancer (Leung and Choi, 2007; Choi et al., 2007; Mandai et al., 2007).
The GpHRs contain two major domains approximately the same size: (a) a relatively large glycosylated N-terminal ectodomain (ECD) containing leucine-rich repeats (LRRs) capped by Cys-rich regions, the latter forming a portion of a hinge region, and (b) a transmembrane domain (TM) with seven membrane spanning helices, three extracellular loops (ecls), three intracellular loops (icls) and a short icl 4, an eighth cytoplasmic helix parallel to the plasma membrane, and a cytoplasmic tail (Ascoli and Puett, 2009). The ECD and TM domains have important and distinct functional roles, namely hormone binding and signal transduction, respectively. Most of the sequential steps involved after hormone binding to the ECD until G protein activation on the inner face of the plasma membrane remain poorly understood. In many experimental systems, LH or hCG binding to LHR results in activation of both protein kinase A and protein kinase C. At relatively low concentrations of LH and hCG, Gsα appears to be the preferred signaling pathway, resulting in a rapid increase in the intracellular concentration of cAMP.
Following the earlier purification and characterization studies of the hormones and receptors at the protein and gene levels, advances in structural biology of these complex glycoprotein hormones and the ECDs of two of the three GpHRs have added a critical new dimension to our understanding of hormone and receptor structure-function relationships. Crystal structures are now available for deglycosylated hCG (Lapthorn et al., 1994; Wu et al., 1994), an antibody-bound glycosylated hCG (Tegoni et al., 1999), and a partially deglycosylated human FSH, both free (Fox et al., 2001) and bound to a large N-terminal fragment of the FSHR ECD (Fan and Hendrickson, 2005, 2007). Moreover, the NMR solution structure of the deglycosylated human α subunit has been determined (De Beer et al., 1996; Erbel et al., 1999). The heterodimeric hormones, members of the cystine-knot growth factor protein family, were found to be highly asymmetric with intertwined subunits forming a large surface area of subunit-subunit contact. A most unusual feature was the presence of a “seatbelt” in hCG and FSH in which an intramolecular disulfide loop in the β subunit (Cys-90-Cys-110 in hCGβ and Cys-84-Cys-104 in FSHβ) is wrapped around a region of the α subunit; contained within this seatbelt is a determinant loop (Cys-93-Cys-100 in hCGβ and Cys-87-Cys-94 in FSHβ) that appears to confer hormone specificity.
Crystal structures are now available for several natural and engineered recombinant GPCRs (see reviews by Mustafi and Palczewski, 2009; Rosenbaum et al., 2009 with references to the original reports). For example, there are several structures of bovine and squid rhodopsin (inactive) and of opsin (an active form of rhodopsin). Structures have also been published for the turkey β1-adrenergic receptor, the human β2-adrenergic receptor, and the human A2A adenosine receptor, all with bound antagonist or inverse agonist. As shown in Fig. 1, the four GPCRs have similar overall structures; the root mean square deviation of the TM regions is < 3 A for these receptors in the inactive state. The similarity in the relative orientations of the TM helices in the GPCRs of known crystallographic structure engenders confidence that these can be used for comparative modeling of the GpHRs. Although not presented in Fig. 1, a comparison of the rhodopsin and opsin structures shows that subtle changes occur in the TM regions, but the most significant changes are at or near the cytoplasmic surface. Here, the cytosolic region of TM6 shifts more than 6 A from the center of the bundle in the inactive state toward TM5. This reorientation involves a break of an ionic “lock” in which the Arg of the (Asp/Glu)Arg(Tyr/Trp) (DRY) motif at the cytoplasmic end of TM3, bound to a Glu in the cytosolic extension of TM6 in rhodopsin, binds to a Tyr on TM5 in opsin. Other alterations at or near the cytoplasmic surface accompany the inactive → active state conformation.
Fig. 1.
Crystal structures of four GPCRs in the inactive state. (A) Structures are shown for rhodopsin (purple), the β1-adrenergic receptor (1AR, orange), the β2-adrenergic receptor (2AR, blue), and the A2A adenosine receptor (green) in two orientations. In the upper and lower rows, the structures of rhodopsin, the β1-adrenergic receptor, and the A2A adenosine receptor are each compared to that of the β2-adrenergic receptor. The lower row shows the same structures depicted in the upper row after a rotation by 90° about the vertical axis. (B) Views of the same structures as in panel A following a 90° rotation about the horizontal axis (same coloring scheme as in A). The root mean square deviation of the TM regions is < 3A in these receptors. The structures emphasize both the highly conserved nature of major portions of these four GPCRs and the subtle differences that also exist. The figure was kindly provided by Dr. B.K. Kobilka and is reprinted with permission from Macmillan Publishers Ltd.: Nature (Rosenbaum et al., 2009).
While no structures are available of the intact GpHRs, Fig. 2 shows the crystal structure of a complex of a partially deglycosylated FSH bound to a partially degylcosylated FSHR ECD (amino acid residues 1–268) (Fan and Hendrickson, 2005, 2007). Also, a structure is available of a deglycosylated complex of a monoclonal antibody bound to a large N-terminal fragment of the human TSHR ECD (Sanders et al., 2007). These seminal studies have delineated important structural features of the hormones, receptor ECDs, and hormone-receptor complexes.
Fig. 2.
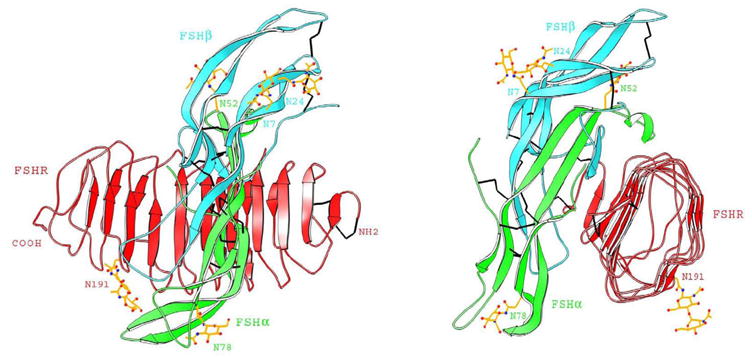
Crystal structure of the FSH-FSHR ECD complex. Two views of the complex, rotated by 90° about the vertical axis, are shown. The ECD is presented as red, and the FSH α and β chains are in green and cyan, respectively. The structure of the complex depicts the large contact surface area accompanying hormone-receptor binding and shows that both subunits contribute to receptor interaction. The figure was kindly provided by Dr. W.A. Hendrickson and is reproduced with permission from Macmillan Publishers Ltd.: Nature (Fan and Hendrickson, 1995).
Of the various gynecological malignancies, ovarian cancer is the most lethal, in part because it is usually diagnosed at a late stage (Choi et al., 2007). Epidemiological studies have shown that infertility and nulliparity are risk factors for ovarian cancer while oral contraceptive use and pregnancy correlate with a lower incidence of ovarian cancer. These and other studies have led to the suggestion that gonadotropins and their receptors may function in some manner to contribute to the etiology of ovarian cancer or to the progression of the disease (Leung and Choi, 2007; Choi et al., 2007; Mandai et al. 2007). Such an effect would be particularly pronounced in postmenopausal women where gonadotropin levels are high. The majority of ovarian cancers are epithelial in nature, arising from the ovarian surface epithelium which has been shown to express LHR and FSHR and to respond to gonadotropins (Choi et al., 2007).
This review focuses on LHR from the perspective of structure-function relationships and its putative role in ovarian cancer. Not intended to be comprehensive, only selected salient findings of the past several years are discussed.
2. Structure and structure-function relationships of LHR
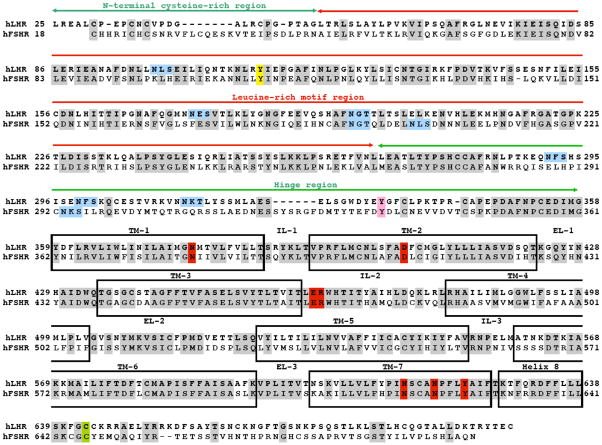
The amino acid sequences of the two human gonadotropin receptors, LHR and FSHR, are shown in Fig. 3, with several important regions denoted. The ECD, encompassing over 330 amino acid residues, contains the N-terminal Cys-rich region, the LRR region, and a hinge region terminating with a Cys-rich sequence. The seven TM helices are depicted, along with the extracellular and intracellular loops, and the eighth (non-TM) helix, the structure being terminated by the C-terminal tail.
Fig. 3.
Amino acid sequences of human LHR and FSHR. Different portions of the receptor are indicated, including the N-terminal Cys-rich region, the LRR region, the hinge region with a Cys-rich component before TM1, the seven TM helices, the three ecls, the three icls, and helix 8 followed by the C-terminal tail. Gray shading: identical residues; blue boxes: consensus sequences for N-linked glycosylation; yellow: the Tyr that participates in dimerization for FSHR and is conserved in LHR; pink: potential site of sulfation; green: potential site of palmitoylation; and red: highly conserved residues of the rhodopsin/β2-adrenergic family of GPCRs. The sequence was obtained from the web site, http://www.ensembl.org/index.html, and reproduced with permission from Elsevier (Ascoli and Puett, 2009).
Since no structural data are available for LHR, comparative modeling with other LRR proteins has been used to generate working models of the ECD (Bhowmick et al., 1996; Vassart et al., 2004; Bogerd, 2007; Puett et al., 2005, 2007; Caltabiano et al., 2008 and references therein for earlier models). The more recent models are similar to each other, not surprising since all were derived from a small subset of LRR proteins. Two very similar models of the LHR ECD were found (Puett et al., 2007) based on comparative modeling using the Nogo receptor (He et al., 2003) and glycoprotein Ibα (Huizinga et al., 2002) as templates. These LHR ECD models were very similar to the model obtained when the crystal structure of the truncated FSHR ECD with bound FSH (Fan and Hendrickson, 2005) served as the template; the major differences being in LRRs 5 and 9 (Puett et al., 2007). A working model of hCG bound to the LHR ECD is shown in Fig. 4 and was obtained by rigid body docking using the crystal structureof hCG and the predicted structure of the LHR ECD (Puett et al., 2007).
Fig. 4.
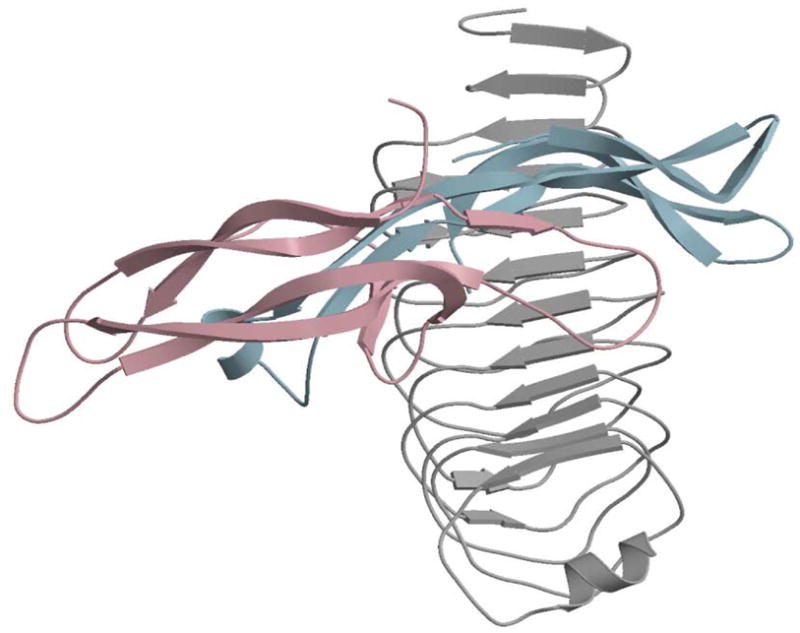
Model of hCG bound to the LHR ECD obtained by rigid body docking with the 3D DOCK program (Gabb et al., 1997). The model of the LHR ECD was based on comparative modeling using the MODELLER program (Sali and Blundell, 2003; Fiser and Sali, 2003) and the structures of the Nogo receptor (He et al. 2003), glycoprotein Ibα (Huizinga et al., 2002), and the FSHR ECD in complex with FSH (Fan and Hendrickson, 1995) and is thus devoid of the hinge region. The α and β subunits are shown in light blue and light pink, respectively, and it can be seen that both subunits, in particular loops 2 and 3 of α, loop 2 of β, and the β seatbelt loop, contact several LRRs of the ECD. The figure is reproduced with permission from Elsevier (Puett et al., 2007).
Also using comparative modeling, proposed structures are available for the TM domain of LHR. Fig. 5 depicts models for LHR in the basal state and when activated by a gain-of-function mutation (Angelova et al., 2002). This model identified a number of intrahelical charge-reinforced H-bonding interactions that maintain the receptor in the inactive state, including the Arg in the (Asp/Glu)Arg(Tyr/Trp) or DRY motif, Arg-464(3.50 in the Ballesteros and Weinstein (1995) numbering scheme) and Asp-564(6.30), located in the cytosolic extensions of TM helices 3 and 6, respectively; Asp-578(6.44) and Asn-615(7.45)/Asn-619(7.49), and others. The solvent accessible surface area (SAS), measured over cytosolic amino acid residues, has proven to be a good indicator of constitutive receptor activation with mutants having SAS values above a threshold exhibiting constitutive activation and those with SAS values below a threshold characterized with basal-type signaling (Zhang et al., 2007; Angelova et al., 2008; Feng et al., 2008; Fanelli et al. 2009).
Fig. 5.
Cytosolic ends of TM3 and TM6 in wild type (left) LHR and the constitutively active LHR mutant, Asp-564-Gly (right). The interaction pattern of Arg-464 (3.50) is shown, and dots indicate that the SAS is 28 A2 for wild type LHR and 120 A2 for the Arg-464-Gly mutant.
Numerous naturally occurring gain-of-function and loss-of-functions mutations have been described (Segaloff, 2009); also, single, double, and triple mutants throughout LHR have been made and characterized, as have various chimeric receptors. Two websites on the GpHRs, http://gris.ulb.ac.be/ (Van Durme et al., 2006) and http://www.ssfa-gphr.de/main/ssfa.php (Kleinau et al., 2007), are excellent sources for this vast literature. These studies have provided important information on the nature of the LHR amino acid residues that participate in hormone binding to the ECD, those responsible for maintaining the receptor in an inactive state in the absence of hormone, and those involved in G protein binding and activation. Naturally occurring activating mutations have been described in transmembrane helices 1, 2, 3, 5, and 6, and naturally occurring inactivating mutations have been reported in exons 1, 4–8, 10, and 11 of the ECD, in icls 1 and 3, and in transmembrane helices 1 and 4–7. These and the many hundreds of engineered mutations have provided considerable insight into the hormone-receptor contact sites and residues involved in signal transduction.
A number of critical amino acid residues, many serving as both positive and negative hormone specificity determinants (Moyle et al., 1994), have been identified in the gonadotropin receptor ECDs (Bhowmick et al. 1996, 1999; Song et al., 2001a, b; Smits et al., 2003; Vischer et al., 2003a, b, 2006; Vassart et al., 2004; Zhang et al., 2007; Caltabiano et al., 2008; Feng et al., 2008). It was recently suggested by Caltabiano et al. (2008) that the determinant loop of hCGβ, which contains two positively charged residues, Arg-94 and Arg-95, and one conserved negatively charged residue, Asp-99, interact with LHR as follows: Arg-94 with Glu-206, Arg-95 with Glu-154, and Asp-99 with Asn-107. (The identical residues are also present in LHβ.) The determinant loop in FSHβ, in contrast, contains three negatively charged residues, Asp-88, Asp-90, and Asp-93. Other hCG-LHR interactions proposed in this model are hCGβ Asp-105 with Tyr-58 and hCGβ Lys-104 with Glu-79. Lastly, the hinge region of LHR has been demonstrated to be important in hormone binding and hormone-mediated signaling (Zeng et al., 2001; Bruysters et al., 2008), but its exact function remains elusive.
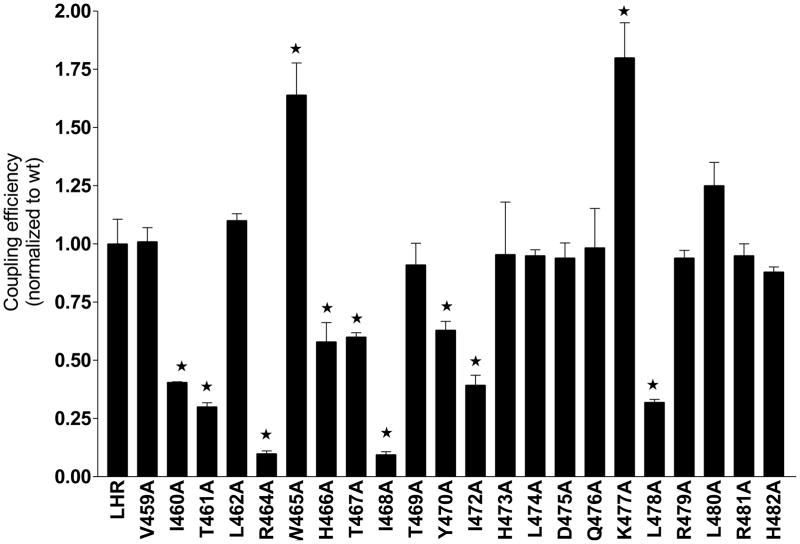
In a recent study the contributions of individual amino acid residues in the cytosolic extensions of TM helices 3, 5, and 6 and of icls 2 and 3 of the LHR were mapped for functional significance using Ala-scanning mutagenesis and computational modeling (Angelova et al., 2008). It was found that residues comprising the cytosolic extension of TM3 and the N-terminal portion of icl2 contributed significantly to G protein coupling and activation; in contrast, icl3 contributed very little. Compared to that of wild type LHR, the coupling efficiencies of the Ala mutants in the cytosolic extension of TM3 and icl2 are shown in Fig. 6 (Glu-463-Ala failed to express and residue 471 is a naturally occurring Ala). The coupling efficiency is a parameter that accounts for the level of receptor expression, hormone-receptor binding affinity, the ED50 for cAMP production and the maximal cAMP produced at a saturating concentration of hCG (Ballesteros and Weinstein, 1995) as defined in the legend to Fig. 6. A perusal of the data shows that a number of amino acid residues are involved, either directly or indirectly, in G protein coupling and activation. Of the 10 residues in the cytosolic extension of TM3, six appear to be important in productive coupling; not surprisingly, Arg-464 of the DRY motif is one of them. In this study, the Ala replacements had no appreciable effect on the hormone binding affinity, which is not surprising since these are located on an icl. The expression levels did vary somewhat, but the major impact on the coupling efficiencies arose from a significant increase in the ED50 and a significant decrease in Rmax, as found particularly with R464A and I468A. In contrast to the findings documented in Fig. 6, Ala-scanning mutagenesis of the cytosolic extensions of TM5/6 and icl 3 revealed only a few residues that appear to be important in effective LHR-G-protein coupling. Of the many residues evaluated in the cytosolic extensions of TM helices 3, 4, 5, and 6, and of icls 2 and 3, only D564 in the cytosolic extension of TM6 seems to maintain LHR in an inactive state.
Fig. 6.
Coupling efficiencies of icl2 LHR mutants following Ala scanning mutagenesis. The coupling efficiency was determined from measurements of expression, binding, and signaling (cAMP production) in human embryonic kidney 293 cells. The coupling efficiency is defined as follows, 0.5[1 + (Kd/ED50)][Rmax/Bmax], where Kd is the hormone-receptor dissociation constant, ED50 represents the concentration of hCG required to achieve 50% maximal production of cAMP, Rmax is the maximum amount of cAMP produced at a saturating concentration of hCG, and Bmax is the receptor expression level; all values are normalized to that of wild type LHR. *, significantly different from wild type LHR (P ≤ 0.05). Reproduced from Angelova et al. (2008) with the permission of The Endocrine Society, Copyright 2008.
Yoked (tethered) forms of hormone and receptor have been prepared in which a single chain hCG, either in the N-α-β-C or N-β-α-C orientation, was fused with the N-terminus of the full-length LHR and expressed and characterized in cultured cells (Wu et al., 1996; Puett et al., 1998; Narayan et al., 2000) and in transgenic mice (Meehan et al., 2005; Meehan and Narayan, 2007; Coonce et al., 2009). The receptor was found to be constitutively active due to the covalently attached hormone, although it is unknown if the hormone binds to and activates the receptor to which it is attached or to a neighboring receptor, i.e. cis or trans activation (Ji et al., 2002). This engineered version of a constitutively activated LHR complements the studies being done with constitutively active mutants of LHR arising from single base changes in lhr (Themmen, 2005; Segaloff, 2009; Ascoli and Puett, 2009). Parallel studies using yoked hCG-FSHR and hCG-TSHR showed that each of the receptors could be activated by hCG, albeit to a small extent with FSHR (Schubert et al., 2003). This model system was also used with yoked α-LHR and yoked hCGβ-LHR to show that the individual subunits are incapable of activating LHR under conditions mimicking high concentrations (Narayan et al., 2002); likewise, yoked versions of minimized forms of hCGβ fused to α-LHR identified minimal responses at best (Schubert and Puett, 2003). This system has also been used to prepare and characterize soluble yoked hCG-LHR ECD C-terminal truncation complexes for circular dichroic spectroscopy in which it was demonstrated that secondary structure is lost as the hinge region is removed (Fralish et al., 2003).
A number of recent studies have addressed the important issue of LHR dimerization and formation of higher order oligomers. Self association of the receptor has been suggested for some time, however considerable controversy existed over whether such association was simply artifactual or not. Different investigators, using a variety of experimental and computational approaches, have within the past few years made a compelling case for LHR dimerization and oligomerization. Using a combination of Western blotting and immunoprecipitation of transiently and stable transformed cells, Tao et al. (2004) reported bands of 67, 84, 166, and 240 kDa, consistent with other reports. The 67 kDa band represents the immature precursor to LHR, and the 84 kDa band corresponds to the mature cell surface monomeric form. The 166 and 240 kDa bands are probably attributable to dimers and higher order oligomers, although the possible involvement of other, non-LHR proteins has not been excluded. Interestingly, it was found that hCG increased the relative amounts of dimers and higher order structures. Several reports have appeared in which fluorescence photobleaching (Roess et al., 2000) and bioluminescence resonance energy transfer (BRET) (Urizar et al., 2005; Zhang et al., 2009; Guan et al., 2009) experiments were done on wild-type and mutant receptors showing the presence of LHR dimers/oligomers on the cell surface. Complementing these reports, a series of elegant experiments was conducted to demonstrate trans-activation of LHR (Ji et al., 2002; Lee et al., 2002; Jeoung et al. 2007). These studies utilized two forms of LHR, one deficient in hormone binding and the other deficient in hormone-mediated signaling. When co-expressed in cells, it was shown that receptor function could be restored. A recent report described the use of transgenic mice co-expressing signaling deficient and binding deficient mutants of LHR to demonstrate the rescue of LHR and restore normal physiological actions of LH (Rivero-Muller et al., 2010). These in vivo results argue strongly for LHR association. Lastly, computational studies have suggested that TM4 in particular (as well as TM5 and TM6) is important in mediating LHR dimerization (Fanelli, 2007).
3. LHR and ovarian cancer
A number of ovarian cancer epithelial cell lines are available, some of which have been shown to express the LHR mRNA and some that do not, the results on SKOV-3 cells being controversial. For example, in two studies PCR failed to detect any mRNA; moreover, no functional LHR was present as judged by a lack of both [125I]hCG specific binding and increased DNA synthesis (Parrott et al., 2001; Warrenfeltz et al., 2008). Another study, however, reported the presence of LHR mRNA in SKOV-3 cells and showed an LH-mediated increase in cell invasiveness and effects on mRNA levels of matrix metalloproteinases (MMP-2 and MMP- 9), tissue inhibitor of metalloproteinase (TIMP-1 and TIMP-2), and plasminogen activator inhibitor (Choi et al., 2006). The reason(s) for the discrepancies are unknown, although the study in which a positive effect was reported found effects only at high levels of human LH, 100 and 1,000 ng/mL. In a recent study, stable transformants of SKOV-3 cells were prepared that expressed about 12,000 LH receptors/cell, binding hCG with a Kd of 0.3–0.8 nM (Warrenfeltz et al., 2008). The LHR+ cells exhibited increased cAMP and inositol phosphate levels in response to hormone. cAMP up-regulates ErbB-2 (Her-2) in Schwann cells (Friegen et al. 2005), and over-expression of ErbB-2 in breast, ovarian, and other tumors correlates with a poorer prognosis for the patient (Holbro et al., 2003). Experiments were devised to determine if LH addition to the LHR+ SKOV-3 cells up-regulated ErbB-2 gene expression, resulting in increased concentrations of protein and phosphorylated ErbB-2, i.e. the active form (Warrenfeltz et al., 2008).
Gene expression was measured using purified cellular RNA that was converted to cDNA, followed by a Taqman Low Density Array with 18S RNA as an internal control. Results from this real-time expression array format, after filtering out very low expressing genes (Ct ≤ 34) and genes with changes between control and experimental samples of < 2-fold, are summarized for selected genes in Fig. 7. The genes were chosen for having been reported to have some association with proliferation, cell adhesion, invasion, or LHR signaling. Panel A compares the LHR+ SKOV-3 cells with mock-transfected (LHR-) cells and shows that several genes are up-regulated, e.g. BRCA1, EGFR, IGF2R, LHR, and others, while IGFBP 5 and 6, MMP 1 and 13, TIMP3, and others are down-regulated, presumably due to a slight elevation in the concentrations of the intracellular second messengers or perhaps to a gonadotropin-independent effect of LHR. A time course of the changes in gene expression following addition of LH to the LHR-expressing cells is given in Panel B (Fig. 7). Expression of ErbB-2, as postulated, is increased by LH-mediated activation of LHR. In addition, genes for LHR, MMP25, IGFBP 1 and 3, and others are also up-regulated, while gene expression of hCGβ, TIMP3, and others are down-regulated. Interestingly, expression of LHβ and hCGβ are both down-regulated.
Fig. 7.
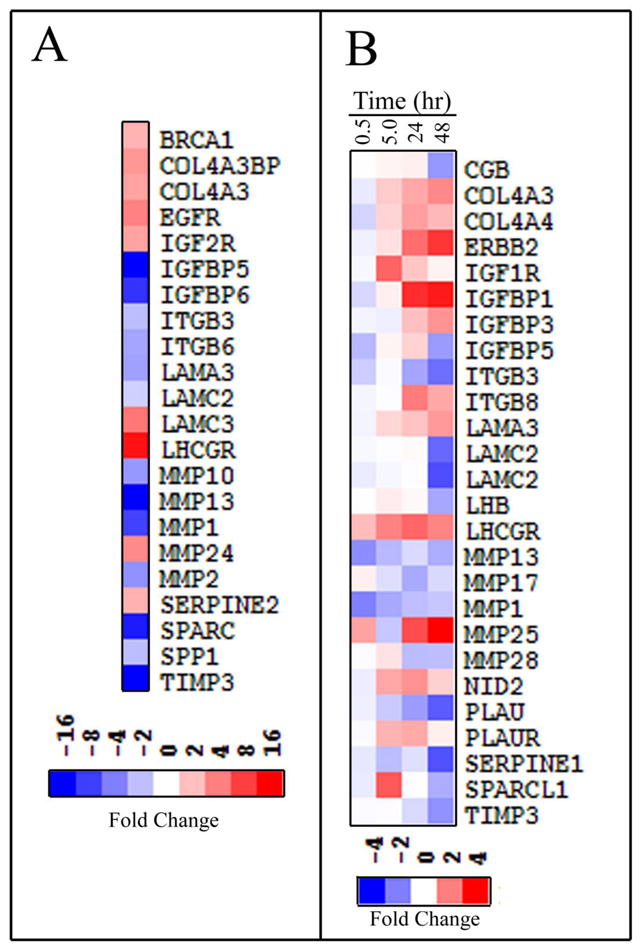
Gene expression in SKOV3 cells. RNA, prepared from mock transfected SKOV3 cells and stable transformants expressing about 12,000 receptors/cell, was converted to cDNA and gene expression determined by quantitative RT-PCR using ABI Taqman Expression Arrays. The data are presented using TreeView (http://rana.lbl.govEisenSoftware.htm). (A) Comparison of LHR+ cells to mock transfected cells. (B) Time-dependent changes in gene expression following incubation of LHR+ cells with LH (13 nM) for various times. The changes are relative to those of LHR+ cells at zero time. From Warrenfeltz et al. (2008) and reproduced with permission granted from the American Association of Cancer Research to authors publishing original figures in their sponsored journals.
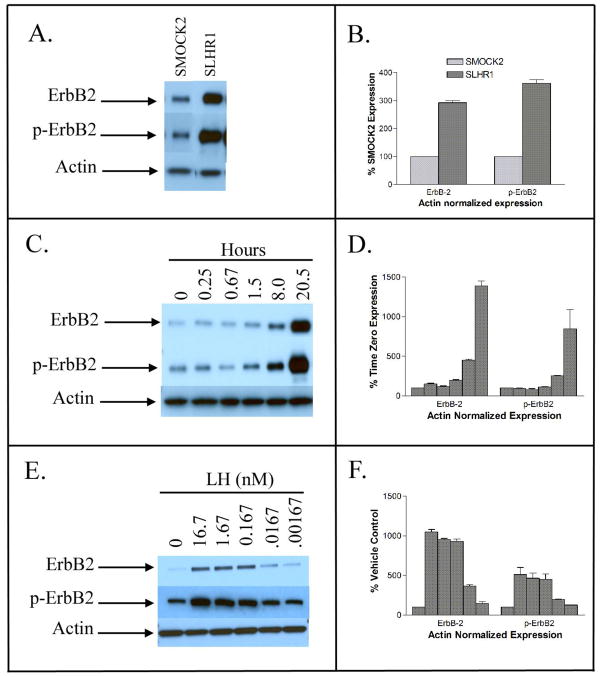
Increased levels of ErbB-2 mRNA were shown to correlate with increased levels of protein and phosphorylated protein (Fig. 8). Other studies demonstrated that forskolin and 8-Br-cAMP increased the intracellular protein concentration of ErbB-2 in both mock transfected and LHR-expressing SKOV-3 cells, while inhibitors of protein kinase A, protein kinase C, EGFR, and the kinase activity of ErbB-2 decreased ErbB-2 protein levels in LHR-expressing cells in the presence of LH (Warrenfeltz et al. 2008).
Fig. 8.
Protein expression (Western blots) in SKOV3 cells. Cellular proteins were separated on 4–20% polyacrylamide gels, then transferred to membranes, immunoblotted, and visualized using chemiluminescence. Actin was used as control in all experiments. (A) Western blots of LHR- cells (SMOCK2) and LHR+ cells (SLHR1) showing expression of ErbB-2 and phosphorylated ErbB-2 (p-ErbB2); (C) Western blots showing expression of ErbB-2 and p-ErbB-2 in LHR+ cells incubated with 16.7 nM LH for various time periods; and (E) Western blots showing expression of ErbB-2 and p-ErbB-2 after incubation for 18 h with different concentrations of LH. Panels (B), (D), and (F) show the results of densitometric measurements of ErbB-2 and p-ErbB-2 after normalization to actin; these panels correspond, respectively, to the blots presented in panels (A), (C), and (E). From Warrenfeltz et al. (2008) and reproduced with permission granted from the American Association of Cancer Research to authors publishing original figures in their sponsored journals.
Interestingly, despite the increase in expression of ErbB-2 in LHR+ SKOV-3 cells, cell proliferation, invasiveness, and migration were reduced by LH. This finding is surprising in view of the reports that increased ErbB-2 expression correlates with increase proliferation ((Yu et al., 1993; Hsieh et al., 2000). Others, however, have reported that LH and hCG reduce proliferation of ovarian epithelial cells and tumors, as well as the breast cancer cell line, MCF-7 (Zheng et al., 2000; Tourgeman et al., 2002; Rao et al., 2004). The low density array data by and large support the observation that LH-mediated LHR activation of the ovarian cancer cells decreases invasiveness and migration. For example, with the exception of MMP25, the expression of the other matrix metalloproteinases on the array were down-regulated, as was the protease, PLAU, while several genes encoding cell adhesion and basement membrane proteins, e.g. COL4A3, COL4A4, NID2, ITGB8, and LAMA3, were up-regulated. The time course over which gene expression was measured shows that, for this limited number of genes, most are appreciably altered after incubation with LH for longer than 30 min; the only early response genes altered 2-fold or more within 30 min were those for LHR, MMP1, MMP13, and MMP25.
The literature is rather confusing on the roles of gonadotropins in ovarian and breast cancer (cf. reviews by Leung and Choi, 2007; Choi et al., 2007; Mandai et al., 2007 and earlier studies referenced therein), probably reflecting the heterogeneity of the tumors and the established cell lines being used. The recent results on an engineered LHR+ ovarian epithelial cancer cell line show that LH slightly reduces proliferation, invasiveness, and migration in short term assays in spite of an LH-mediated increase in a protein such as ErbB-2 that is usually associated with increased proliferation. Other studies on a variety of ovarian cancer cell lines have shown, however, that gonadotropins can increase proliferation, migration, and invasiveness. Since many ovarian cancer express LHR and FSHR, this area of investigation requires additional research to sort out the myriad, and at times conflicting, results that have been published.
4. Conclusions
Tremendous gains have been made in our knowledge of LHR at the molecular level and its mode of ligand binding and subsequent activation. Nonetheless, major gaps remain that must be addressed for a better understanding of this important GpHR. The similar structures of the FSHR and TSHR ECDs engenders confidence that that of LHR will not be drastically different; yet, a structure is needed and particularly one with bound hormone to the full length ECD to ensure accuracy and completeness in identifying hormone-receptor contact sites. Elucidation of the structure and function of the hinge region, known to be important in LHR function, is required, as is the mechanism by which hormone binding to the ECD is transmitted via the TM helices resulting in G protein activation (see Ascoli and Puett, 2009; Kleinau and Krause, 2009). In time, as methods for crystallizing membrane proteins become more developed, it is anticipated that structures will become available for full-length LHR with and without bound hormone. Until such time, however, much can be done to elucidate the many areas that are poorly understood. The roles of LH, hCG, and LHR are reasonably well delineated in the reproductive axis, although their contributions to cancer, particularly breast and ovarian, begs to be clarified. Such studies should utilize genomics, proteomics, and indices of proliferation, migration, and invasiveness in cell studies, and animal models and primary tumors as fully as possible. Lastly, further research is required on the importance and consequences of LHR expression in non-gonadal tissues. The future is indeed very bright for continued investigations.
Acknowledgments
This paper is dedicated to the memory of Yongsheng Li who contributed much to the field in his relatively few years of graduate research. The research was supported by NIH grants (DK33973 and DK69711; D.P.), a Georgia Cancer Coalition grant (S.W.W.), and a Telethon-Italy grant (S00068TELU; F.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angelova K, Fanelli F, Puett D. A model for constitutive lutropin receptor activation based on molecular simulation and engineered mutations in transmembrane helices 6 and 7. J Biol Chem. 2002;277:32202–32213. doi: 10.1074/jbc.M203272200. [DOI] [PubMed] [Google Scholar]
- Angelova K, Fanelli F, Puett D. Contributions of intracellular loops 2 and 3 of the lutropin receptor in Gs coupling. Mol Endocrinol. 2008;22:126–138. doi: 10.1210/me.2007-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli M, Puett D. The gonadotropin hormones and their receptors. In: Strauss JF III, Barbieri R, Yen R, Jaffe’s, editors. Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 6. Saunders/Elsevier; Philadelphia: 2009. pp. 35–55. [Google Scholar]
- Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocrine Rev. 2002;23:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relationships in G protein-coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- Bhowmick N, Huang, Puett D, Isaacs NW, Lapthorn AJ. Determinants of residues important in hormone binding to the extracellular domain of the luteinizing hormone/chorionic gonadotropin receptor by site-directed mutagenesis and modeling. Mol Endocrinol. 1996;10:1147–1159. doi: 10.1210/mend.10.9.8885249. [DOI] [PubMed] [Google Scholar]
- Bhowmick N, Narayan P, Puett D. Identification of ionizable residues on the extracellular domain of the lutropin receptorinvolved in ligand binding. Endocrinology. 1999;140:4558–4563. doi: 10.1210/endo.140.10.7077. [DOI] [PubMed] [Google Scholar]
- Bogerd J. Ligand-selective determinants in gonadotropin receptors. Mol Cell Endocrinol. 2007;260–262:144–152. doi: 10.1016/j.mce.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Bruysters MWP, Verhoef-Post M, Themmen APN. Asp(330) and Tyr(331) in the C-terminal cysteine-rich region of the luteinizing hormone receptor are key residues in hormone-induced receptor activation. J Biol Chem. 2008;283:25821–25828. doi: 10.1074/jbc.M804395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caltabiano G, Campillo M, De Leener A, Smits G, Vassart G, Costagliola S, Pardo L. The specificity of binding of glycoprotein hormones to their receptors. Cell Mol Life Sci. 2008:1–9. doi: 10.1007/s00018-008-8002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Choi KC, Auersperg N, Leung PCK. Gonadotropins activate proteolysis and increase invasion through protein kinase A and phosphatidylinositol 3-kinase pathways in human epithelial ovarian cancer cells. Cancer res. 2006;66:3912–3920. doi: 10.1158/0008-5472.CAN-05-1785. [DOI] [PubMed] [Google Scholar]
- Choi JH, Wong AST, Huang HF, Leung PCK. Gonadotropins and ovarian cancer. Endocrine Rev. 2007;28:440–461. doi: 10.1210/er.2006-0036. [DOI] [PubMed] [Google Scholar]
- Coonce MM, Rabideau AC, McGee S, Smith K, Narayan P. Impact of a constitutively active luteinizing hormone receptor on testicular gene expression and postnatal Leydig cell development. Mol Cell Endocrinol. 2009;298:33–41. doi: 10.1016/j.mce.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beer T, Van Zuylen CWEM, Leeflang BR, Had K, Boelens R, Kaptein R, Kamerling JP, Vliegenthart JFG. NMR studies of the free alpha subunit of human chorionic gonadotropin: Structural influences of N-glycosylation and the beta subunit on the conformation of the alpha subunit. Eur J Biochem. 1996;241:229–242. doi: 10.1111/j.1432-1033.1996.0229t.x. [DOI] [PubMed] [Google Scholar]
- Erbel PJA, Karimi-Nejad Y, De Beer T, Boelens R, Kamerling JP, Vliegenthart JFG. Solution structure of the alpha-subunit of human chorionic gonadotropin. Eur J Biochem. 1999;260:490–498. doi: 10.1046/j.1432-1327.1999.00188.x. [DOI] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433:269–277. doi: 10.1038/nature03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA. Assembly and structural characterization of an authentic complex between human follicle stimulating hormone and a hormone-binding ectodomain of its receptor. Mol Cell Endocrinol. 2007;260–262:73–82. doi: 10.1016/j.mce.2005.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli F. Dimerization of the lutropin receptor: Insights from computational modeling. Mol Cell Endocrinol. 2007;260–262:59–64. doi: 10.1016/j.mce.2005.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli F, De Benedetti PG, Raimondi R, Seeber M. Computational modeling of intramolecular and intermolecular communication in GPCRs. Curr Prot Pept Sci. 2009;10:173–185. doi: 10.2174/138920309787847554. [DOI] [PubMed] [Google Scholar]
- Feng XY, Muller T, Mizrachi D, Fanelli F, Segaloff DL. An intracellular loop (IC2) residue confers different basal constitutive activities to the human lutropin receptor and human thyrotropin receptor through structural communication between IL2 and helix 6, via helix 3. Endocrinology. 2008;149:1705–1717. doi: 10.1210/en.2007-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiser A, Sali A. Modeller: Generation and refinement of homology-based protein structure models. Meth Enzymol. 2003;374:461–491. doi: 10.1016/S0076-6879(03)74020-8. [DOI] [PubMed] [Google Scholar]
- Fox KM, Dias JA, Van Roey P. Three-dimensional structure of human follicle-stimulating hormone. Mol Endocrinol. 2001;15:378–389. doi: 10.1210/mend.15.3.0603. [DOI] [PubMed] [Google Scholar]
- Fralish GB, Dattilo B, Puett D. Structural analysis of yoked chorionic gonadotropin-luteinizing hormone receptor ectodomain complexes by circular dichroic spectroscopy. Mol Endocrinol. 2003;17:1192–1202. doi: 10.1210/me.2002-0349. [DOI] [PubMed] [Google Scholar]
- Fregien NL, White LA, Bunge MB, Wood PM. Forskolin increases neuregulin receptors in human Schwann cells without increasing receptor mRNA. Glia. 2005;49:24–35. doi: 10.1002/glia.20091. [DOI] [PubMed] [Google Scholar]
- Gabb HA, Jackson RM, Sternberg MJ. Modelling protein docking using shape complementarity, electrostatics and biochemical information. J Mol Biol. 1997;272:106–120. doi: 10.1006/jmbi.1997.1203. [DOI] [PubMed] [Google Scholar]
- Guan R, Feng X, Wu X, Zhang M, Zhang X, Hebert TE, Segaloff DL. Bioluminescence resonance energy transfer studies reveal constitutive dimerization of the human lutropin receptor and a lack of correlation between receptor activation and the propensity for dimerization. J Biol Chem. 2009;284:7483–7494. doi: 10.1074/jbc.M809150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XL, Bazan JF, McDermott G, Park JB, Wang K, Tessier-Lavigne M, He Z, Garcia KC. Structure of the Nogo receptor ectodomain: A recognition module implicated in myelin inhibition. Neuron. 2003;38:177–185. doi: 10.1016/s0896-6273(03)00232-0. [DOI] [PubMed] [Google Scholar]
- Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res. 2003;284:99–110. doi: 10.1016/s0014-4827(02)00099-x. [DOI] [PubMed] [Google Scholar]
- Hsieh SS, Malerczyk C, Aigner A, Czubayko F. ErbB-2 expression is rate limiting for epidermal growth factor-mediated stimulation of ovarian cancer cell proliferation. Int J Cancer. 2000;86:644–651. doi: 10.1002/(sici)1097-0215(20000601)86:5<644::aid-ijc7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Huizinga EG, Tsuji S, Romijn RAP, Schiphorst ME, de Groot PG, Sixma JJ, Gros P. Structures of glycoprotein Ibα and its complex with von Willebrand Factor A1 domain. Science. 2002;297:1176–1179. doi: 10.1126/science.107355. [DOI] [PubMed] [Google Scholar]
- Jeoung MK, Lee CW, Ji I, Ji TH. Trans-activation, cis-activation and signal selection of gonadotropin receptors. Mol Cell Endocrinol. 2007;260–262:137–143. doi: 10.1016/j.mce.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji I, Lee C, Song Y, Conn PM, Ji TH. Cis- and trans-activation of hormone receptors: The LH receptor. Mol Endocrinol. 2002;16:1299–1308. doi: 10.1210/mend.16.6.0852. [DOI] [PubMed] [Google Scholar]
- Kleinau G, Krause G. Thyrotropin and homologous glycoprotein hormone receptors: Structural and functional aspects of extracellular signaling mechanisms. Endocrine Rev. 2009;30:133–151. doi: 10.1210/er.2008-0044. [DOI] [PubMed] [Google Scholar]
- Kleinau G, Brehm M, Wiedemann U, Labudde D, Leser U, Krause G. Implications for molecular mechanisms of glycoprotein hormone receptors using a new sequence-structure-function analysis resource. Mol Endocrinol. 2007;21:574–580. doi: 10.1210/me.2006-0309. [DOI] [PubMed] [Google Scholar]
- Lapthorn AJ, Harris DC, Littlejohn A, Lustbader JW, Canfield RE, Machin KJ, Morgan FJ, Isaacs NW. Crystal structure of human chorionic gonadotropin. Nature. 1994;369:455–461. doi: 10.1038/369455a0. [DOI] [PubMed] [Google Scholar]
- Lee C, Ji I, Ryu K, Song Y, Conn PM, Ji TH. Two defective heterozygous luteinizing hormone receptors can rescue hormone action. J Biol Chem. 2002;277:15795–15800. doi: 10.1074/jbc.M111818200. [DOI] [PubMed] [Google Scholar]
- Leung PCK, Choi JH. Endocrine signaling in ovarian surface epithelium and cancer. Human Reprod Update. 2007;13:143–162. doi: 10.1093/humupd/dml002. [DOI] [PubMed] [Google Scholar]
- Mandai M, Konishi I, Kuroda H, Fujii S. LH/hCG action and development of ovarian cancer-A short review on biological and clinical/epidemiological aspects. Mol Cell Endocrinol. 2007;269:61–64. doi: 10.1016/j.mce.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Meehan TP, Narayan P. Constitutively active luteinizing hormone receptors: Consequences of in vivo expression. Mol Cell Endocrinol. 2007;260–262:294–300. doi: 10.1016/j.mce.2006.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan TP, Harmon BG, Overcast ME, Yu KK, Camper SA, Puett D, Narayan P. Gonadal defects and hormonal alterations in transgenic mice expressing a single chain human chorionic gonadotropin-lutropin receptor complex. J Mol Endocrinol. 2005;34:489–503. doi: 10.1677/jme.1.01669. [DOI] [PubMed] [Google Scholar]
- Moyle WR, Campbell RK, Myers RV, Bernard MP, Han Y, Wang X. Co-evolution of ligand-receptor pairs. Nature. 1994;368:251–255. doi: 10.1038/368251a0. [DOI] [PubMed] [Google Scholar]
- Mustafi D, Palczewski K. Topology of Class A G protein-coupled receptors: Insights gained from crystal structures of rhodopsins, adrenergic, and adenosine recptors. Mol Pharmacol. 2009;75:1–12. doi: 10.1124/mol.108.051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P, Wu C, Puett D. Genetic engineering of single chain gonadotropins and hormone-receptor fusion proteins. Methods. 2000;21:59–66. doi: 10.1006/meth.2000.0975. [DOI] [PubMed] [Google Scholar]
- Narayan P, Gray J, Puett D. Yoked complexes of human choriogonadotropin and the lutropin receptor: Evidence that monomeric individual subunits are inactive. Mol Endocrinol. 2002;16:2733–2745. doi: 10.1210/me.2002-0208. [DOI] [PubMed] [Google Scholar]
- Parrott JA, Doraiswamy V, Kim G, Mosher R, Skinner MK. Expression and actions of both the follicle stimulating hormone receptor and the luteinizing hormone receptor in normal ovarian surface epithelium and ovarian cancer. Mol Cell Endocrinol. 2001;172:213–222. doi: 10.1016/s0303-7207(00)00340-3. [DOI] [PubMed] [Google Scholar]
- Puett D, Wu C, Narayan P. The tie that binds: Design of biologically active single chain chorionic gonadotropins and and a gonadotropin-receptor complex using protein engineering. Biol Reprod. 1998;58:1337–1342. doi: 10.1095/biolreprod58.6.1337. [DOI] [PubMed] [Google Scholar]
- Puett D, Li Y, Angelova K, DeMars G, Meehan TP, Fanelli F, Narayan P. Structure-function relationships of the luteinizing hormone receptor. Ann NY Acad Sci. 2005;1061:41–54. doi: 10.1196/annals.1336.006. [DOI] [PubMed] [Google Scholar]
- Puett D, Li Y, DeMars G, Angelova K, Fanelli F. A functional transmembrane complex: The luteinizing hormone receptor with bound ligand and G protein. Mol Cell Endocrinol. 2007;260–262:126–136. doi: 10.1016/j.mce.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao CHV, Li X, Manna SK, Lei ZM, Aggarwal BB. Human chorionic gonadotropin decreases proliferation and invasion of breast cancer MCF-7 cells by inhibiting NF-κB and AP-1 activation. J Biol Chem. 2004;279:25503–25510. doi: 10.1074/jbc.M400683200. [DOI] [PubMed] [Google Scholar]
- Rivero-Muller A, Chou YY, Ji I, Lajic S, Hanyaloglu AC, Jonas K, Rahman N, Ji TH, Huhtaniemi I. Rescue of defective G protein-coupled receptor function in vivo by intermolecular cooperation. Proc Natl Acad Sci USA. 2010;107:2319–2324. doi: 10.1073/pnas.0906695106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roess DA, Horvat RD, Munnelly H, Barisas BG. Luteinizing hormone receptors are self-associated in the plasma membrane. Endocrinology. 2000;141:4518–4523. doi: 10.1210/endo.141.12.7802. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SGF, Kobilka BK. The structure and function of G protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A, Blundell TL. Comparative protein modeling by satisfaction of spatial restraints. J Mol Biol. 2003;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Sanders J, Chirgadze DY, Sanders P, Baker S, Sullivan A, Bhardwaja A, Bolton J, Reeve M, Nakatake N, Evans M, Richards T, Powell M, Nunez Miguel R, Blundell TL, Furmaniak J, Rees Smith B. Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid. 2007;17:395–410. doi: 10.1089/thy.2007.0034. [DOI] [PubMed] [Google Scholar]
- Schubert RL, Puett D. Single-chain human chorionic gonadotropin analogs containing the determinant loop of the β-subunit linked to the α-subunit. J Mol Endocrinol. 2003;31:157–168. doi: 10.1677/jme.0.0310157. [DOI] [PubMed] [Google Scholar]
- Schubert RL, Narayan P, Puett D. Specificity of cognate ligand-receptor interactions: Fusion proteins of human chorionic gonadotropin and the heptahelical receptors for human luteinizing hormone, thyroid-stimulating hormone, and follicle-stimulating hormone. Endocrinology. 2003;144:129–137. doi: 10.1210/en.2002-220829. [DOI] [PubMed] [Google Scholar]
- Segaloff DL. Diseases associated with mutations of the human lutropin receptor. In: Tao Y-X, editor. Progress in Molecular Biology and Translational Science: G Protein-Coupled Receptors in Health and Disease, Part B. Elsevier; London: 2009. pp. 97–114. [DOI] [PubMed] [Google Scholar]
- Smits G, Campillo M, Govaerts C, Janssens V, Richter C, Vassart G, Pardo L, Costagliola S. Glycoprotein hormone receptors: Determinants in leucine-rich repeats responsible for ligand specificity. EMBO J. 2003;22:2692–2703. doi: 10.1093/emboj/cdg260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YS, Ji I, Beauchamp J, Isaacs NW, Ji TH. Hormone interactions to Leu-rich repeats in the gonadotropin receptors (I) J Biol Chem. 2001a;276:3426–3435. doi: 10.1074/jbc.M003772200. [DOI] [PubMed] [Google Scholar]
- Song YS, Ji I, Beauchamp J, Isaacs NW, Ji TH. Hormone interactions to Leu-rich repeats in the gonadotropin receptors (II) J Biol Chem. 2001b;276:3436–3442. doi: 10.1074/jbc.M003773200. [DOI] [PubMed] [Google Scholar]
- Tao YX, Johnson NB, Segaloff DL. Constitutive and agonist-dependent self-association of the cell surface human lutropin receptor. J Biol Chem. 2004;279:5904–5914. doi: 10.1074/jbc.M311162200. [DOI] [PubMed] [Google Scholar]
- Tegoni M, Spinelli S, Verhoeyen, Davis P, Cambillau C. Crystal structure of a ternary complex between human chorionic gonadotropin (hCG) and two Fv fragments specific for the alpha and beta-subunits. J Mol Biol. 1999;289:1375–1385. doi: 10.1006/jmbi.1999.2845. [DOI] [PubMed] [Google Scholar]
- Themmen APN. An update of the pathophysiology of human gonadotrophin subunit and receptor gene mutations and polymorphisms. Reproduction. 2005;130:263–274. doi: 10.1530/rep.1.00663. [DOI] [PubMed] [Google Scholar]
- Tourgeman DE, Lu JJ, Boostanfar R, Amezcua C, Felix JC, Paulson RJ. Human chorionic gonadotropin suppresses ovarian epithelial neoplastic cell proliferation in vitro. Fertil Steril. 2002;78:1096–1099. doi: 10.1016/s0015-0282(02)03367-8. [DOI] [PubMed] [Google Scholar]
- Urizar E, Montanelli L, Loy T, Bonomi M, Swillens S, Gales C, Bouvier M, Smits G, Vassart G, Costagliola S. Glycoprotein hormone receptors: Link between receptor homodimerization and negative cooperativity. The EMBO J. 2005;24:1954–1964. doi: 10.1038/sj.emboj.7600686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Durme J, Horn F, Costagliola S, Vriend G, Vassart G. GRIS: Glycoprotein-hormone receptor information system. Mol Endocrinol. 2006;20:2247–2255. doi: 10.1210/me.2006-0020. [DOI] [PubMed] [Google Scholar]
- Vassart G, Pardo L, Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci. 2004;29:119–126. doi: 10.1016/j.tibs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Vischer HF, Granneman JCM, Bogerd J. Opposite contribution of two ligand-selective determinants in the N-terminal hormone-binding exodomainof human gonadotropin receptors. Mol Endocrinol. 2003a;17:1972–1981. doi: 10.1210/me.2003-0172. [DOI] [PubMed] [Google Scholar]
- Vischer HF, Granneman JCM, Noordam MJ, Mosselman S, Bogerd J. Ligand selectivity of gonadotropin receptors. Role of the beta-strands of extracellular leucine-rich repeats 3 and 6 of the human luteinizing hormone receptor. J Biol Chem. 2003b;278:15505–15513. doi: 10.1074/jbc.M300634200. [DOI] [PubMed] [Google Scholar]
- Vischer HF, Granneman JCM, Bogerd J. Identification of follicle-stimulating hormone-selective β-strands in the N-terminal hormone-binding exodomain of human gonadotropin receptors. Mol Endocrinol. 2006;20:1880–1893. doi: 10.1210/me.2005-0202. [DOI] [PubMed] [Google Scholar]
- Warrenfeltz SW, Lott SA, Palmer TM, Gray JC, Puett D. Luteinizing hormone-induced up-regulation of ErbB-2 is insufficient stimulant of growth and invasion in ovarian cancer cells. Mol Cancer Res. 2008;6:1775–1785. doi: 10.1158/1541-7786.MCR-08-0214. [DOI] [PubMed] [Google Scholar]
- Wu C, Narayan P, Puett D. Protein engineering of a novel constitutively active hormone-receptor complex. J Biol Chem. 1996;271:31638–31642. doi: 10.1074/jbc.271.49.31638. [DOI] [PubMed] [Google Scholar]
- Wu H, Lustbader JW, Liu Y, Canfield RE, Hendrickson WA. Structure of human chorionic gonadotropin at 2.6 resolution from MAD analysis of the selenomethionylprotein. Structure. 1994;2:545–558. doi: 10.1016/s0969-2126(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Yu D, Wolf JK, Scanlon M, Price JE, Hung MC. Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res. 1993;53:891–898. [PubMed] [Google Scholar]
- Zeng H, Phang T, Song YS, Ji I, Ji TH. The role of the hinge region of the luteinizing hormone receptor in hormone interaction and signal generation. J Biol Chem. 2001;276:3451–3458. doi: 10.1074/jbc.M007488200. [DOI] [PubMed] [Google Scholar]
- Zhang ML, Tao YX, Ryan GL, Feng XY, Fanelli F, Segaloff DL. Intrinsic differences in the response of the human lutropin receptor versus the human follitropin receptor to activating mutations. J Biol Chem. 2007;282:25527–25539. doi: 10.1074/jbc.M703500200. [DOI] [PubMed] [Google Scholar]
- Zhang M, Feng X, Guan R, Hebert TE, Segaloff DL. A cell surface inactive mutant of the human lutropin receptor (hLHR) attenuates signaling of wild-type or constitutively active receptors via heterodimerzation. Cell Signal. 2009;21:1663–1671. doi: 10.1016/j.cellsig.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Lu JJ, Luo F, Zheng Y, Feng YJ, Felix JC, Lauchlan SC, Pike MC. Ovarian epithelial tumor growth promotion by follicle-stimulating hormone and inhibition of the effect by luteinizing hormone. Gynecol Oncol. 2000;76:80–88. doi: 10.1006/gyno.1999.5628. [DOI] [PubMed] [Google Scholar]