Summary
Plants produce naturally occurring insect repellents, such as citronellal, which is the main component of citronellal oil and is among the most widely-used-naturally-occurring insect repellents. However, the molecular pathways through which insects sense botanical repellents are unknown. Here, we showed that Drosophila used two pathways for direct avoidance of citronellal. The olfactory co-receptor, Or83b, which is required for the response to the synthetic repellent DEET, contributed to citronellal repulsion, and was essential for citronellal-evoked action potentials. Mutations affecting the Ca2+-permeable cation channel, TRPA1 resulted in a comparable defect in avoiding citronellal vapor. The TRPA1-dependent aversion to citronellal relied on a G protein/phospholipase C (PLC) signaling cascade, rather than direct detection of citronellal by TRPA1. Loss of TRPA1, Gq or PLC caused an increase in the frequency of citronellal-evoked action potentials in olfactory receptor neurons. Absence of the Ca2+-activated K+ channel, Slowpoke, resulted in a similar impairment in citronellal avoidance, and an increase in the frequency of action potentials. These results suggest that TRPA1 is required for activation of a BK channel to modulate citronellal-evoked action potentials, and for aversion to citronellal. In contrast to Drosophila TRPA1, Anopheles gambiae TRPA1 was directly and potently activated by citronellal, thereby raising the possibility that mosquito TRPA1 may be a target for developing improved repellents to reduce insect-borne diseases such as malaria.
In Drosophila, a trap-based assay has been employed to investigate the mechanism of action of the most commonly used man-made repellent, DEET [1, 2]. The assay does not monitor behavioral avoidance of a volatile repellent. Rather, it tests for inhibition of the attraction to food odors.
To measure repulsion to chemical vapors, we developed a binary-choice assay (Figure S1A). We applied a repellent and a control chemical to the bottoms of two test tubes, inserted screens to prevent physical contact with the compounds and introduced ~100 flies into the connected tubes. After an incubation period, we counted the flies in the two test zones, and calculated the avoidance index (A.I.). This “direct airborne repellent test” (DART) has two advantages over the trap assay. First, it measures direct repulsion to an airborne chemical vapor. Second, it monitors the response to a repellent in minutes, compared to 24 – 72 hours used in the trap assay [1].
To evaluate the DART assay, we tested benzaldehyde. This odorant caused dose-dependent avoidance, while the vehicle (DMSO) or a non-volatile aversive tastant (quinine) did not (Figure S1C). Thus, the DART assay effectively measures evasive behavior, rather than the masking of a food odor. Benzaldehyde (0.1 or 1%) elicited avoidance within 15 minutes, and the A.I.s peaked between 30 and 60 minutes (Figure S1D). After four hours, there was still strong avoidance, which then declined, possibly due to desensitization or a reduction in the chemical gradient.
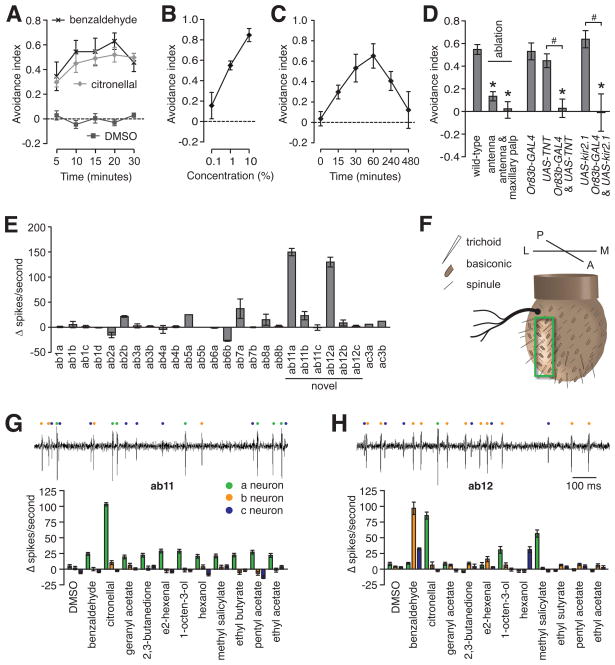
Citronellal is used commonly in lotions, sprays and candles to repel mosquitoes, fleas and ticks [3]. Indeed, using a variation of the DART assay (Figure S1B), Anopheles gambiae avoided citronellal and benzaldehyde (Figure 1A). To address whether citronellal repels Drosophila, we performed the DART assay. We found that flies avoided citronellal in a dose-dependent manner (Figure 1B), and there was no difference between males and females (Figure S1E). As with benzaldehyde, the aversion to citronellal peaked between 30 and 60 minutes (Figure 1C).
Figure 1.
Avoidance to airborne citronellal vapor. (A) Variation of DART assay (Figure S1B) used to assay repulsion of female Anopheles gambiae to citronellal and benzaldehyde (10% each). (B) Avoidance to 0.1 – 10% citronellal vapor. (C) Time-dependence for avoidance to 1% citronellal. (D) Requirement of antenna and Or83b-expressing ORNs for citronellal repulsion. The asterisks indicate significant differences from the wild-type control (ANOVA, p<0.05). (E) Survey of SSR responses of the chemosensory neurons in the 3rd antennal segment to 10% citronellal. (F) Cartoon indicating location of novel ab11 and ab12 sensilla (green box). (G and H) Representative SSR traces of spontaneous activity demonstrating presence of 3 ORNs (top) and unique odorant response profiles for ab11 (G) and ab12 (H) sensilla to 1% odorants (bottom). Mean ±SEMs are shown. The number (n) of experiments for the SSRs shown in Figures 1E, 1G and 1H are indicated in Table S1.
The synthetic repellent DEET has been reported to function through masking the response to attractive odors in a trap assay [2]. Addition of DEET to the entrance of a food-containing vial results in a larger proportion of flies selecting the food vial without the DEET [2] (Figure S1F). 1% citronellal also reduced the attractive response to food in the trap and DART assays (Figures S1F and G). As with citronellal, DEET functioned as a direct repellent using the DART assay (Figure S1H).
Citronellal avoidance was through airborne vapor rather than contact chemosensation, since a screen separated the flies and the repellent (Figure S1A). The hair-like sensilla that house the olfactory receptor neurons (ORNs) are located on the main olfactory organ, the antenna, and the maxillary palp [4, 5]. Consistent with the detection of citronellal through the sense of smell, animals with ablated antenna and maxillary palps, or antennal only, showed dramatic decreases in avoidance (Figure 1D). To address a requirement for ORNs for repulsion to citronellal, we inactivated the ORNs using either an inward rectifying K+ channel, Kir2.1 (UAS-kir2.1) [6], which hyperpolarizes neurons thereby interfering with production of action potentials, or tetanus toxin (UAS-TNT) [7], which prevents synaptic transmission. We expressed UAS-kir2.1 and UAS-TNT in ORNs under control of the Or83b promoter (Or83b-GAL4) [8]. OR83b is a co-receptor required for detecting a broad range of odorants since it promotes trafficking of other olfactory receptors to dendrites [8]. We found that inactivation of ORNs by expression of Kir2.1 or tetanus toxin strongly impaired citronellal avoidance (Figure 1D).
To identify the ORNs mediating citronellal detection, we surveyed the electrophysiological responses of the olfactory sensilla located on the 3rd antennal segment. Olfactory sensilla are classified into three morphological types, basiconic, coeloconic and trichoid, and each contains the odor-sensitive dendrites of one to four ORNs [reviewed in 9, 10]. Using single-sensillum recordings (SSR), we assayed the sensitivity of previously characterized sensilla to citronellal, none of which responded strongly to citronellal [11, 12] (Figure 1E). Therefore, we surveyed two previously uncharacterized basiconic sensilla, ab11 and ab12, located on the lateral surface of the 3rd antennal segment below the aristae (Figure 1F). The ab11 and ab12 sensilla each contained three neurons that had spontaneous activity (activity in the absence of odorants) with distinct spike amplitudes. We named these neurons ab11a-c and ab12a-c, in descending order of relative amplitude (Figures 1G and H). Ab11 and ab12 contained one olfactory neuron each (ab11a and ab12a respectively) that was activated strongly by citronellal (Figures 1G and H). Among 11 odorants tested, only citronellal induced a high frequency of action potentials in ab11 (Figures 1G), while ab12 also responded strongly to benzaldehyde (ab12b) and moderately to methyl salicylate (ab12a; Figure 1H).
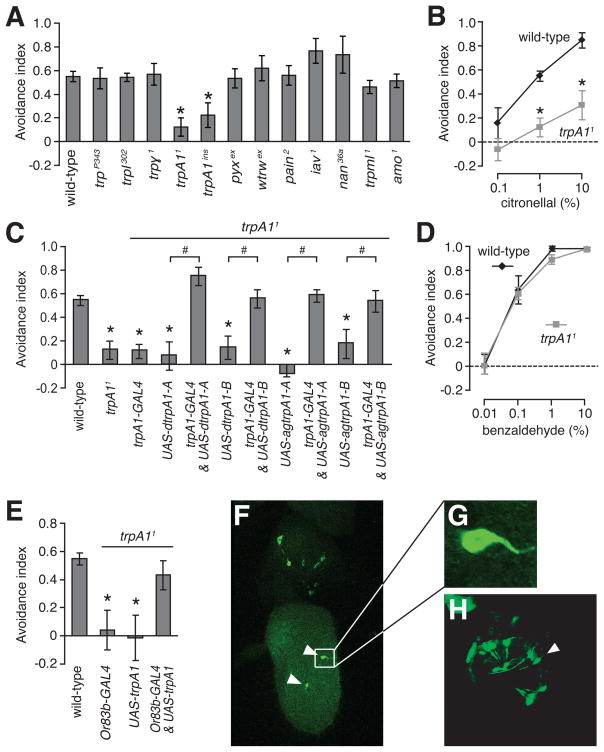
To address the identities of proteins required for citronellal avoidance, we considered TRP channels. These cation channels are candidates as many are modulated in vitro by noxious compounds, some of which induce pain or have repellent properties [13–22]. The Drosophila genome encodes 13 TRP channels, 11 of which are dispensable for adult viability. To address whether a Drosophila TRP channel was essential for citronellal avoidance behavior, we tested all 11 viable mutants. The responses of 10 were indistinguishable from wild-type (Figure 2A). In contrast, the repellent reaction to 1% citronellal was reduced greatly in trpA11 [23] and trpA1ins [24] mutant flies (Figures 2A and B). At a concentration of 10%, citronellal aversion was reduced but not eliminated (Figure 2B). Expression of a wild-type transgene (UAS-trpA1+) under the control of trpA1-GAL4 rescued the trpA11 phenotype (Figure 2C and Figure S2A). The trpA11 defect was not due to a general impairment in olfaction since the avoidance to benzaldehyde was unaltered (Figure 2D).
Figure 2.
Requirement for trpA1 for avoidance to airborne citronellal vapor. (A) Avoidance of trp mutants to 1% citronellal. (B) Wild-type and trpA11 responses to 0.1 – 10% citronellal. (C) Rescue of the trpA11 phenotype with either Drosophila (dtrpA1-A and dtrpA1-B) or Anopheles gambiae (agtrpA1-A and agtrpA1-B) trpA1 transgenes using the GAL4/UAS system. (D) Wild-type and trpA11 responses to 0.01 – 10% benzaldehyde. (E) Expression of UAS-trpA1 in ORNs using the Or83b-GAL4 restored citronellal repulsion in trpA11. (F – H) Expression of the trpA1 reporter in 2nd and 3rd antennal segments using the trpA1-GAL4 and UAS-GFP (green). (F) Staining in a coronal plane of an antenna from a late-stage pupae. GFP positive neurons in the 3rd antennal segment are indicated with arrowheads. (G) High magnification view of the inset in panel (F). (H) Staining in a transverse plane of an antenna from a late-stage pupae. Error bars represent ±SEMs. The asterisks indicate significant differences from the wild-type control (ANOVA, p<0.05). The pound signs denote significant differences between the indicated flies (unpaired Student t-test, p<0.05).
To address whether the mosquito TRPA1 homolog might function in citronellal repulsion, we tested for rescue of the Drosophila trpA11 phenotype with a mosquito trpA1+ transgene. We cloned the Anopheles gambiae trpA1 gene (Figure S2D and E) and introduced the transgene (UAS-agtrpA1-A) into the Drosophila trpA11 background. Expression of the UAS-agtrpA1-A transgene using the trpA1-GAL4 rescued the defect in trpA11 (Figure 2C and Figure S2B). During the isolation of mosquito trpA1 cDNAs, we found that Anopheles expressed an alternative mRNA (agtrpA1-B), encoding 34 residues distinct from those in TRPA1-A (666 to 699, Figure S2G). A corresponding mRNA isoform was also produced in Drosophila (Figure S2F and G). We expressed UAS-agtrpA1-B or UAS-dtrpA1-B in trpA11 flies under control of the trpA1-GAL4 and found that either variant rescued the citronellal impairment (Figure 2C). These results indicate that a role for TRPA1 in citronellal avoidance is conserved in Anopheles.
The finding that inactivation of ORNs with Kir2.1 or tetanus toxin impaired citronellal avoidance (Figure 1D) raised the possibility that trpA1 may function in these neurons. To address this question, we expressed UAS-trpA1-A under the control of the Or83b-GAL4 and found that it restored citronellal avoidance in trpA11 (Figure 2E). This functional rescue strongly supports the conclusion that trpA1 is required in ORNs for sensing citronellal. Consistent with this finding, the trpA1-GAL4, which rescued the trpA11 defect in combination with the UAS-trpA1 transgenes, drove expression in 2~6 neurons in the 3rd antennal segment, in addition to several neurons in the 2nd antennal segment (Figures 2F, 2G and 2H). Moreover, expression of UAS-kir2.1 using the trpA1-GAL4 caused a reduction in citronellal avoidance (Figure S2C).
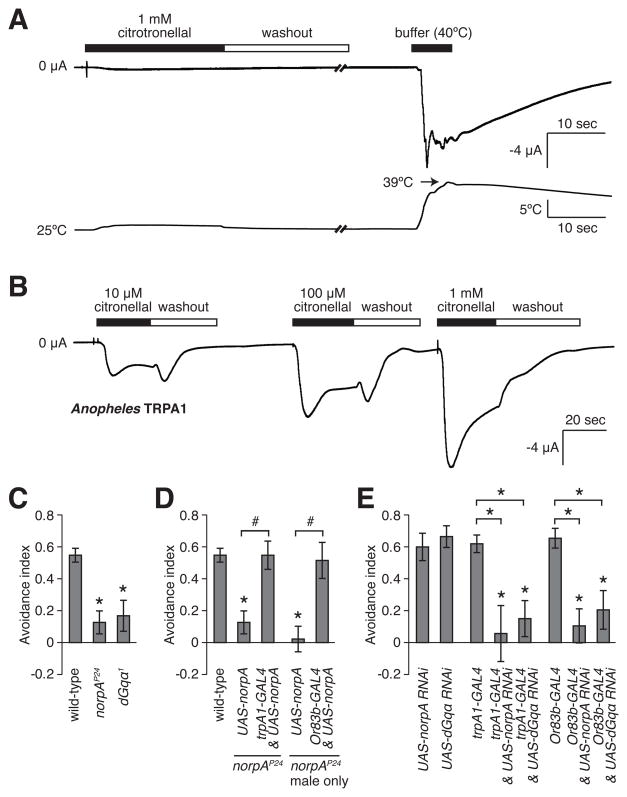
To address whether TRPA1 may be modulated directly by citronellal, we expressed Drosophila TRPA1 in Xenopus oocytes. Consistent with a previous report, TRPA1 was activated transiently by a temperature increase from 25°C to 39°C (Figure 3A) [25], and more slowly by addition of increasing concentrations of allyl isothiocyanate (AITC; Figure S3A) [26]. However, concentrations of citronellal as high as 1 mM induced little current (Figure 3A), and citronellal did not inhibit temperature-induced activity (Figure S3B). A higher concentration (2 mM) induced a relatively small current in TRPA1 injected oocytes, but not in controls injected with the vehicle (Figures S3C and S3D). This current was reversed rapidly in a citronellal-free buffer (Figure S3C). Thus, citronellal activated Drosophila TRPA1 directly, but with low potency. In contrast, we found that 10 μM citronellal robustly activated TRPA1 from Anopheles gambiae (Figure 3B).
Figure 3.
Expression of TRPA1 in Xenopus oocytes and requirement for PLC and Gqα for avoiding 1% citronellal. Expression of Drosophila TRPA1 (A) and Anopheles TRPA1 (B) in oocytes. (A and B) Filled bars indicate the duration of a stimulus and open bars indicate washouts with stimulus-free buffers. (A) Representative effects of 1 mM citronellal (Mean ΔI SEM= −0.16±0.03 μA, n=6) and a heat shift (~25 to 39 °C, ΔI = −11.0±2.7 μA, n=3) on Drosophila TRPA1. (B) Activation of Anopheles gambiae TRPA1 by increasing concentrations of citronellal (10 μM, ΔI= −3.00±0.68 μA, n=7; 100 μM, ΔI= −5.48±0.78 μA, n=10; and 1 mM, ΔI= −12.0±3.4 μA, n=5). (C) Requirement for PLC (NORPA) and dGqα for citronellal avoidance. (D) Expression of UAS-norpA using the trpA1-GAL4 or Or83b-GAL4 rescued the norpAP24 phenotype. (E) Requirement of NORPA and dGqα in ORNs for citronellal avoidance. RNAi knockdown of norpA and dGqα using the trpA1-GAL4 or Or83b-GAL4. Asterisks indicate statistically significant differences from wild-type unless indicated with a bracket (ANOVA, p<0.05). The pound sign indicates significant differences between the indicated measurements (unpaired Student t-test, p<0.05). Error bars represent ± SEMs.
Because Drosophila TRPA1 was activated poorly by citronellal, we considered the possibility that it might function downstream of a G protein (Gq) and phospholipase C (PLC) signaling cascade. Multiple TRP channels are linked to Gq/PLC signaling [18] and TRPA1 contributes to the avoidance of some noxious tastants [27] and in larval temperature discrimination in the 18 – 24 °C range through a Gq/PLC signaling cascade [23]. Of significance here, the evasive behavior induced by citronellal was reduced significantly in the dGqα1 and norpAP24 mutants (Figure 3C). The defect in norpAP24 was rescued by expressing UAS-norpA using either the trpA1-GAL4 or Or83b-GAL4 (Figure 3D). RNAi knockdown of dGqα(UAS-dGqα-RNAi) and norpA (UAS-norpA-RNAi) under control of either the trpA1-GAL4 or Or83b-GAL4 significantly reduced citronellal avoidance (Figure 3E). These results indicate that dGq, PLC and TRPA1 function in the same ORNs and support the model that they act in a common signaling pathway.
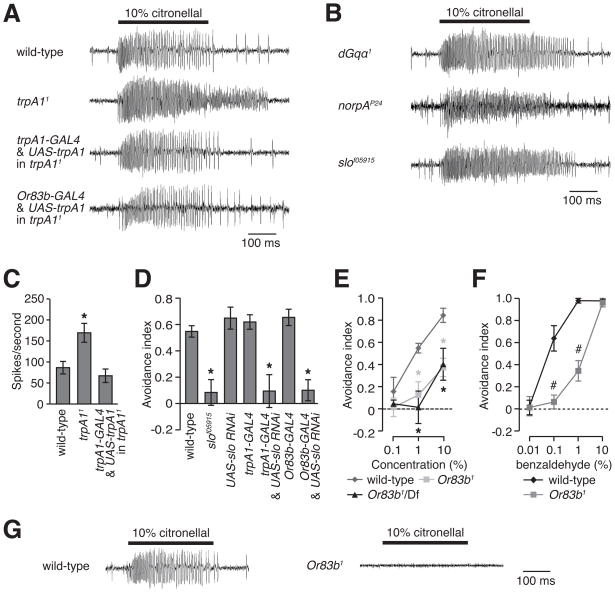
To characterize the consequences on citronellal-induced ORN activity resulting from loss of TRPA1, we performed SSRs. For nearly all ORN types, there were no significant differences in citronellal responses between wild-type controls and trpA11 mutants, including the ab12a neurons (Figure S4A and data not shown), which also respond to benzaldehyde (Figure S4B). However, rather than being reduced, citronellal-evoked action potential frequencies in trpA11 ab11a neurons were significantly higher than controls (Figure 4A and Figure S4C). The increased activity in trpA11 ab11a neurons was reversed by expressing a wild-type trpA1 transgene using either the trpA1-GAL4 or the Or83b-GAL4 (Figure 4A and Figure S4C).
Figure 4.
Increased action potential frequency in trpA11. (A and B) Representative SSR traces from ab11 sensilla stimulated with 10% citronellal. (C) Quantification of deactivation defect observed in ab11a ORNs during 200 ms immediately following citronellal stimulus (n=10). (D) Impairment of citronellal avoidance by mutation or RNAi knockdown of slo. (E) Reduction of citronellal avoidance in the or83b1 mutant. (F) Dose response to benzaldehyde in Or83b1 flies. (G) Representative SSR traces from ab11 sensilla in control and Or83b1. Error bars represent ± SEMs. The asterisks indicate significant differences from the wild-type control (ANOVA, p<0.05). The pound signs denote significant differences between the indicated flies (unpaired Student t-test, p<0.05).
We found two features of the citronellal responses that were abnormal in the trpA11 ab11a neurons. First, there was a higher citronellal-evoked action potential frequency than in wild-type (Figures 4A and S4C). Second, there was a defect in deactivation in trpA11 ab11a neurons (Figures 4A and 4C). We observed the same two defective phenotypes in ab11 neurons in the dGqα1 and norpAP24 mutants (Figure 4B), although only the increase in the evoked responses was clearly different when testing significance using ANOVA (Figure S4D). These results support the conclusion that the dGqα1, norpAP24 and trpA11 mutations affect the citronellal-response in an ORN in ab11.
The findings that there were increases in the frequency of citronellal-evoked action potentials were unexpected and raised a question as to the basis for these defects. TRPA1 is a Ca2+-permeable channel, and since reduced activity of Ca2+-activated K+ channels (BK channels) increase the frequency of action potential firing [28, 29], we wondered whether loss of TRPA1 caused reduced BK channel activity. If so, then a mutation in the gene (slowpoke, slo) encoding the fly BK channel might phenocopy the trpA1 phenotype. In support of this model, the slof05915 mutation caused an increase in the frequency of citronellal-evoked action potentials (Figures 4B and Figure S4D) and impaired citronellal avoidance (Figure 4D). Introduction of the UAS-slo-RNAi in combination with either the trpA1-GAL4 or the Or83b-GAL4 resulted in a similar defect in citronellal avoidance (Figure 4D).
We propose that TRPA1 is required for activity of Slo, which in turn modulates citronellal-induced firing of action potentials. Slo might be required in many ORNs, and be regulated by additional TRP channels. In support of this proposal, ab12 also responded to citronellal, and displayed a higher frequency of action potentials in slof05915, but did not function through a Gqα//PLC/TRPA1 pathway (Figure S4A). Knockout of a mammalian TRP channel, TRPC1, also disrupts the activity of a Ca2+-activated K+ channel (KCa) in salivary gland cells, and mutations affecting either TRPC1 or KCa result in similar defects in salivary gland secretion [30, 31]. Thus, a role for TRP channels in activating Ca2+-activated K+ channels might be a common but poorly appreciated general phenomenon, which is evolutionarily conserved.
The finding that loss of TRPA1 causes an increase rather than a decrease in citronellal-induced action potentials, suggests that there might be a TRPA1 independent-pathway required for generating action potentials in response to citronellal. Or83b is a candidate for functioning in such a pathway since mutation of Or83b interferes with the ability of the synthetic repellent, DEET, to inhibit the attraction to food odors [2]. We found that Or83b1 mutant flies, or Or83b1 in trans with a deficiency that uncovers the locus, exhibited a similar impairment in citronellal avoidance as in trpA1 mutant flies (Figure 4E). An Or83b1 defect in the DART assay was not specific to citronellal, as they were also impaired in the response to benzaldehyde (Figure 4F). We tested whether the frequency of citronellal-induced action potentials was altered in Or83b1 ab11 sensilla. In contrast to the trpA1 mutant phenotype, none of the mutant Or83b1 ab11 neurons responded to citronellal (Figure 4G).
Our data indicate that there are dual pathways required for the response to citronellal. Or83b is necessary for producing citronellal-induced action potentials, while a Gq/PLC/TRPA1 pathway appears to function in the modulation of action potential frequency by activating BK channels. We suggest that an abnormally high frequency of action potentials may lead to rapid depletion of the readily releasable pools of neurotransmitter, thereby muting the citronellal response. Interestingly, a loss-of-function mutation affecting a worm BK channel also results in a behavioral phenotype– increased resistance to ethanol [32]. Although, Drosophila TRPA1 functions downstream of a Gq/PLC signaling pathway, citronellal can also directly activate TRPA1, but with low potency. Nevertheless, since Anopheles gambiae TRPA1 is also expressed in the antenna [33], and is activated directly by citronellal with high potency, we suggest that mosquito TRPA1 represents a new potential target for in vitro screens for volatile activators, which might serve as new types of insect repellents.
Highlights.
Fruit flies use dual TRPA1- and Or83b-dependent pathways for avoiding citronellal.
Opposite requirements for TRPA1 and Or83b for citronellal-induced action potentials.
Flies avoid citronellal through a Gq/PLC/TRPA1 pathway, coupled to a BK channel.
Anopheles but not Drosophila TRPA1 is directly and potently activated by citronellal.
Supplementary Material
Acknowledgments
We thank the Bloomington Stock Center for fly stocks and FlyBase for critical information. We also thank Dr. P. Garrity for the UAS-trpA1 flies and trpA1 plasmid constructs, Drs. S. Das and G. Dimopoulos for the Anopheles cDNA pool and providing female Anopheles, H. S. Shim for fly husbandry, Dr. K. Venkatachalam for suggesting that loss of a BK channel (Slowpoke) might phenocopy the trpA1 phenotype and Dr. M. Fowler for help with functional expression of the Anopheles TRPA1. SHK was supported in part by a Samsung Fellowship and BA was supported by a NINDS (NS064684) postdoctoral fellowship. WBG was supported by the NIDDK (DK032753). This work was supported by grants to DPS from the NIDCD (DC02539) and to CM from the NIGMS (GM085335) and the Bill and Melinda Gates Foundation (53126).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reeder NL, Ganz PJ, Carlson JR, Saunders CW. Isolation of a deet-insensitive mutant of Drosophila melanogaster (Diptera: Drosophilidae) J Econ Entomol. 2001;94:1584–1588. doi: 10.1603/0022-0493-94.6.1584. [DOI] [PubMed] [Google Scholar]
- 2.Ditzen M, Pellegrino M, Vosshall LB. Insect odorant receptors are molecular targets of the insect repellent DEET. Science. 2008;319:1838–1842. doi: 10.1126/science.1153121. [DOI] [PubMed] [Google Scholar]
- 3.Katz TM, Miller JH, Hebert AA. Insect repellents: historical perspectives and new developments. J Am Acad Dermatol. 2008;58:865–871. doi: 10.1016/j.jaad.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 5.Smith DP. Odor and pheromone detection in Drosophila melanogaster. Pflugers Arch. 2007;454:749–758. doi: 10.1007/s00424-006-0190-2. [DOI] [PubMed] [Google Scholar]
- 6.Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baines RA, Robinson SG, Fujioka M, Jaynes JB, Bate M. Postsynaptic expression of tetanus toxin light chain blocks synaptogenesis in Drosophila. Curr Biol. 1999;9:1267–1270. doi: 10.1016/s0960-9822(99)80510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Ronderos DS, Smith DP. Diverse signaling mechanisms mediate volatile odorant detection in Drosophila. Fly (Austin) 2009;3:290–297. doi: 10.4161/fly.9801. [DOI] [PubMed] [Google Scholar]
- 10.Ha TS, Smith DP. Odorant and pheromone receptors in insects. Front Cell Neurosci. 2009;3:10. doi: 10.3389/neuro.03.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmore T, Ignell R, Carlson JR, Smith DP. Targeted mutation of a Drosophila odor receptor defines receptor requirement in a novel class of sensillum. J Neurosci. 2003;23:9906–9912. doi: 10.1523/JNEUROSCI.23-30-09906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 13.Bandell M, Macpherson LJ, Patapoutian A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr Opin Neurobiol. 2007;17:490–497. doi: 10.1016/j.conb.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parnas M, Peters M, Dadon D, Lev S, Vertkin I, Slutsky I, Minke B. Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium. 2009;45:300–309. doi: 10.1016/j.ceca.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stotz SC, Vriens J, Martyn D, Clardy J, Clapham DE. Citral sensing by Transient Receptor Potential channels in dorsal root ganglion neurons. PLoS ONE. 2008;3:e2082. doi: 10.1371/journal.pone.0002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 18.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 20.McKemy DD, Nenhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 21.Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Peier A, Moqrich A, Hergarden A, Reeve A, Andersson D, Story G, Earley T, Dragoni I, McIntyre P, Bevan S, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 23.Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat Neurosci. 2008;11:871–873. doi: 10.1038/nn.2170. [DOI] [PubMed] [Google Scholar]
- 24.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 26.Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci USA. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Gao SB, Lv CX, Wu Y, Guo ZH, Ding JP, Xu T. Characterization of voltage-and Ca2+-activated K+ channels in rat dorsal root ganglion neurons. J Cell Physiol. 2007;212:348–357. doi: 10.1002/jcp.21007. [DOI] [PubMed] [Google Scholar]
- 29.Gover TD, Moreira TH, Weinreich D. Role of calcium in regulating primary sensory neuronal excitability. Handb Exp Pharmacol. 2009:563–587. doi: 10.1007/978-3-540-79090-7_16. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, et al. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc Natl Acad Sci USA. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambudkar IS. Regulation of calcium in salivary gland secretion. Crit Rev Oral Biol Med. 2000;11:4–25. doi: 10.1177/10454411000110010301. [DOI] [PubMed] [Google Scholar]
- 32.Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Qiu YT, Lu T, Kwon HW, Pitts RJ, Van Loon JJ, Takken W, Zwiebel LJ. Anopheles gambiae TRPA1 is a heat-activated channel expressed in thermosensitive sensilla of female antennae. Eur J Neurosci. 2009;30:967–974. doi: 10.1111/j.1460-9568.2009.06901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.