Summary
The mitotic checkpoint maintains genomic stability by blocking the metaphase-anaphase transition until all kinetochores attach to spindle microtubules [1, 2]. However, some defects are not detected by this checkpoint. With low concentrations of microtubule targeting agents the checkpoint eventually becomes satisfied though the spindles may be short and/or multipolar[3, 4] and the fidelity of chromosome distribution and cleavage completion are compromised. In real life, environmental toxins, radiation, or chemotherapeutic agents may lead to completed but inaccurate mitoses. Once the checkpoint is satisfied and cells divide it has been assumed that the daughter cells would proliferate regardless of prometaphase duration. However, when continuously exposed to microtubule inhibitors, untransformed cells eventually slip out of mitosis after 12-48 hours and arrest in G1[5-8] (also see [9]). Interestingly, transient but prolonged treatments with nocodazole allow completion of mitosis but the daughter cells arrest in interphase [10, 11](also see [9, 12]). We characterize the relationship between prometaphase duration and the proliferative capacity of daughter cells. Our results reveal the existence of a mechanism that senses prometaphase duration; if prometaphase lasts >1.5 hours, this mechanism triggers a durable p38 -p53 dependent G1 arrest of the daughter cells despite normal division of their mothers.
Keywords: cell cycle, G1, mitotic checkpoint, p38, prometaphase
Results and Discussion
Asynchronous cultures of RPE1 (untransformed human cells) were mounted in observation chambers with drugs that prevent satisfaction of the mitotic checkpoint. Individual cells were continuously followed by time lapse video microscopy. After 6 hours the drugs were removed and replaced with BrdU containing medium for further observation of the same fields for up to 100 hours. Then preparations were fixed for analysis of BrdU incorporation or p53/p21 expression in the progeny of the mother cells previously followed in vivo. Since cells entered mitosis at different times, those that entered mitosis early spent several hours in prometaphase before drug washout. Those entering mitosis shortly before drug removal spent little extra time in prometaphase. All cells were exposed to the drug for the same amount of time and none slipped into interphase during prolonged prometaphase (see [5]).
In 0.08 μM nocodazole cells assembled short bipolar spindles or monopolar spindles, both with monooriented chromosomes (Figure 1A). After drug removal, all cells rapidly recovered and divided in a normal fashion (Figure S1A). All daughters of mother cells spending <~1.5 hours in prometaphase proliferated with normal kinetics (Figure 1B and Movie S1). All daughters of mothers that spent >~1.5 hours in prometaphase arrested in G1 (Figure 1B and Movie S2) as determined by interphase arrest and lack of BrdU incorporation. Two hours after nocodazole removal, all daughters of mothers spending >1.5 hours in prometaphase showed elevated nuclear p53 expression, and at 48 hours 100% showed p21 expression indicating an enforced arrest (Figure 2A). Primary human fibroblasts behaved the same except that they needed to be held in prometaphase for >2 hours for their daughters to arrest in G1 (Figure S1B).
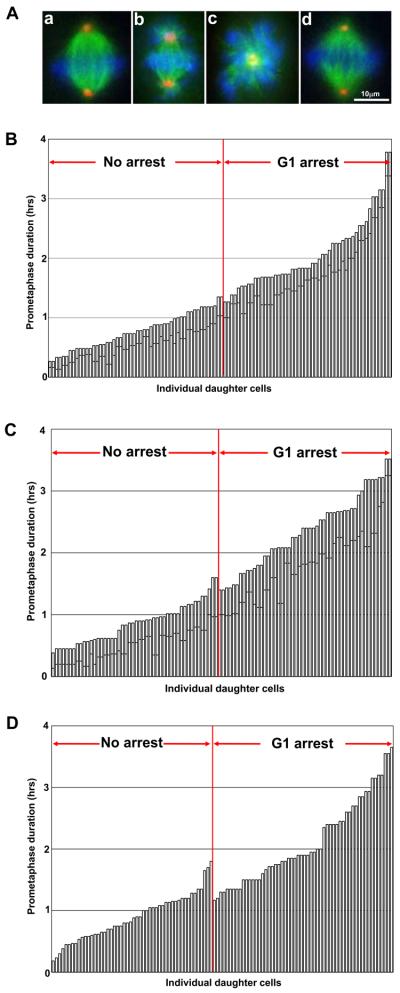
Figure 1.
A. Prometaphase spindles in RPE1 cells. (a) Control. (b) 0.08μM nocodazole. (c) 100 μM monastrol. (d) 4μM MG132 at 30 minutes after drug application. Fluorescence microscopy: DNA (blue); alpha-tubulin (green); pericentrin (red).
B. Duration of mother cell prometaphase and the proliferative capacity of daughter cells; 0.08μM nocodazole. Each vertical bar represents a daughter cell and the height of the bar indicates the duration of prometaphase for its mother cell. The dark line across each vertical bar represents the time the mother cell spent in prometaphase in the presence of drug. The vertical bars are rank ordered by the duration of prometaphase for the mother cells. Daughters that proliferate are to the left of the vertical red line and those that arrest in G1 are to the right of this line. Prometaphase in control cells (cell rounding/nuclear envelope breakdown to anaphase onset) averages 18 minutes (range 9-30 minutes, n=117).
C. Duration of mother cell prometaphase and the proliferative capacity of the daughter cells; 100μM monastrol.
D. Duration of mother cell prometaphase and the proliferative capacity of the daughter cells. RPE1 cells were treated with 5μM Taxol for 10 minutes before mitosis. Satisfaction of the mitotic checkpoint was variable giving a range of prometaphase durations.
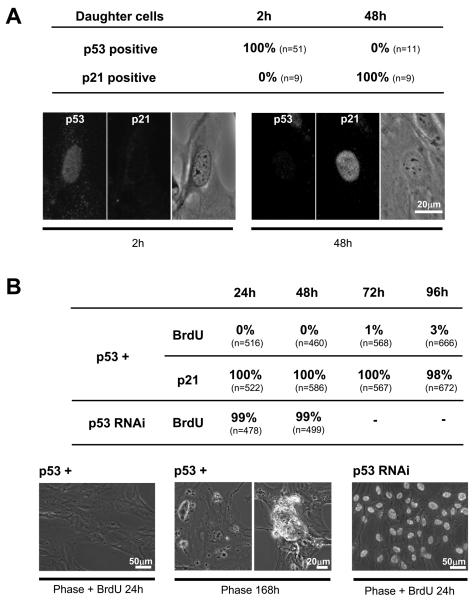
Figure 2.
A. p53 and p21 expression in the daughters of RPE1 mother cells spending >1.5 hrs in prometaphase; 0.08μM nocodazole. Individual mother cells and their progeny were continuously followed; daughter cells were fixed 2 and 48 hours after nocodazole removal. Nine cells in each category were double labeled with anti-p53 and anti-p21 antibodies; the rest were single labeled. Table: percent daughters that expressed nuclear p53 or p21 at the indicated times. Fluorescence and phase contrast images.
B. Proliferative capacity of daughter RPE1 cells cultured in dishes after their mothers were held in prometaphase for 2-6 hours by 0.08μM nocodazole. The p53+ category shows percentage of cells with BrdU incorporation and p21 expression at the indicated times after drug removal for p53 normal cells. The p53 RNAi category shows the percentages of p53 knockdown cells incorporating BrdU at the indicated times after drug removal. First image: Overlain phase contrast and BrdU fluorescence image of p53 normal cells 24 hours after drug removal. Second image panels: Phase contrast image of two fields of p53 normal cells 168 hours after drug removal showing dead cells. Third image: Overlain phase contrast and BrdU fluorescence image of p53 knockdown cells 24 hours after drug removal.
The same experiments were conducted using 100μM monastrol, a kinesin Eg5 inhibitor that prevents centrosome separation (Figure 1A). Daughters of mothers held in prometaphase for <1.5 hours progressed through interphase and divided repeatedly with normal kinetics. Daughters of mothers held in prometaphase for >1.5 hours arrested (Figure 1C); none incorporated BrdU and all tested exhibited elevated p21 expression (not shown). Primary human fibroblasts behaved the same except that prometaphase durations >2 hours caused the daughters to arrest in G1 (Figure S1C).
We prolonged prometaphase with Taxol, which delays satisfaction of the mitotic checkpoint by promoting microtubule assembly/stability[4, 13, 14]. We exposed cultures to 5μM Taxol for 10 minutes and followed individual cells as they entered mitosis. The cells took 11 to 219 minutes to satisfy the mitotic checkpoint. Mother cells spending shorter amounts of time in prometaphase produced daughters that proliferated while long times in prometaphase produced daughters that arrested in G1 as determined by lack of BrdU incorporation (Figure 1D). Two tripolar divisions were not counted.
To control for filming conditions and provide a larger sample size we treated cultures with 0.08μM nocodazole for 4 hours; shook off mitotic cells, and cultured them in drug plus BrdU for another 2 hours (prometaphase durations thus ranged 2-6 hours). After nocodazole removal, cells were cultured in dishes before fixation for BrdU and p21 immunolocalization at 24 - 96 hours. Almost all daughters arrested in G1 and expressed p21 (Figure 2B). At 168 hours (7 days) the dishes contained some dead daughter cells (Figure 2B). To test if this arrest was p53 dependent, we transfected cultures with p53 siRNA and 48 hours later treated them with nocodazole as described above. At 24 and 48 hours after drug removal 99% of the daughter cells and their progeny incorporated BrdU (Figure 2B).
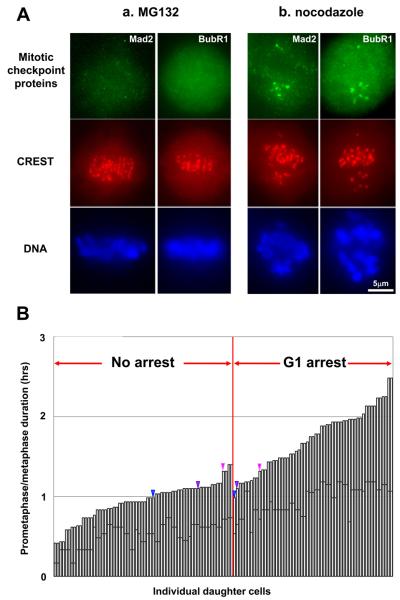
We also prolonged prometaphase with 4μM MG132, a proteasome inhibitor that blocks the metaphase-anaphase transition by preventing cyclin B destruction. Mitotic cells uniformly showed normal bipolar spindles with all chromosomes aligned on the metaphase plate within 30 minutes of drug application (Figure 1A) (also see [15]). Mad2 and BubR1 were not present at the kinetochores (Figure 3A) (Mad2 n=18; BubR1 n=21). Thus, the checkpoint is promptly satisfied in the presence of MG132. Control cells treated with 0.08μM nocodazole showed robust localization of both checkpoint proteins at the centromeric regions of monooriented chromosomes (Figure 3A). We treated cultures with 4μM MG132 for 10-70 minutes, times well short of those inducing spindle pole fragmentation [16]. We followed individual cells already in mitosis because MG132 inhibits mitotic entry. After drug removal all cells divided in a normal bipolar fashion. Prometaphase durations < ~1.5 hour allowed the daughters to proliferate while prometaphase durations > ~1 hour led to a durable G1 arrest (Figure 3B).
Figure 3.
A. The mitotic checkpoint is promptly satisfied in MG132 treated RPE1 cells. Panel (a): Cells fixed and immunostained for the checkpoint proteins Mad2 and BubR1 30 minutes after drug application. These proteins are not detectable on centromeres and all chromosomes are aligned on the metaphase plate. Panel (b): To validate our methodology cells were treated with 0.08μM nocodazole. Mad2 and BubR1 were localized to unattached kinetochores of monooriented chromosomes but not localized to bioriented chromosomes at the metaphase plate.
B. Duration of mother cell prometaphase and the proliferative capacity of their daughter cells. RPE1 cells in mitosis were held in prometaphase for an additional 10-70 minutes with 4μM MG132. In a few cases (color coded arrowheads) one daughter proliferated while its sister arrested in G1.
These results can be interpreted in two ways that are not mutually exclusive. First, the temporal separation between mitotic checkpoint satisfaction and prometaphase durations that arrest the daughters indicates that the proliferation block of the daughters can become activated after mitotic checkpoint satisfaction. Also, prolonged activity of the mitotic checkpoint in the mother cell is not required for daughter cells to arrest in G1. Second, G1 arrest of daughters after prometaphase prolongation by microtubule targeting drugs depends on the prolonged activity of the mitotic checkpoint while MG132 prolongation of prometaphase leads to a G1 arrest through a distinct mechanism, such as stress due to the lack of protein turnover.
Testing possible causes for G1 arrest after prolonged prometaphase
When cells slip out of grossly prolonged mitosis, phospho-H2AX foci, indicative of DNA damage [17], are seen in the reformed nuclei [18, 19]. We tested if prometaphase durations of <6 hours produce DNA damage that could activate p53 and arrest daughter cells. Since control cells in mitosis contained sparse phospho-H2AX foci at the chromosomes (not shown, see [18, 20]), we assayed for foci in early G1 daughter cells. Cultures were treated with 0.08 μM nocodazole for 4 hours; mitotic cells were shaken off and treated for an additional 2 hours with drug (2-6 hour prometaphase). None of the daughter cells showed nuclear phospho-H2AX foci 1 hour after drug removal even though all would have arrested in G1 had they not been fixed (Figure S2A). For cells held in 2-6 hours prometaphase with monastrol 2% of the daughters showed foci even though all would have arrested in G1 (Figure S2A). This is consistent with a report that 8 hour monastrol treatments do not lead to phospho-H2AX expression [21].
To validate our ability to detect sparse DNA damage, we irradiated control cultures for 5 seconds with UV light, shook off mitotic cells and fixed the daughters 2 hours thereafter to allow time for mitosis completion and p53 expression (see Figure 2B). Phospho-H2AX foci form and spread at sites of DNA damage and p53 expression indicates activation of the DNA damage checkpoint. Most cells showed neither phospho-H2AX foci nor nuclear p53 expression as expected from this low UV exposure (Figure S2B). Importantly, 44 cells showed just a few phospho-H2AX foci and p53 expression indicating the detectability of low levels of DNA damage (Figure S2B). The 18 cells with few phospho-H2AX foci but no p53 expression suggest that low levels of DNA damage in a mother cell may not always be sufficient to arrest its daughters in G1. This strengthens the case that after sufficiently prolonged prometaphase, the uniform G1 arrest of the daughters is independent of DNA damage.
We analyzed time lapse films of individual mother cells to determine if the time taken for the completion of mitosis correlates with the proliferative capacity of daughter cells. For daughters that arrest compared to those that proliferate, the interval from their mother cells metaphase-anaphase transition to the start of daughter cell flattening is essentially the same (Figure S2C).
Nocodazole or monastrol treatments (3-8 hours) lead to an incidence of lagging, merotelically attached chromosomes after drug removal [21, 22]. Consequent chromosome gains/losses can inhibit daughter cell proliferation[21, 23]. We treated cultures with 0.08μM nocodazole or 100μM monastrol for 6 hours and followed individual cells so we knew how long each was held in prometaphase. The drugs were washed out with fresh medium rather than phosphate buffered saline and the cultures were fixed 50-60 minutes later when cells were in late anaphase/early telophase. The 3-4% incidence of lagging chromosomes for cells held >1.5 hours in prometaphase does not explain the expected uniform G1 arrest of their daughters (Figure S3A).
We tested if increased p53 expression during prolonged mitosis[24, 25] could explain the G1 arrest of daughter cells. We found no systematic increase in p53 expression levels for cells held in prometaphase for 3.75 hours relative to those held in prometaphase for 45 minutes (Figure S3B). Nevertheless, these observations do not rule out the possibility that p53 could be activated, regardless of its expression levels, during prolonged prometaphase.
P38 activity participates in the G1 arrest of daughter cells
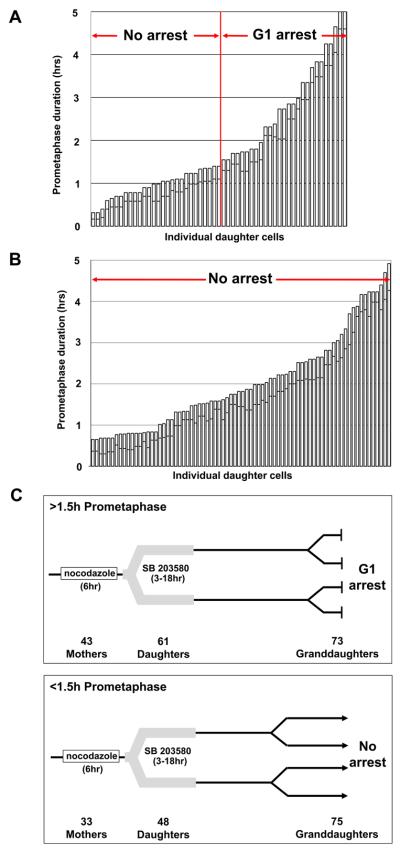
We treated cultures with 0.08 μM nocodazole plus 10μM SB 203580 and after 6 hours washed both out. SB 203580 blocks p38 activity by binding to the ATP pocket but does not prevent its activation[26]. Prometaphase durations <1.5 hours allowed daughters to proliferate while longer prometaphase durations uniformly led to a G1 arrest (Figure 4A). Thus, p38 activity during prolonged prometaphase is not required for the G1 arrest of the daughter cells. However, when we continuously applied SB 203580 just after we washed out the nocodazole, all daughters proliferated repeatedly regardless of their mothers' prometaphase durations (Figure 4B). We obtained the same results using monastrol to hold cells in prometaphase (not shown). For MG132 treated cells continuous p38 inhibition moved the break point between prometaphase durations that do and do not allow daughter cell proliferation to approximately one hour longer prometaphase times for the mother cells (Figure S3C).
Figure 4.
A. Duration of mother cell prometaphase and the proliferative capacity of the daughter cells when p38 activity is inhibited during prolonged prometaphase; 0.08μM nocodazole plus SB 203580. The p38 inhibitor was washed out concurrently with the nocodazole.
B. Duration of mother cell prometaphase and the proliferative capacity of the daughter cells when p38 activity is inhibited after prolonged prometaphase. Cells were held in prometaphase with 0.08μM nocodazole alone and SB 203580 was continuously applied after nocodazole removal.
C. Consequence of prolonged prometaphase are irreversible. We followed individual cells exposed to 0.08μM nocodazole for 6 hours. After drug removal, SB 203580 was transiently introduced for 3-18 hours to allow daughters that otherwise would have arrested in G1 to become committed to the next cell cycle. Top panel diagrammatically shows the behavior of the progeny of 43 mothers that were held in prometaphase for >1.5 hours. The number of progeny still in view is indicated. All progeny showed the same behavior. All “Granddaughters” arrested in G1 despite being born of mitoses of ~normal duration (average 24 minutes, range 10-49 minutes, from cell rounding to daughter cell flattening; controls average 27 minutes, range 18-36 minutes). Bottom panel shows behavior of the progeny of 33 same preparation mothers held in prometaphase <1.5 hours. All “Granddaughters” proliferated.
Lastly, we treated cultures with 0.08μM nocodazole for 6 hours and followed individual cells. After drug removal we transiently introduced SB 203580 to allow the daughter cells, which otherwise would have arrested in G1, to become committed to the next cell cycle. For mothers held >1.5 hours in prometaphase, all daughters remaining in view divided again with normal timing. Remarkably, all of their daughters (the “Granddaughters”) still in view arrested in G1 for at least ~149 hours despite having been born from mitoses of normal duration (Figure 4C). In the same preparations we also followed the progeny of mother cells held in prometaphase for <1.5 hours; all remaining in view divided several times before the film runs were terminated.
Our results reveal the existence of a mechanism that senses prometaphase duration, and if it lasts >~1.5 hours in RPE and >~2 hours in primary fibroblasts, an irreversible p38-p53 dependent block to daughter cell proliferation is decisively activated. What activates the p38-p53 pathway is not clear though it does not appear to be DNA damage, chromosome missegregation, increased p53 expression, or prolonged time for mitosis completion after anaphase onset. The facts that similar results were obtained with four drugs with completely different modes of action and that all cells for any given experiment were exposed to the drug for the same duration argue against the possibility that our results are due to pleiotropic effects of the drugs. Our experiments are self-controlling for drug application and transient reversion of prophase cells into interphase when exposed to microtubule poisons[27].
The cell has a remarkably limited tolerance for prometaphase duration even though the mitotic checkpoint can hold them in mitosis for 12-48 hours before slippage into interphase. Since prometaphase/metaphase for RPE1 cells lasts up to 30 minutes and at most 72 minutes for primary cells, the mechanism that senses prolonged prometaphase is not seemingly directed towards normal resolvable problems in kinetochore attachment during spindle assembly. Rather it could serve as a backup for the mitotic checkpoint to handle persistent spindle assembly defects caused by environmental toxins, which are never completely resolved but eventually allow mitotic checkpoint satisfaction[3, 4]. Short and/or multipolar spindles degrade the fidelity of chromosome distribution and cleavage, thereby compromising genomic integrity and promoting transformation[28].
Experimental procedures
Cell culture, drugs, and immunofluorescence
hTERT-RPE1 cells and human primary foreskin fibroblasts (BJ strain from American Type Culture Collection) were cultured as described previously[29]. For the transient p38 inhibitor experiments we used RPE1 cells stably expressing GFP-centrin 1 (a centriole marker, see [29]) to determine the cell cycle stage at which the granddaughter cells arrested by counting centrioles (two in G1). Nocodazole, monastrol, paclitaxel (Taxol), BrdU and SB 203580 were purchased from Sigma-Aldrich; MG132 was obtained from A. G. Scientific. Validated and annealed siRNA against p53 was purchased from Ambion (Silencer®, #51193) and diluted into a 50μM stock solution with nuclease-free water. 2μl of stock solution was mixed with 5μl of Lipofectamine (Invitrogen) and then introduced onto RPE1 cells for 2 hours; then the cells were washed and cultured for 48 hrs. For the nocodazole and monastrol experiments involving the inhibition of p38, the SB 203580 was either introduced along with the other drugs and washed out along with them or alternatively, the SB 203580 was introduced along with BrdU just after the nocodazole or monastrol were washed out.
Monoclonal anti BrdU antibody (BD Biosciences; 555627), monoclonal alpha-tubulin antibody (Sigma-Aldrich; T5168), polyclonal pericentrin antibody (Abcam; ab4448), human CREST autoimmune serum (Immunovision), monoclonal Ki-67 antibody (BD Bioscience; K72820), monoclonal p53 antibody (Santa Cruz; DO-1, sc-126), polyclonal p21 antibody (Abcam; ab796), monoclonal BubR1 antibody (BD Bioscience; 612502), and polyclonal phospho-H2AX (Ser139) antibody (Millipore; 07-164) were used as previously described[29]. Polyclonal Xenopus Mad2 antibody was a gift from Dr. Gary J. Gorbsky (University of Oklahoma Health Sciences Center). Observations were made with a Leica DMR-series microscope equipped with phase contrast and fluorescence optics. Images were recorded with a Retiga Exi camera (Qimaging Corp.) and Slidebook software (Intelligent Imaging Innovations, Inc).
Prolonging prometaphase
Coverslips bearing asynchronous cells were assembled into observation chambers [30] with medium containing 0.08μM nocodazole or 100μM monastrol, and fields of cells were continuously followed by video time lapse microscopy at 37°C for 6 hrs. After 6 hrs, the view field was marked with diamond scribe, the bottom of the observation chamber was removed, the drug washed out with fresh medium several times, and the chamber was reassembled with fresh medium containing BrdU for further observation of the same microscope fields for up to 100 hours. During the film runs, the medium was changed every two days. The duration of prometaphase is here defined as the interval from when the cells rounded up/nuclear envelope breakdown to anaphase onset as seen by chromosome disjunction or the sudden start of cell elongation. Completion of mitosis is here defined as the interval from anaphase onset to the start of daughter cell flattening. At the end of the time lapse runs, the cultures were fixed for analysis of BrdU incorporation or p53/p21 expression in the progeny of the cells previously followed in vivo. Live cell imaging was conducted with Olympus BH-2 or Leica DMRXE microscopes equipped with phase-contrast optics. Images were taken with Orca ER, Orca 100 (Hamamatsu), and Retiga EXi cameras (Qimaging), acquired with Simple PCI software (Hamamatsu), and exported as AVI movies.
For the Taxol experiments, asynchronous cells on coverslips were exposed to 5μM Taxol for 10 minutes; the drug was washed out and replaced with medium containing BrdU; and individual cells were followed by time lapse video microscopy for up to 100 hours at 37°C. For the MG132 experiments, asynchronous cells on coverslips were exposed to 4μM MG132 for 12-70 min; the drug was washed out with medium containing BrdU; and individual cells were followed by time lapse video microscopy for up to 100 hours. Since 4μM MG132 blocks cells from entering mitosis, we followed only those cells already in mitosis. At the end of the time lapse runs, the cultures were fixed for analysis of BrdU incorporation in the progeny of the cells previously followed in vivo.
Highlights.
Our results reveal the cell has a mechanism that senses prometaphase duration
Prometaphase >1.5 hr irreversibly blocks daughter cell proliferation despite normal division of their mothers
Block to daughter cell proliferation is p38 and p53 dependent
This block protects against spindle defects that allow mitotic checkpoint satisfaction
Supplementary Material
Acknowledgements
We first thank Dan McCollum for useful discussions and comments on the manuscript. We are also grateful to Gary Gorbsky and Steve Doxsey for providing antibodies. We also thank Jason Swedlow, Joshua Nordberg and Eric Veien for advice and assistance with technical matters. This work was supported by National Institute of Health GM30758 to GS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rieder CL, Cole RW, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 3.Brito DA, Yang Z, Rieder CL. Microtubules do not promote mitotic slippage when the spindle assembly checkpoint cannot be satisfied. J Cell Biol. 2008;182:623–629. doi: 10.1083/jcb.200805072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Z, Kenny AE, Brito DA, Rieder CL. Cells satisfy the mitotic checkpoint in Taxol, and do so faster in concentrations that stabilize syntelic attachments. J Cell Biol. 2009;186:675–684. doi: 10.1083/jcb.200906150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brito DA, Rieder CL. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr Biol. 2006;16:1194–1200. doi: 10.1016/j.cub.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cross SM, Sanchez CA, Morgan CA, Schimke MK, Ramel S, Idzerda RL, Raskind WH, Reid BJ. A p53-dependent mouse spindle checkpoint. Science. 1995;267:1353–1356. doi: 10.1126/science.7871434. [DOI] [PubMed] [Google Scholar]
- 7.Lanni JS, Jacks T. Characterization of the p53-dependent postmitotic checkpoint following spindle disruption. Mol Cell Biol. 1998;18:1055–1064. doi: 10.1128/mcb.18.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minn AJ, Boise LH, Thompson CB. Expression of Bcl-xL and loss of p53 can cooperate to overcome a cell cycle checkpoint induced by mitotic spindle damage. Genes Dev. 1996;10:2621–2631. doi: 10.1101/gad.10.20.2621. [DOI] [PubMed] [Google Scholar]
- 9.Demidenko ZN, Kalurupalle S, Hanko C, Lim CU, Broude E, Blagosklonny MV. Mechanism of G1-like arrest by low concentrations of paclitaxel: next cell cycle p53-dependent arrest with sub G1 DNA content mediated by prolonged mitosis. Oncogene. 2008;27:4402–4410. doi: 10.1038/onc.2008.82. [DOI] [PubMed] [Google Scholar]
- 10.Ciciarello M, Mangiacasale R, Casenghi M, Zaira Limongi M, D'Angelo M, Soddu S, Lavia P, Cundari E. p53 displacement from centrosomes and p53-mediated G1 arrest following transient inhibition of the mitotic spindle. J Biol Chem. 2001;276:19205–19213. doi: 10.1074/jbc.M009528200. [DOI] [PubMed] [Google Scholar]
- 11.Tritarelli A, Oricchio E, Ciciarello M, Mangiacasale R, Palena A, Lavia P, Soddu S, Cundari E. p53 localization at centrosomes during mitosis and postmitotic checkpoint are ATM-dependent and require serine 15 phosphorylation. Mol Biol Cell. 2004;15:3751–3757. doi: 10.1091/mbc.E03-12-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannakakou P, Robey R, Fojo T, Blagosklonny MV. Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: molecular determinants of paclitaxel-induced cytotoxicity. Oncogene. 2001;20:3806–3813. doi: 10.1038/sj.onc.1204487. [DOI] [PubMed] [Google Scholar]
- 13.Brito DA, Rieder CL. The ability to survive mitosis in the presence of microtubule poisons differs significantly between human nontransformed (RPE-1) and cancer (U2OS, HeLa) cells. Cell Motil Cytoskeleton. 2009;66:437–447. doi: 10.1002/cm.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikui AE, Yang CP, Matsumoto T, Horwitz SB. Low concentrations of taxol cause mitotic delay followed by premature dissociation of p55CDC from Mad2 and BubR1 and abrogation of the spindle checkpoint, leading to aneuploidy. Cell Cycle. 2005;4:1385–1388. doi: 10.4161/cc.4.10.2061. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman DB, Pearson CG, Yen TJ, Howell BJ, Salmon ED. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol Biol Cell. 2001;12:1995–2009. doi: 10.1091/mbc.12.7.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrhardt AG, Sluder G. Spindle pole fragmentation due to proteasome inhibition. J Cell Physiol. 2005;204:808–818. doi: 10.1002/jcp.20335. [DOI] [PubMed] [Google Scholar]
- 17.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 18.Dalton WB, Nandan MO, Moore RT, Yang VW. Human cancer cells commonly acquire DNA damage during mitotic arrest. Cancer Res. 2007;67:11487–11492. doi: 10.1158/0008-5472.CAN-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quignon F, Rozier L, Lachages AM, Bieth A, Simili M, Debatisse M. Sustained mitotic block elicits DNA breaks: one-step alteration of ploidy and chromosome integrity in mammalian cells. Oncogene. 2007;26:165–172. doi: 10.1038/sj.onc.1209787. [DOI] [PubMed] [Google Scholar]
- 20.Ichijima Y, Sakasai R, Okita N, Asahina K, Mizutani S, Teraoka H. Phosphorylation of histone H2AX at M phase in human cells without DNA damage response. Biochem Biophys Res Commun. 2005;336:807–812. doi: 10.1016/j.bbrc.2005.08.164. [DOI] [PubMed] [Google Scholar]
- 21.Thompson SL, Compton DA. Examining the link between chromosomal instability and aneuploidy in human cells. J Cell Biol. 2008;180:665–672. doi: 10.1083/jcb.200712029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Cell Biol. 2001;153:517–527. doi: 10.1083/jcb.153.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson SL, Compton DA. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J Cell Biol. 2010;188:369–381. doi: 10.1083/jcb.200905057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blagosklonny MV. Prolonged mitosis versus tetraploid checkpoint: how p53 measures the duration of mitosis. Cell Cycle. 2006;5:971–975. doi: 10.4161/cc.5.9.2711. [DOI] [PubMed] [Google Scholar]
- 25.Blagosklonny MV. Mitotic arrest and cell fate: why and how mitotic inhibition of transcription drives mutually exclusive events. Cell Cycle. 2007;6:70–74. doi: 10.4161/cc.6.1.3682. [DOI] [PubMed] [Google Scholar]
- 26.Lee JC, Kumar S, Griswold DE, Underwood DC, Votta BJ, Adams JL. Inhibition of p38 MAP kinase as a therapeutic strategy. Immunopharmacology. 2000;47:185–201. doi: 10.1016/s0162-3109(00)00206-x. [DOI] [PubMed] [Google Scholar]
- 27.Rieder CL, Cole R. Microtubule disassembly delays the G2-M transition in vertebrates. Curr Biol. 2000;10:1067–1070. doi: 10.1016/s0960-9822(00)00678-3. [DOI] [PubMed] [Google Scholar]
- 28.Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Uetake Y, Loncarek J, Nordberg JJ, English CN, La Terra S, Khodjakov A, Sluder G. Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J Cell Biol. 2007;176:173–182. doi: 10.1083/jcb.200607073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uetake Y, Sluder G. Cell-cycle progression without an intact microtuble cytoskeleton. Curr Biol. 2007;17:2081–2086. doi: 10.1016/j.cub.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.