Introduction
Specialized cell polarity is a hallmark of neurons – axons and dendrites differ in their morphology, the proteins that they contain and their electrical properties. However, the mechanisms that regulate neuronal polarity must operate differently in neurons that release transmitter from their dendrites, such as mitral and granule cells of the vertebrate olfactory bulb. In these neurons, both axons and dendrites contain vesicles and the proteins responsible for calcium-dependent vesicle fusion. Recent work has provided considerable insight into the mechanisms neurons use to establish polarity. Here we discuss how analysis of the exceptional case of neurons that release transmitter dendritically may shed further light on the molecular and functional mechanisms that generate neuronal polarization.
Cell polarity in neurons
Many of the molecular differences between axons and dendrites can be explained by the function of these two cellular compartments as pre- and postsynaptic structures, respectively. The idea of functional specialization (that dendrites receive input and axons transmit output) was first stated by Cajal as his law of dynamic polarization and is a key principle in neuroscience. However, a number of neuron types are now known to violate this usual rules of neuronal polarity in that they release neurotransmitter from their dendrites [1;2]. Olfactory bulb mitral and granule cells, thalamic interneurons, dentate granule cells, substantia nigra dopamine neurons, auditory brainstem neurons, cortical pyramidal cells, and others all have been shown to release various transmitters from dendrites [1;2]. This observation leads to two questions about basic neuronal biology that will be discussed below: Do dendritic and axonal release use common mechanisms, and if so, how do the normal mechanisms of polarity differ in these cells to allow dendrites to provide functional output? We propose that studying the physiology as well as the cell biology and development of these ‘exceptions’ may illuminate general principles of functional specialization.
Physiology and cell biology of dendrodendritic synapses
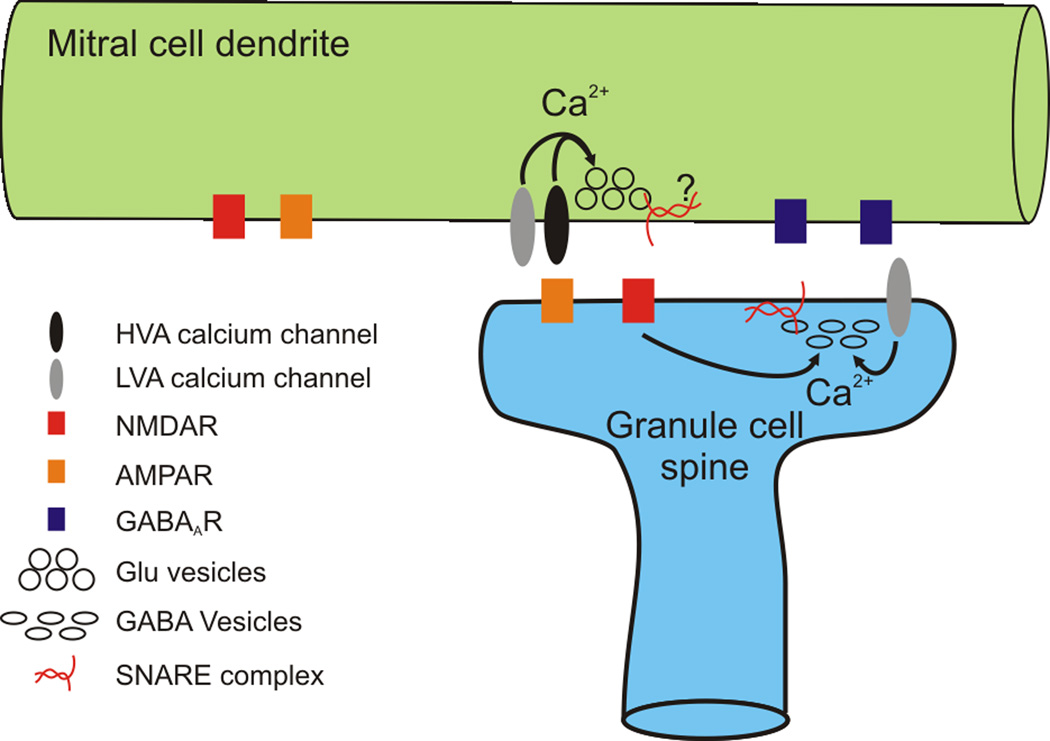
Nowhere in the brain is dendritic release of transmitter as prominent as in the vertebrate olfactory bulb [1;3–5]. The major classes of excitatory neurons (mitral and tufted cells) and inhibitory neurons (granule cells) in main and accessory olfactory bulbs release neurotransmitters (glutamate and GABA respectively) from hundreds of sites along their dendrites [6–8]. This release occurs at highly specialized reciprocal dendrodendritic synapses (figure 1). Mitral cell dendrites make excitatory synapses onto granule cell spines, which in turn form inhibitory synapses back onto the same dendritic shaft [9]. The mechanisms of transmitter release at both these synapses are thought to be in many respects similar to those of classical synapses made by axons (see below). Indeed, mitral cells also release transmitter from conventional axonal synapses, although the mechanisms of transmitter release at these synapses have not been well characterized. Interestingly, granule cells do not have axons at all and so all of the output that they provide is via dendritic release sites [1]. Below we will discuss the physiology and cell biology of these atypical synapses, highlighting those features that may be exploited to provide insight into the development and function of synapses across the brain.
Figure.
Synaptic transmission at olfactory bulb reciprocal dendrodendritic synapses shares many properties with release from axon terminals. Clusters of vesicles containing glutamate are found in mitral cell dendrites and are associated with asymmetric synaptic junctions [3;9]. Release of glutamate from these dendritic sites depends strongly on elevation of intracellular calcium **[10–12]. Action potentials propagate in the dendrites of mitral cells *[13;14] some of which have remarkably high sodium channel densities [14], and trigger release by opening of high voltage activated calcium channels, specifically those of the P/Q type** [10;15]. Paired intracellular recordings between dendritically coupled mitral cells and from mitral cells synapsing onto granule cells have shown that action potentials elicit short latency EPSCs that are essentially indistinguishable from those observed in cells coupled by standard axodendritic synapses [16–19]. Release from mitral cells also can be measured by detecting currents through NMDA and AMPA receptors localized on the same dendrite as the release sites, allowing recording of autapse-like responses [12;20;21]. This approach also has demonstrated that glutamate release from mitral cells results in fast, short latency EPSPs[21;22]. Extracellular stimulation of mitral cells while recording from granule cells suggests that release from mitral cell dendrites exhibits short term depression of a magnitude and time course similar to that observed at axonal synapses [23].
Interestingly, even though many properties of dendritic output are consistent with the established paradigm for axonal release (and may involve similar molecular mechanisms), dendritic release can also be triggered by a variety of subthreshold events ** [24–26]. Such sensitivity to dendritic depolarization may allow dendritic release to be coupled to local subthreshold excitatory input, effectively giving a single neuron multiple outputs that can be regulated independently . Release rates during subthreshold depolarization are enhanced when paired with activation of group I metabotropic glutamate receptors** [24]. While such asynchronous release is not typically observed at axonal release sites, recent experiments have shown that subthreshold depolarizations can modulate the probability of action potential-dependent release from axons of cortical neurons [27–29]. In mitral cells, asynchronous release depends on elevation of intracellular free calcium and is coupled to opening of low voltage activated (LVA) calcium channels such as T-type calcium channels. Subthreshold release has been studied in mitral cells of the mouse accessory olfactory bulb (AOB), which also form reciprocal synapses with granule cells **[24]. Group I mGluRs also increase LVA calcium currents in mitral cells of the main olfactory bulb, although subthreshold release has not yet been reported from these cells [30]. Interestingly AOB mitral cells specifically contain very high levels of doc2b mRNA (Allen Brain Atlas). The doc2b protein has been proposed as a mediator of calcium-dependent spontaneous release. Doc2b has very high calcium affinity (~200 nM) and competes with synaptotagmin at snare complexes to cause vesicle fusion [31]. The potential coupling of axonal release to such high-affinity sensors is of considerable interest, especially in light of recent reports showing slow and sustained calcium elevations in axons evoked by subthreshold synaptic input [32]. One possibility is that like their dendritic counterparts, axons can signal in a variety of modes ranging from tonic and asynchronous to highly phasic output that is tightly coupled to spikes. If the relative efficacy of these modes is subject to modulation, neurons may be able to alternate between analog (subthreshold) and digital (pulse-coupled) signaling at both axons and dendrites.
On the other side of olfactory bulb reciprocal dendrodendritic synapses, the release of GABA from granule cell dendrites occurs at sites on large dendritic spines (figure 1). This release is calciumdependent and is believed to occur in two modes: global and local [33]. Global release, likely mediated by T-type calcium channels, [8;34] occurs when these cells fire sodium and/or calcium spikes. Despite the fact that they have no axons, granule cells do express sodium channels and fire action potentials. These action potentials backpropagate extensively in granule cell dendrites [34] and result in calcium influx that is coupled to release[35]. Such robust dendritic excitability has been observed in other presynaptic dendrites [36;37], and may be generally important for dendritic release. This raises the interesting question of whether local excitability (dendritic or axonal) and release competence are coregulated at the molecular level. Under one attractively simple hypothesis, the recruitment and/or insertion of specific voltage gated channels and presynaptic machinery may be coupled, providing a simple way to ensure that distal release sites have local sources of depolarization and calcium. The local mode of granule cell release occurs when excitatory input from mitral cells to single granule cell spines causes depolarization in a single spine that then initiates GABA release [33;38]. This release may depend in part on calcium influx by NMDA receptors[39;40] and possibly also mGluRs [25;41].
Despite being axonless, granule cells do express transcripts for proteins that are typically associated with axonal release, including synaptotagmin I [42]. Since these proteins cannot be involved in axonal release in these cells, they presumably play a role in dendritic release. Comparisons of mechanisms involved in dendritic and axonal GABA release are difficult because few details are available, but most data are consistent with dendritic release of GABA from olfactory bulb granule cells being mechanistically very similar to GABA release from axonal synapses made by interneurons in other brain areas *[43]. The fact that all neurotransmitter release from granule cells is mediated by their dendrites has recently allowed for the investigation of the behavioral consequences of manipulating dendritic release ** [44]. This work has demonstrated that enhancing or reducing dendritc release from a subset of granule cells alters both mitral cell inhibition and odor discrimination time. This is the first demonstration of a measurable behavioral effect of a specific manipulation of dendritic release.
Cell polarity and dendritic release
Establishing cell polarity depends critically on differential localization of proteins. For neurons in particular, functional polarization requires efficient targeting of proteins involved in neurotransmitter release to axons and those involved in detection of neurotransmitter to dendrites. This targeting results in the specific accumulation of relevant proteins to presynaptic release sites such as synaptic boutons and to postsynaptic sites such as dendritic spines [45]. Until recently, our understanding of how neuronal cell polarity is established was largely based on experiments performed in young cultured neurons in which proteins were identified whose localization was an early predictor of whether a neurite would become an axon or a dendrite. This approach led to the identification of kinesins *[46;47] and the kinase GSKβ [48] as playing an important role in the early stages of neuronal polarization. This model has suggested that selection of one neurite from the group of immature neurites as the early axon is a stochastic process. The connection between these earliest symmetry breaking events and the subsequent targeting of proteins important in synaptic function is not well understood. Recently, limitations of the study of polarity in cultured neurons have become apparent, and the importance of local environmental cues encountered by migrating neurons has been highlighted. This in vivo work also has identified the PAR3/PAR6 complex and the serine/threonine kinase LRK1 as playing critical roles in specifying polarity of pyramidal neurons in cortex [49] and highlighted the relationship between migration, cell shape and specification of the early axon. Essentially this work has shown that the trailing process of a migrating neuron is already beginning to differentiate into an axon.
A missing link in much of this analysis has been an understanding of how the molecular factors that contribute to morphological and molecular polarity lead ultimately to functional polarity. For example, how is the localization of kinesin linked to the redistribution of synaptic vesicle associated proteins? Similarly, how is targeting of receptors and PSD proteins linked to the early stages of specification of dendritic polarity? Do the mechanisms involved in these processes cause exclusion of inappropriate proteins or promote the delivery of appropriate ones? Possible clues to this linkage have been provided by recent work showing that netrin signaling results in specific exclusion of synaptic vesicle associated proteins from regions of dendrite in c. eleganssensory neurons **[50]. This work shows the linkage between morphological development and localized exclusion of presynaptic proteins and suggests that analyzing specification of cell polarity in vivo will be critical. A second clue arises from the observation that knockout of the axon initial segment protein AnkyrinG results in Purkinje cell axons developing spines and postsynaptic densities [51]. These spines are innervated by presynaptic boutons and apparently form functional synapses. This suggests that AnkyrinG normally functions to excluding dendritic proteins from axons.
Despite progress in this work on the development of neuronal polarity, the connection between these mechanisms and the formation of functional synapses remains incomplete. Because of their unusual mixed polarity, neurons that release transmitter from their dendrites, especially those that do so using the standard vesicle fusion apparatus may be very useful model systems for analyzing the connection between initial specification of polarity and functional specialization. In this regard, we propose several key questions for future work on dendrodendritic synapses:
-
-
To what extent do mitral and granule cell dendrites use the same vesicle release and recycling machinery typically found at axonal release sites?
-
-
Are any of the molecular markers of axonal structure or axonal transport found in mitral and granule cell dendrites? Identifying these markers may give clues to how trafficking of the synaptic vesicles release machinery is directed to dendrites in these neurons.
-
-
How and when are polarity decisions made in neurons that release transmitter from their dendrites?
Conclusions
Synapses are complex structures, the assembly and maintenance of which requires delivery of specific protein complexes to both pre and postsynaptic elements and this process is critical to neuronal polarization [52]. Much of this analysis of the signaling and protein trafficking associated with synapse formation has been performed in the context of neuronal polarity. However, the links between the specification of axon/dendrite polarity and the functional assembly of synapses are not very clear. The analysis of neurons having dendritic release sites, such as olfactory bulb mitral and granule cells, may allow the key links between molecular and functional polarity to be understood.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nathaniel N. Urban, Department of Biological Sciences, Center for the Neural Basis of Cognition Carnegie Mellon University
Jason B Castro, Center for Neuroscience and Center for the Neural Basis of Cognition, University of Pittsburgh.
References List
- 1.Margrie TW, Urban NN. Dendrites as Transmitters. In: Stuart GJ, Spruston N, Hausser M, editors. Dendrites. edn 2. Oxford University Press; 2007. [Google Scholar]
- 2.Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 3.Rall W, Shepherd GM, Reese TS, Brightman MW. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exp.Neurol. 1966;14:44–56. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- 4.Jahr CE, Nicoll RA. Dendrodendritic inhibition: demonstration with intracellular recording. Science. 1980;207:1473–1475. doi: 10.1126/science.7361098. [DOI] [PubMed] [Google Scholar]
- 5.Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci. 2003;26:501–506. doi: 10.1016/S0166-2236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- 6.Price JL, Powell TP. The mitral and short axon cells of the olfactory bulb. J.Cell Sci. 1970;7:631–651. doi: 10.1242/jcs.7.3.631. [DOI] [PubMed] [Google Scholar]
- 7.Woolf TB, Shepherd GM, Greer CA. Serial reconstructions of granule cell spines in the mammalian olfactory bulb. Synapse. 1991;7:181–192. doi: 10.1002/syn.890070303. [DOI] [PubMed] [Google Scholar]
- 8.Egger V, Urban NN. Dynamic connectivity in the mitral cell-granule cell microcircuit. Seminars in Cell and Developmental Biology. 2006:17. doi: 10.1016/j.semcdb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Price JL, Powell TP. The synaptology of the granule cells of the olfactory bulb. J.Cell Sci. 1970;7:125–155. doi: 10.1242/jcs.7.1.125. [DOI] [PubMed] [Google Scholar]
- 10. Isaacson JS, Strowbridge BW. Olfactory reciprocal synapses: dendritic signaling in the CNS. Neuron. 1998;20:749–761. doi: 10.1016/s0896-6273(00)81013-2. This paper provides the first detailed description of the properties of dendritic release in the olfactory bulb and establishes that dendritic release in this circuit is in many says similar to classical release from axons.
- 11.Isaacson JS. Mechanisms governing dendritic gamma-aminobutyric acid (GABA) release in the rat olfactory bulb. Proc.Natl.Acad.Sci.U.S.A. 2001;98:337–342. doi: 10.1073/pnas.021445798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaacson JS. Glutamate spillover mediates excitatory transmission in the rat olfactory bulb [see comments] Neuron. 1999;23:377–384. doi: 10.1016/s0896-6273(00)80787-4. [DOI] [PubMed] [Google Scholar]
- 13.Chen WR, Midtgaard J, Shepherd GM. Forward and backward propagation of dendritic impulses and their synaptic control in mitral cells. Science. 1997;278:463–467. doi: 10.1126/science.278.5337.463. [DOI] [PubMed] [Google Scholar]
- 14.Bischofberger J, Jonas P. Action potential propagation into the presynaptic dendrites of rat mitral cells. J.Physiol. 1997;504(Pt 2):359–365. doi: 10.1111/j.1469-7793.1997.359be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong W, Chen WR. Dynamic gating of spike propagation in the mitral cell lateral dendrites. Neuron. 2002;34:115–126. doi: 10.1016/s0896-6273(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 16.Urban NN, Sakmann B. Reciprocal intraglomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells. J.Physiol. 2002;542:355–367. doi: 10.1113/jphysiol.2001.013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoppa NE, Westbrook GL. AMPA autoreceptors drive correlated spiking in olfactory bulb glomeruli. Nat.Neurosci. 2002;5:1194–1202. doi: 10.1038/nn953. [DOI] [PubMed] [Google Scholar]
- 18.Pimentel DO, Margrie TW. Glutamatergic transmission and plasticity between olfactory bulb mitral cells. J.Physiol. 2008;586:2107–2119. doi: 10.1113/jphysiol.2007.149575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma J, Lowe G. Calcium permeable AMPA receptors and autoreceptors in external tufted cells of rat olfactory bulb. Neuroscience. 2007;144:1094–1108. doi: 10.1016/j.neuroscience.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicoll RA, Jahr CE. Self-excitation of olfactory bulb neurones. Nature. 1982;296:441–444. doi: 10.1038/296441a0. [DOI] [PubMed] [Google Scholar]
- 21.Margrie TW, Sakmann B, Urban NN. Action potential propagation in mitral cell lateral dendrites is decremental and controls recurrent and lateral inhibition in the mammalian olfactory bulb. Proc.Natl.Acad.Sci.U.S.A. 2001;98:319–324. doi: 10.1073/pnas.011523098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy GJ, Darcy DP, Isaacson JS. Intraglomerular inhibition: signaling mechanisms of an olfactory microcircuit. Nat.Neurosci. 2005;8:354–364. doi: 10.1038/nn1403. [DOI] [PubMed] [Google Scholar]
- 23.Dietz SB, Murthy VN. Contrasting short-term plasticity at two sides of the mitral-granule reciprocal synapse in the mammalian olfactory bulb. J.Physiol. 2005;569:475–488. doi: 10.1113/jphysiol.2005.095844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castro JB, Urban NN. Subthreshold glutamate release from mitral cell dendrites. J.Neurosci. 2009;29:7023–7030. doi: 10.1523/JNEUROSCI.5606-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castro JB, Hovis KR, Urban NN. Recurrent dendrodendritic inhibition of accessory olfactory bulb mitral cells requires activation of group I metabotropic glutamate receptors. J.Neurosci. 2007;27:5664–5671. doi: 10.1523/JNEUROSCI.0613-07.2007. This paper describes a novel form of subthreshold release from dendrites that is enhanced by activation of group I mGluRs and is coupled to activation of T-type calcium channels.
- 26.Heinbockel T, Laaris N, Ennis M. Metabotropic glutamate receptors in the main olfactory bulb drive granule cell-mediated inhibition. J.Neurophysiol. 2007;97:858–870. doi: 10.1152/jn.00884.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alle H, Geiger JR. Analog signalling in mammalian cortical axons. Curr.Opin.Neurobiol. 2008;18:314–320. doi: 10.1016/j.conb.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. doi: 10.1126/science.1119055. [DOI] [PubMed] [Google Scholar]
- 29.Shu Y, Yu Y, Yang J, McCormick DA. Selective control of cortical axonal spikes by a slowly inactivating K+ current. Proc.Natl.Acad.Sci.U.S.A. 2007;104:11453–11458. doi: 10.1073/pnas.0702041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston J, Delaney K. Synaptic activation of T-type Ca2+ channels via mGluR activation in the primary dendrite of mitral cells. J.Neurophysiol. 2010 doi: 10.1152/jn.00796.2009. [DOI] [PubMed] [Google Scholar]
- 31.Groffen AJ, Martens S, Diez AR, Cornelisse LN, Lozovaya N, de Jong AP, Goriounova NA, Habets RL, Takai Y, Borst JG, Brose N, McMahon HT, Verhage M. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science. 2010;327:1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christie JM, Jahr CE. Dendritic NMDA receptors activate axonal calcium channels. Neuron. 2008;60:298–307. doi: 10.1016/j.neuron.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egger V, Svoboda K, Mainen ZF. Dendrodendritic synaptic signals in olfactory bulb granule cells: local spine boost and global low-threshold spike. J.Neurosci. 2005;25:3521–3530. doi: 10.1523/JNEUROSCI.4746-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egger V, Svoboda K, Mainen ZF. Mechanisms of lateral inhibition in the olfactory bulb: efficiency and modulation of spike-evoked calcium influx into granule cells. J.Neurosci. 2003;23:7551–7558. doi: 10.1523/JNEUROSCI.23-20-07551.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egger V, Svoboda K, Mainen ZF. Dendrodendritic synaptic signals in olfactory bulb granule cells: local spine boost and global low-threshold spike. J.Neurosci. 2005;25:3521–3530. doi: 10.1523/JNEUROSCI.4746-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bischofberger J, Jonas P. Action potential propagation into the presynaptic dendrites of rat mitral cells. J.Physiol.(Lond.) 1997;504:359–365. doi: 10.1111/j.1469-7793.1997.359be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hausser M, Stuart G, Racca C, Sakmann B. Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron. 1995;15:637–647. doi: 10.1016/0896-6273(95)90152-3. [DOI] [PubMed] [Google Scholar]
- 38.Zelles T, Boyd JD, Hardy AB, Delaney KR. Branch-specific Ca2+ influx from Na+-dependent dendritic spikes in olfactory granule cells. J.Neurosci. 2006;26:30–40. doi: 10.1523/JNEUROSCI.1419-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen WR, Xiong W, Shepherd GM. Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron. 2000;25:625–633. doi: 10.1016/s0896-6273(00)81065-x. [DOI] [PubMed] [Google Scholar]
- 40.Halabisky B, Friedman D, Radojicic M, Strowbridge BW. Calcium influx through NMDA receptors directly evokes GABA release in olfactory bulb granule cells. J.Neurosci. 2000;20:5124–5134. doi: 10.1523/JNEUROSCI.20-13-05124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong HW, Hayar A, Ennis M. Activation of group I metabotropic glutamate receptors on main olfactory bulb granule cells and periglomerular cells enhances synaptic inhibition of mitral cells. J.Neurosci. 2007;27:5654–5663. doi: 10.1523/JNEUROSCI.5495-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berton F, Iborra C, Boudier JA, Seagar MJ, Marqueze B. Developmental regulation of synaptotagmin I, II, III, and IV mRNAs in the rat CNS. J.Neurosci. 1997;17:1206–1216. doi: 10.1523/JNEUROSCI.17-04-01206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron. 2008;57:536–545. doi: 10.1016/j.neuron.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 44. Abraham NM, Egger V, Shimshek DR, Renden R, Fukunaga I, Sprengel R, Seeburg PH, Klugmann M, Margrie TW, Schaefer AT, Kuner T. Synaptic inhibition in the olfactory bulb accelerates odor discrimination in mice. Neuron. 2010;65:399–411. doi: 10.1016/j.neuron.2010.01.009. In this paper, the authors selectively interfere with and augment excitabillity of olfactory bulb granule cells and show specific effects on odor discrimination time. This is the first demonstration of a specific functional role of dendritic release in a vertebrate system.
- 45.Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron. 2000;26:465–472. doi: 10.1016/s0896-6273(00)81178-2. [DOI] [PubMed] [Google Scholar]
- 46.Jacobson C, Schnapp B, Banker GA. A change in the selective translocation of the Kinesin-1 motor domain marks the initial specification of the axon. Neuron. 2006;49:797–804. doi: 10.1016/j.neuron.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Lewis TL, Jr, Mao T, Svoboda K, Arnold DB. Myosin-dependent targeting of transmembrane proteins to neuronal dendrites. Nat.Neurosci. 2009;12:568–576. doi: 10.1038/nn.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Barnes AP, Solecki D, Polleux F. New insights into the molecular mechanisms specifying neuronal polarity in vivo. Curr.Opin.Neurobiol. 2008;18:44–52. doi: 10.1016/j.conb.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poon VY, Klassen MP, Shen K. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature. 2008;455:669–673. doi: 10.1038/nature07291. In this paper the authors show that UNC-6/Netrin signaling controls both neurite guidance and also molecular/functional polarity. This demonstrates an important coordination between between guidance/trophic factors and functional polarity.
- 51.Sobotzik JM, Sie JM, Politi C, Del TD, Bennett V, Deller T, Schultz C. AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proc.Natl.Acad.Sci.U.S.A. 2009;106:17564–17569. doi: 10.1073/pnas.0909267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat.Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]