Summary
How cave animals adapt to life in darkness is a poorly understood aspect of evolutionary biology [1]. Here we identify a behavioral shift and its morphological basis in Astyanax mexicanus, a teleost with a sighted surface dwelling form (surface fish) and various blind cave dwelling forms (cavefish) [2–4]. Vibration attraction behavior (VAB) is the ability of fish to swim toward the source of a water disturbance in darkness. VAB was typically seen in cavefish, rarely in surface fish, and advantageous for feeding success in the dark. The potential for showing VAB has a genetic component and is linked to the mechanosensory function of the lateral line. VAB was evoked by vibration stimuli peaking at 35 Hz, blocked by lateral line inhibitors, appeared after developmental increases in superficial neuromast (SN) number and size [5–7], and was significantly reduced by bilateral ablation of SN. We conclude that VAB and SN enhancement co-evolved to compensate for loss of vision and help blind cavefish find food in darkness.
Results and Discussion
Vibration attraction behavior
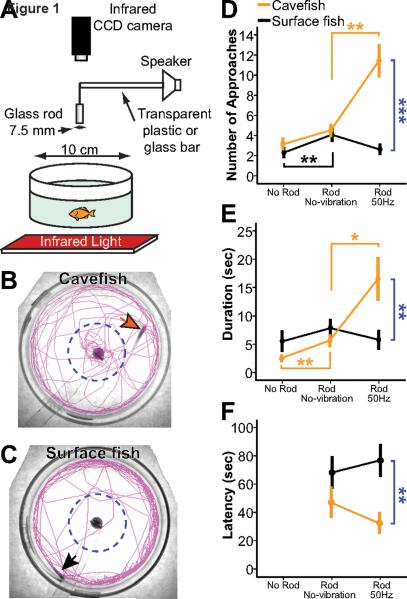
Cavefish swim toward water disturbances created by small clay particles dropped into cave pools [8]. In contrast, surface fish scatter and flee when faced with the same type of stimulus in a natural lighted habitat. In the laboratory, cavefish are attracted to an oscillating object in water [9], prompting us to quantify this behavior using a vibrating rod as a stimulus (Figure 1 and Movie S1). In the absence of the rod, cavefish and surface fish usually swam along the edges of the assay chamber. When the rod was inserted into the water, but not vibrated, both forms of Astyanax responded weakly by occasionally exploring the center of the chamber. However, when the rod was vibrated at 50 Hz, a frequency often used for analyzing fish orientation behaviors [10, 11], cavefish, but not surface fish, were strongly attracted, as determined by an increased mean number of approaches (NOA) to the rod, a higher mean duration of swimming in the vicinity of the rod, and a shorter mean latency before beginning to swim toward the rod (Figure 1D–1F and Table S1). We defined vibration attraction behavior (VAB) as the increase in attraction to a vibrating rod above a background level of 4 NOA – observed with the non-vibrating rod – during a 3 min assay period (Figure 1D and 2A). VAB is stable in laboratory raised cavefish, as determined by replicated values in assays on the same individuals conducted a month apart (mean NOA ± SEM of first and second assays: 12.7 ± 3.0 and 12.0 ± 2.4, respectively; see Table S1).
Figure 1. Quantification of VAB.
(A) Diagram showing the assay system used to record VAB in darkness. An infrared light illuminates the cylinder-shaped assay chamber from below. Swimming is recorded with a CCD infrared camera positioned above the assay chamber. A glass rod is inserted into the assay chamber to produce vibration stimuli. (B and C) Path (purple lines) of swimming in Pachón cavefish (B) and surface fish (C) during the 3 min assay period. Dotted lines indicate 2 cm-diameter quantification area surrounding the grass rod (dark spot in center of the chamber). Arrows indicate the starting positions of the fish. (D–F) VAB quantified in cavefish (orange lines and points) and surface fish (black lines and points) as NOA (D), duration (E), and latency (F) in the absence of a rod (left), the presence of a stationary rod (middle), and the presence of a vibrating rod (50 Hz) (right). Values are means ± standard error of the mean (SEM). Cavefish: N = 40. Surface fish: N = 44. Orange, black and blue asterisks indicate significant differences among cavefish, surface fish, and between cavefish and surface fish, respectively (*: p < 0.05. **: p < 0.01. ***: p < 0.001). See also Movie S1, Figure S1, and Table S1 and S2.
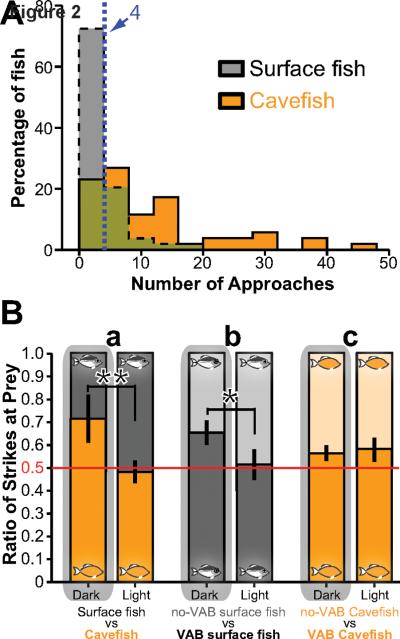
Figure 2. Significance of VAB in feeding determined by competitive prey-capture experiments.
(A) VAB levels in surface fish and Pachón cavefish measured as NOA. Surface fish: N = 54, gray area. Cavefish: N = 52, orange area. Vertical dashed line represents NOA cut-off value for classifying fish with (above 4) and without (below 4) VAB with a stimulus of 50 Hz. (B) Prey-capture competition assays. Bars show the proportion of strikes at prey between pairs of surface fish (gray bars) and cavefish (orange bars) with or without VAB during a 1 min assay period in darkness (a, b and c; left bars) and in light (a, b and c; right bars). A total of eight pairs of cavefish versus surface fish (a), five pairs of surface fish with and without VAB (b), and ten pairs of cavefish with and without VAB (c) in the dark and light are shown. Values are mean ratio of strikes ± 95% confidence intervals of the mean. Significance levels: *: p < 0.05. **: p < 0.01. See also Table S1.
Laboratory and/or field conducted behavioral assays showed that Pachón, Los Sabinos, and Piedras cavefish exhibit VAB, whereas Molino cavefish, which evolved independently [12, 13], and most surface fish did not show VAB (Table S2). VAB was more robust in field-collected Pachón cavefish compared to those raised in the laboratory, suggesting that VAB may decline over generations in the laboratory. The complete absence of light and fluctuating food resources are hallmarks of the cave environment [1] that are not experienced by cavefish raised in the laboratory. To determine the roles of these environmental factors, VAB assays were conducted with surface fish raised in the dark or after starvation. Neither manipulation resulted in the induction of VAB (Figure S1A and Table S1), prompting us to investigate the possibility of a genetic basis for VAB. Accordingly, VAB was examined in the F1 progeny of a surface fish and Pachón cavefish cross. The mean distribution of VAB in the offspring was intermediate between those of the parents (Figure S1B and Table S1), suggesting that VAB is an inherited trait. Thus VAB has a genetic basis, although the contribution of unknown environmental factors cannot be excluded. This conclusion is consistent with previous results showing that both environmental and genetic factors contribute to animal behavior [14, 15].
Significance of VAB in finding food
Laboratory raised Pachón cavefish showed a wide range of VAB values, and some individuals did not display this behavior (Figure 2A). Additionally, most laboratory raised surface fish lacked VAB, although some individuals were identified with relatively low VAB (Figure 2A). These differences allowed for testing the significance of VAB in feeding by competitive prey-capture experiments using pairs of fish with and without VAB (Figure 2B). Cavefish are better competitors than surface fish for limited quantities of food in the dark [16]. Consistent with this observation, cavefish dominated over surface fish in frequency of strikes at prey in the dark, but this advantage disappeared in the light (Figure 2Ba). Key findings of the competition experiments were (1) that surface fish with VAB showed significantly more strikes in the dark than surface fish without VAB, a difference that disappeared in the light (Figure 2Bb), and (2) that cavefish with VAB predominated over those without VAB in light or darkness (Figure 2Bc). The results suggest that VAB has a role in feeding and may be beneficial for survival of Astyanax in caves, although additional factors, such as enhanced olfaction, may also be involved in prey capture by cavefish. Small invertebrates such as copepods disturb the water at 30–40 Hz when swimming [17], which is in the detection range of the lateral line system (see below and [18]). Cavefish themselves create 30–90 Hz turbulence when in motion [19], suggesting that moving cavefish produce water disturbances that other cavefish might be able to follow [16], either to a source of food or perhaps to find mates.
In the absence of macroscopic predators, cavefish could be free to express VAB in caves, which could be a risky endeavor for surface fish outside of caves. Surface fish expressing VAB in their natural surface environment could be under negative selective pressure because of the risk of predation. Nevertheless, a small proportion of laboratory raised surface fish showed low levels of VAB, which were abolished by lateral line inhibitors (see below and Figure S2B), suggesting that the VAB phenotype is present at low frequencies in natural populations. When introduced into a dark cave, surface fish with VAB would have an advantage over those lacking this phenotype, and thus could serve as the founders of cavefish populations. As VAB levels gradually increased through selection in subsequent generations, the ability of cavefish to successfully find food in the darkness would be enhanced. Therefore, natural selection for VAB may be one of the ways in which surface fish-like ancestors became adapted to caves and evolved into cavefish, emphasizing the importance of behavioral diversity in adapting animals to new environmental challenges.
Superficial neuromasts underlie VAB
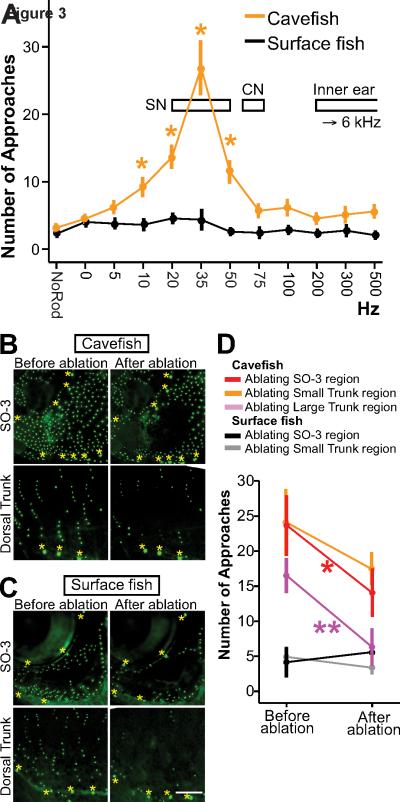
We determined the tuning of VAB by measuring responses to stimulus frequencies ranging from 5 to 500 Hz. This allowed us to discriminate between possible roles of the lateral line and inner ear sensory systems. The results showed that VAB has a relatively low frequency range (10 to 50 Hz) with a peak at 35 Hz (Figure 3A), considerably lower than the best sensing range of the Astyanax inner ear (200–6000 Hz) [20], but within the frequencies detectable by the lateral line (20–80 Hz) [18]. To confirm the role of the lateral line, we measured VAB after treatment with cobalt or gentamicin, which inhibit the function of neuromasts without detectable effects on the inner ear [21–24]. The results showed that these inhibitors abolished VAB (Figure S2), indicating that VAB is mediated by the lateral line. This conclusion is supported by the absence of changes in auditory structure and capacity between the two forms of Astyanax [20, 25].
Figure 3. VAB is controlled by superficial neuromasts.
(A) The relationship between VAB and vibration frequencies (Hz) in Pachón cavefish and surface fish. Values are mean NOA ± SEM. Cavefish: N = 40 at no rod and 0 Hz, 35 at 5 Hz, 10 Hz, 20 Hz, 100 Hz and 200 Hz, 19 at 35 Hz and 75 Hz, 51 at 50 Hz, 8 at 300 Hz, and 27 at 500 Hz. Asterisks: frequencies at which cavefish VAB is significantly above the 0 Hz condition at p < 0.05 (see Table S1). Surface fish: N = 44 at no rod and 0 Hz, 38 at 5 Hz, 10 Hz, 20 Hz and 100Hz, 19 at 35 Hz and 75 Hz, 54 at 50 Hz, 35 at 200 Hz, 8 at 300 Hz, and 27 at 500 Hz. Open horizontal boxes indicate the best sensing range of superficial neuromasts (SN), canal neuromasts (CN), and inner ear (right) in Astyanax [18, 20]. See also Figures S2 and S3. (B–D) DASPEI-stained Pachón cavefish (B) and surface fish (C) before and after SN ablation. Scale bar is 1.0 mm; magnification is the same in (B) and (C). (D) VAB in cavefish and surface fish before SN ablation and 2 days after bilateral SN ablation. Values are means ± SEM. Significance levels: *: p < 0.05. **: p < 0.01. Cavefish: N = 16 for SO-3, N = 10 for small region of the trunk, and N = 11 for large region of the trunk. Surface fish: N = 13 for SO-3 and N = 6 for small region of the trunk. A vibrating rod at 35 Hz was used. See also Table S1 and Figure S4.
The lateral line system consists of canal neuromasts (CN) and superficial neuromasts (SN) [26]. Adult cavefish and surface fish have about the same number of CN but cavefish have several fold more SN than surface fish [5–7]. Cavefish CN and SN are also larger and contain more sensory hair cells than those of surface fish [5, 7]. SN are the best candidates for a role in VAB because maximal VAB was evoked at 35 Hz, which coincides with the peak frequency of SN ([18]; see Figure 3A), and previous studies showed that CN ablation did not affect the attraction of cavefish to a vibrating sphere [9]. The following experiments were conducted to determine the type of neuromasts involved in VAB. First, we explored the timing between VAB onset and neuromast development in surface fish and cavefish (Figure S3). The number of CN did not change during development but CN size increased about two-fold from 1 to 4 months post-fertilization (mpf) (Figure S3C and S3D). In contrast, the number and size of SN increased starting at 2 mpf, and this increase was greater in cavefish than in surface fish (Figure S3C and S3D). VAB was first detected at 3 mpf in cavefish (Figure S3B), which is consistent with a role for SN. Second, we determined the effects on VAB of ablating SN bilaterally in the cranial third suborbital bone (SO-3 [27]) and the trunk (Figure S4), body areas with different neuromast densities. Cavefish lacking SN in the SO-3 region (ablated SN: 115 ± 8, mean ± SEM) or in a large trunk region containing about the same SN number as the SO-3 region (ablated SN: 119 ± 6) showed significant reduction in VAB (Figure 3B, 3D, S4A and S4C). Decreased VAB was also observed when SN were ablated in a smaller dorsal trunk region (ablated SN: 50 ± 3), but this change was not significant (Figure 3D and S4B). In contrast, bilateral ablation of SN in similar regions of surface fish controls had no behavioral effects (Figure 3C and 3D). CN were usually not affected in these experiments (Figure 3B and 3C). Therefore, the ablation experiments provide strong evidence that SN enhancement is responsible for cavefish VAB and demonstrate the primary and cooperative effects of SN located in the head and trunk on this behavior.
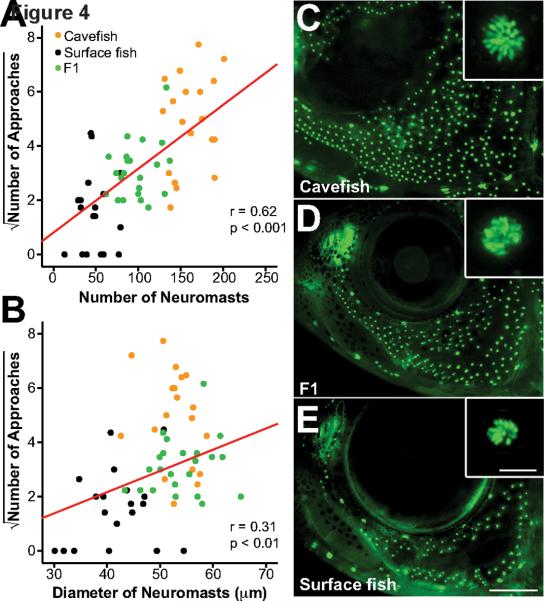
To determine whether increased SN number, size, or both are responsible for VAB, we compared VAB and SN number or diameter in the SO-3 regions of individual cavefish, surface fish, and their F1 progeny (Figure 4). The results showed that VAB was positively correlated with both elevated SN number and size (Figure 4A and 4B). Importantly, F1 hybrids showed intermediate VAB levels and exhibited SN numbers between those of their surface fish and cavefish parents (Figure 4A and 4C–4E), but the size of F1 hybrid SN was similar to large cavefish SN (Figure 4B–4D). The results suggest that both SN number and size are involved in cavefish VAB, although number may be the more important factor in determining the magnitude of VAB. Nevertheless, surface fish with low levels of VAB did not show significant increases in SN number or size (Figure 4A and 4B), implying that SN enhancement may not be necessary for modest levels of VAB.
Figure 4. The relationship between VAB and SN number and size in Pachón cavefish, surface fish, and the F1 progeny of a surface fish × cavefish cross.
(A and B) Scatter plots showing the relationship between the SN number (A) or diameter (B) and the square root of NOA. Both SN number and diameter are significantly correlated with VAB (see Table S1). Cavefish: N = 20. Surface fish: N = 19. F1 hybrids: N = 23. Linear regression line in red. A vibrating rod at 35 Hz was used. (C–E) DASPEI staining of neuromasts in the SO-3 region of a cavefish (C), a F1 hybrid (D), and a surface fish (E). Scale bar in (E) is 1.0 mm; magnification is the same in (C–E). Insets in (C–E) show the size of a typical SN from the SO-3 region of fish in (C), (D), and (E). Scale bar in inset (E) is 50 μm; magnification is the same in each inset.
We have found that enhancement of SN may be responsible for the evolution of relatively high levels of VAB in cavefish. The cavefish SN cupulae (hair cell stereocilia covered by a gelatinous case) are about 300 μm in length compared to about 42 μm in surface fish [7]. The long cavefish cupulae extend beyond the motionless boundary layer, which attenuates water movements along the surface of the body [28, 29]. McHenry et al. [29] determined the thickness of the boundary layer to be 40 μm in low water currents produced by vibration stimuli below 100 Hz. Large cavefish SN could therefore detect low vibration-frequency stimuli (below 50 Hz) in an otherwise calm cave pool. A dense cluster of neuromasts oriented in the same direction likely share common innervation, forming a small sensing unit [7], this neural network could produce low-noise high-quality information by averaging activity patterns within a group of neuromasts [30]. Taking advantage of a dense cluster of large SN, cavefish with sophisticated sensors may be able to evaluate and react to vibrating stimuli better than surface fish.
Astyanax cavefish have evolved a suite of constructive traits, including larger jaws [31], more taste buds [31, 32], modified brain structures [33], changes in behavior [34–39], and larger and more numerous SN [5–7], compared to their surface fish counterparts. Prior to this study, the relationship between the constructive morphological traits and the evolution of behavioral changes had not been examined. The current results have linked one of the constructive traits – SN enhancement – to a behavior that is advantageous for feeding in a dark environment. An increase of neuromasts has also been reported in other cavefish species [40], and thus lateral line enhancement could be a general phenomenon that mediates the adaptation of teleosts to life in caves.
Experimental Procedures
Biological materials
The experiments were performed on laboratory-raised [5, 27, 31, 41] and wild-caught Astyanax mexicanus cavefish and surface fish. Fishes used in the assays ranged from 1 month to 2 years old and were fed living Artemia salina larvae. Wild-caught surface fish were collected from Nacimiento del Río Choy and Río Tampaón, both in San Luis Potosí, Mexico. Wild-caught Pachón and Piedras cavefish were collected at Cueva de El Pachón and at El Sótano de Las Piedras, respectively, in San Luis Potosí, Mexico. All methods described in Experimental Procedures were approved by the University of Maryland Animal Care and Use Committee and conformed to NIH guidelines.
Vibration attraction behavior assays
VAB was assayed in the dark using cylindrical chambers (Pyrex 325 ml glass dish, 10 cm diameter × 5 cm high, Corning, Corning, NY) filled to 30–35 mm with water in the laboratory. Fish were placed in the assay chamber 4 or 5 days before the experiment to acclimate, and 1 day before the assay fish were transferred to a dark room. Before the assay was initiated, the fish were subjected to another 3 min acclimation period on the recording chamber. Stimulus was created by using a 7.5 mm-diameter glass rod inserted 15–18 mm into the water supported by a 1 mm thick and 95 mm long transparent plastic plate (up to 100 Hz stimuli) or a 3 mm thick and 115 mm long glass bar (200–500 Hz stimuli). Manual stimuli were produced at 3–4 Hz (2.9 mm amplitude based on Fourier analysis of captured videos performed with MatLab Software R2007a, The MathWorks Inc., Natick, MA) or mechanically at 5–500 Hz (Table S3). The axis of the vibration was in the horizontal plane. An EISCO function generator (Eisco Lab Supplies, Ambala, Cantt, India) or a Leader LG1301 function generator (Leader Instruments Corp., Cypress, CA) with an audio speaker (Pro Speakers, Apple, Cupertino, CA) were used to produce the stimlus, which were calibrated by Fourier analysis of recorded videos taken with a high speed CCD camera (DRS Lightning RDT, DRS Data & Imaging Systems, Oakland, NJ). Individual fish were subjected to either a single assay in the absence of a rod, in the presence of a non-vibrating rod, or in the presence of a vibrating rod, or three successive assays using a random sequence of these three conditions. Swimming movements were video recorded for a 3 min period under the illumination of an infrared light (880 nm wave length, BL1960-880 black light, Advanced Illumination, Rochester, VT). An infrared CCD camera (Qicam IR, Qimaging, Surrey, Canada) with a zoom lens (Zoom 7000, Navitar, Rochester, NY) was used to capture images at 10 frames/sec using StreamPix 3.36.0 video recording software (NorPix, Montreal, Canada). ImageJ 1.35s software (NIH, Bethesda, MD) was used for video analysis.
Wild-caught fish were maintained for one or two days in 2 L plastic bottles containing water from their original location or purified tap water, and VAB was assayed at a field location. Fish were transferred to the assay chamber at least an hour before the assay, acclimated in the dark, and subjected to a 3 min acclimation period on the recording stage. Manual stimulation was provided by a glass rod for 3 min as described above, and behavior was recorded with an infrared-light-equipped infrared camcorder (DCR-SR200C, Sony, Tokyo, Japan). NOA was counted with reference to a transparent plastic disc (2 cm diameter) attached to the vibrating glass probe.
Genetic and environmental effects on VAB
A female surface fish was crossed with a male Pachón cavefish to obtain F1 hybrids. To test for the effects of darkness on VAB, surface fish were raised from hatched larvae for 3–4 months in constant darkness prior to the behavioral assay. Controls were reared under a normal 14 hr light 10 hr dark diurnal cycle. For starvation experiments, surface fish (four months to a year old) were transferred to an assay chamber and starved for 5 days prior to the assay.
Prey capture competition assay
Surface fish and cavefish with or without VAB were marked by an Alcian Blue tattoo near their dorsal fins. Five days prior to the assay, a pair of tattooed fish was transferred to a test aquarium (12 × 20 × 10 cm), subjected to either continuous darkness or a 14 h-light and 10 h-dark diurnal cycle depending on whether subsequent assays were to be conducted in dark or light, respectively, and fed Artemia larvae once per 2 days. On the day of the assay, three drops of living Artemia larvae were added to the aquarium and strikes at prey were recorded for 1 min with the infrared camcorder. Strikes were counted for every fish, and the total number of strikes for pairs was calculated. Then, the ratio of strike numbers of a dominating fish was calculated. Each competition assay was repeated twice and the ratios of strike numbers were averaged across the two trials.
Vibration frequency analysis
Vibration frequency analysis was conducted in three ways: (1) in the absence of a vibrating glass rod, (2) in the presence of a stationary glass rod (0 Hz), and (3) in the presence of a vibrating rod at 5–500 Hz (Figure 3A). The sequence of vibration frequencies was randomly chosen for each fish, and a resting period of at least 25 min was applied between the assays. VAB was recorded as described above.
Cobalt and gentamicin treatment
Before treatment, fish were assayed for VAB as described above, followed by a 4- or 5-day resting period. The fish were then immersed in a 0.08 mM solution of CoCl2 (Sigma, St. Louis, MO) in Ca2+-free standardized water (25 mM KCl, 50 mM KNO3, 50 mM NaH2PO4, 200 mM NaCl, 100 mM MgSO4, adjusted to pH 7.2 with NaOH; [21, 22]), or in a 0.002% solution of gentamicin sulfate salt (Sigma) in conditioned water (conductivity approximately 600 μS) for 14–19 hours [23] prior to VAB assays. Control fish were immersed in Ca2+-free standardized water containing 3.75 mM Ca2+ for the cobalt experiment or in conditioned water for the gentamicin experiment.
Neuromast vital staining
For vital staining with 2-(4-(dimethylamino)styryl)-N-ethylpyridinium iodide (DASPEI; Invitrogen, Eugene, OR) [42], fish were immersed in 25 μg/ml DASPEI dissolved in conditioned water (conductivity approximately 600 μS) for 1 hr, followed by immersion in ice-cold 66.7 μg/ml Ethyl 3-aminobenzoate methanesulfonate salt (MS222, Sigma) in conditioned water, viewed with a fluorescence microscope (Axioskop 2 equipped with 2.5× Plan-Neofluar lens and a FITC filter set; Zeiss, Göttingen, Germany), and photographed with a Zeiss Axiocam CCD camera. Neuromasts were quantified on images of DASPEI-stained fish using ImageJ software. CN were counted in the infra-orbital lateral line, and SN were counted in the SO-3 region [27]. The longer diameter of CN and SN was measured in 10 largest stained neuromasts in the same area.
Superficial neuromast ablation
Before ablation VAB was measured and neuromasts were stained with DASPEI and photographed. SN were ablated by applying 0.5 μl of Vetbond non-toxic tissue adhesive (3M, St. Paul, MN) along either the SO-3 or a dorsal trunk region using the plastic tip of a 2.5 μl Eppendorf pipetman. Application of Vetbond to CN was avoided. After application of tissue adhesive to one side, the fish was exposed to air for 10 seconds, and tissue adhesive was then applied to the same area on the opposite side, followed by a second cycle of air-drying. Treated fish were placed in a 10 cm-diameter cylindrical chamber containing conditioned water at room temperature. Within a day of active swimming the tissue adhesive usually “peeled off” the body, resulting in a void in the underlying field of SN.
Statistics
The specifics and results of the statistical tests are provided in Table S1. Statistical tests were conducted with PASW 17.0 software (SPSS, Chicago, IL).
Highlights.
Vibration attraction behavior (VAB) was characterized in blind cavefish
VAB has a genetic basis and confers an advantage for feeding in darkness
VAB is based on an increase in superficial neuromasts (SN)
Co-evolution of VAB and SN was likely a critical step in adaptation to cave life
Supplementary Material
Acknowledgements
We thank S. Coombs, B. Casper, E. Tytell and G. Ashida for helpful discussions, D. Fong for collection of Piedras cavefish, E. Tytell for equipment loans and programming, and R. Norman and S. Ahmed for fish care and supporting behavioral assays and analyses. This research was supported by a Japan Society for the Promotion of Science Postdoctoral Fellowship to M.Y. and NIH (R01-EYE014619) and NSF (IBN-052384) grants to W.R.J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Culver DC. Cave life, Evolution and ecology. Harvard University Press; Cambridge: 1982. [Google Scholar]
- 2.Jeffery WR. Cavefish as a model system in evolutionary developmental biology. Dev. Biol. 2001;231:1–12. doi: 10.1006/dbio.2000.0121. [DOI] [PubMed] [Google Scholar]
- 3.Jeffery WR. Emerging model systems in evo-devo: cavefish and microevolution of development. Evol. Dev. 2008;10:265–272. doi: 10.1111/j.1525-142X.2008.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkens H. Evolution and genetics of epigean and cave Astyanax-fasciatus (Characidae, Pisces) - Support for the neutral mutation theory. Evol. Biol. 1988;23:271–367. [Google Scholar]
- 5.Jeffery WR, Strickler AG, Guiney S, Heyser DG, Tomarev SI. Prox 1 in eye degeneration and sensory organ compensation during development and evolution of the cavefish Astyanax. Dev. Genes Evol. 2000;210:223–230. doi: 10.1007/s004270050308. [DOI] [PubMed] [Google Scholar]
- 6.Schemmel C. Vergleichende Untersuchungen an den Hautsinnesorganen oberund unterirdisch lebender Astyanax-Formen. Z. Morph. Tiere. 1967;61:255–316. [Google Scholar]
- 7.Teyke T. Morphological differences in neuromasts of the blind cave fish Astyanax-hubbsi and the sighted river fish Astyanax-mexicanus. Brain Behav. Evol. 1990;35:23–30. doi: 10.1159/000115853. [DOI] [PubMed] [Google Scholar]
- 8.Parzefall J. Field observation in epigean and cave populations of Mexican characid Astyanax mexicanus (Pisces, Characidae) Mém. Biospéléol. 1983;10:171–176. [Google Scholar]
- 9.Abdel-Latif H, Hassan ES, von Campenhausen C. Sensory performance of blind Mexican cave fish after destruction of the canal neuromasts. Naturwissenschaften. 1990;77:237–239. doi: 10.1007/BF01138492. [DOI] [PubMed] [Google Scholar]
- 10.Coombs S. Signal detection theory, lateral-line excitation patterns and prey capture behaviour of mottled sculpin. Anim. Behav. 1999;58:421–430. doi: 10.1006/anbe.1999.1179. [DOI] [PubMed] [Google Scholar]
- 11.Coombs S, Patton P. Lateral line stimulation patterns and prey orienting behavior in the Lake Michigan mottled sculpin (Cottus bairdi) J. Comp. Physiol. A, Neuroethol. Sens. Neural Behav. Physiol. 2009;195:279–297. doi: 10.1007/s00359-008-0405-4. [DOI] [PubMed] [Google Scholar]
- 12.Borowsky R. Restoring sight in blind cavefish. Curr. Biol. 2008;18:R23–R24. doi: 10.1016/j.cub.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Wilkens H. Regressive evolution: ontogeny and genetics of cavefish eye rudimentation. Biol. J. Linn. Soc. 2007;92:287–296. [Google Scholar]
- 14.Gould JL. Genetics and molecular ethology. Z. Tierpsychol. 1974;36:267–292. doi: 10.1111/j.1439-0310.1974.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 15.Pitcher TJ. Behaviour of teleost fishes. Chapman & Hall; London: 1993. [Google Scholar]
- 16.Hüppop K. Food-finding ability in cave fish. Int. J. Speleol. 1987;16:59–66. [Google Scholar]
- 17.Montgomery JC, Macdonald JA. Sensory tuning of lateral line receptors in antarctic fish to the movements of planktonic prey. Science. 1987;235:195–196. doi: 10.1126/science.235.4785.195. [DOI] [PubMed] [Google Scholar]
- 18.Münz H. Functional organization of the lateral line periphery. In: Coombs S, Görner P, Münz H, editors. The mechanosensory lateral line. Springer-Verlag; New York: 1989. [Google Scholar]
- 19.Bleckmann H, Breithaupt T, Blickhan R, Tautz J. The time course and frequency content of hydrodynamic events caused by moving fish, frogs, and crustaceans. J. Comp. Physiol. A. 1991;168:749–757. doi: 10.1007/BF00224363. [DOI] [PubMed] [Google Scholar]
- 20.Popper AN. Auditory capacities of the Mexican blind cave fish (Astyanax jordani) and its eyed ancestor (Astyanax mexicanus) Anim. Behav. 1970;18:552–562. [Google Scholar]
- 21.Hassan ES, Abdel-Latif H, Biebricher R. Studies on the effects of Ca++ and Co++ on the swimming behavior of the blind Mexican cave fish. J. Comp. Physiol. A, Sensory, Neural, Behav. Physiol. 1992;171:413–419. [Google Scholar]
- 22.Karlsen HE, Sand O. Selective and reversible blocking of the lateral line in fresh-water fish. J. Exp. Biol. 1987;133:249–262. [Google Scholar]
- 23.Song JK, Yan HY, Popper AN. Damage and recovery of hair cells in fish canal (but not superficial) neuromasts after gentamicin exposure. Hearing Res. 1995;91:63–71. doi: 10.1016/0378-5955(95)00170-0. [DOI] [PubMed] [Google Scholar]
- 24.Van Trump WJ, Coombs S, Duncan K, McHenry MJ. Gentamicin is ototoxic to all hair cells in the fish lateral line system. Hearing Res. 2010;261:42–50. doi: 10.1016/j.heares.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Popper AN. Morphology of Weberian ossicles of two species of genus Astyanax (Ostariophysi - Characidae) J. Morphol. 1971;133:179–188. doi: 10.1002/jmor.1051330205. [DOI] [PubMed] [Google Scholar]
- 26.Coombs S, Görner P, Münz H. The mechanosensory lateral line. Springer-Verlag; New York: 1989. [Google Scholar]
- 27.Yamamoto Y, Espinasa L, Stock DW, Jeffery WR. Development and evolution of craniofacial patterning is mediated by eye-dependent and -independent processes in the cavefish Astyanax. Evol. Dev. 2003;5:435–446. doi: 10.1046/j.1525-142x.2003.03050.x. [DOI] [PubMed] [Google Scholar]
- 28.Batchelor GK. An introduction to fluid dynamics. Cambridge University Press; New York: 1967. [Google Scholar]
- 29.McHenry MJ, Strother JA, van Netten SM. Mechanical filtering by the boundary layer and fluid-structure interaction in the superficial neuromast of the fish lateral line system. J. Comp. Physiol. A, Neuroethol. Sens. Neural Behav. Physiol. 2008;194:795–810. doi: 10.1007/s00359-008-0350-2. [DOI] [PubMed] [Google Scholar]
- 30.Van Trump WJ, McHenry MJ. The morphology and mechanical sensitivity of lateral line receptors in zebrafish larvae (Danio rerio) J. Exp. Biol. 2008;211:105–2115. doi: 10.1242/jeb.016204. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto Y, Byerly MS, Jackman WR, Jeffery WR. Pleiotropic functions of embryonic sonic hedgehog expression link jaw and taste bud amplification with eye loss during cavefish evolution. Dev. Biol. 2009;330:200–211. doi: 10.1016/j.ydbio.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varatharasan N, Croll RP, Franz-Odendaal T. Taste bud development and patterning in sighted and blind morphs of Astyanax mexicanus. Dev. Dyn. 2009;238:3056–3064. doi: 10.1002/dvdy.22144. [DOI] [PubMed] [Google Scholar]
- 33.Menuet A, Alunni A, Joly JS, Jeffery WR, Rétaux S. Expanded expression of sonic hedgehog in Astyanax cavefish: multiple consequences on forebrain development and evolution. Development. 2007;134:845–855. doi: 10.1242/dev.02780. [DOI] [PubMed] [Google Scholar]
- 34.Burchards H, Dolle A, Parzefall J. Aggressive-behavior of an epigean population of Astyanax mexicanus (Characidae, Pisces) and some observations of three subterranean populations. Behav. Processes. 1985;11:225–235. doi: 10.1016/0376-6357(85)90017-8. [DOI] [PubMed] [Google Scholar]
- 35.de Perera TB. Fish can encode order in their spatial map. Proc. R. Soc. Lond. B, Biol. Sci. 2004;271:2131–2134. doi: 10.1098/rspb.2004.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espinasa L, Yamamoto Y, Jeffery WR. Non-optical releasers for aggressive behavior in blind and blinded Astyanax (Teleostei, Characidae) Behav. Processes. 2005;70:144–148. doi: 10.1016/j.beproc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Plath M, Rohde M, Schröder T, Taebel-Hellwig A, Schlupp I. Female mating preferences in blind cave tetras Astyanax fasciatus (Characidae, Teleostei) Behaviour. 2006;143:15–32. [Google Scholar]
- 38.Sharma S, Coombs S, Patton P, de Perera TB. The function of wall-following behaviors in the Mexican blind cavefish and a sighted relative, the Mexican tetra (Astyanax) J. Comp. Physiol. A, Neuroethol. Sens. Neural Behav. Physiol. 2009;195:225–240. doi: 10.1007/s00359-008-0400-9. [DOI] [PubMed] [Google Scholar]
- 39.Schemmel C. Studies on the genetics of feeding behavior in the cave fish Astyanax mexicanus f. anoptichthys. An example of apparent monofactorial inheritance by polygenes. Z. Tierpsychol. 1980;53:9–22. doi: 10.1111/j.1439-0310.1980.tb00730.x. [DOI] [PubMed] [Google Scholar]
- 40.Poulson TL. Cave adaptation in amblyopsid fishes. Am. Midl. Nat. 1963;70:257–290. [Google Scholar]
- 41.Yoshizawa M, Jeffery WR. Shadow response in the blind cavefish Astyanax reveals conservation of a functional pineal eye. J. Exp. Biol. 2008;211:292–299. doi: 10.1242/jeb.012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jørgensen JM. Evolution of octavolateralis sensory cells. In: Coombs S, Görner P, Münz H, editors. The mechanosensory lateral line. Springer-Verlag; New York: 1989. pp. 115–145. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.