Abstract
Serotonin 2C receptors (5-HT2CR) have been shown to undergo post-transcriptional RNA editing. This modification affects the affinity, coupling and constitutive activity of the receptor. In vivo, manipulations such as stress or antidepressant administration dramatically modify the pattern of 5-HT2CR mRNA editing, suggesting that this phenomenon might be involved in the pathophysiology of stress-related disorders. Indeed, alterations of 5-HT2CR mRNA editing have been observed in depressed patients. Thus, the recent development of mice expressing either the non-edited (5-HT2CR-INI) or the fully-edited form of 5-HT2CR (5-HT2CR - VGV) provides a novel opportunity to investigate the relevance of this phenomenon in the context of stress-related disorders. We observed that both 5-HT2CR-INI and 5-HT2CR-VGV mice exhibit exaggerated anxiety-like behaviors in the elevated plus maze paradigm. This phenotype was observed when the INI or VGV mutations were present in mice on a BALB/c background, as well as non-significant trends in the same direction in mice on a C57BL/6J background. In animal models of antidepressant-like activity, the absence of editing of 5-HT2CR mRNA (5-HT2CR-INI) induced an increase in the time spent immobile in the forced-swim test (FST) and tail suspension test (TST). Complete editing of 5-HT2C receptor mRNA (5-HT2CR-VGV) induced antidepressant-like behavior in the FST and TST, as reflected by a significant decrease in time spent immobile. These phenotypes were unrelated to alterations in locomotor activity in both 5-HT2CR-INI and -VGV. In the TST, these phenotypes were accompanied by a decrease and an increase in response to desipramine in 5-HT2CR-INI and -VGV, respectively. These data constitute the first in vivo demonstration of a role for 5-HT2CR mRNA editing in anxiety- and depression-related behaviors.
Keywords: RNA editing, 5-HT2C receptor, anxiety, antidepressant, transgenic mice
1. Introduction
Human and rodent serotonin 2C receptors (5-HT2CRs) undergo post-transcriptional RNA editing. Five closely spaced adenosines, located within a sequence encoding the second intracellular loop, are converted to inosine to alter the coding potential of three triplet codons (Burns et al., 1997). Through the action of specific deaminase enzymes (ADAR), this post-transcriptional modification alters agonist binding affinity, G-protein coupling and constitutive activity of the receptor (Werry et al., 2008). Thus, editing of 5-HT2CRs represents a functional adaptation of the serotoninergic system and consequently may play a role in the etiology of stress-related disorders.
Dysregulation in 5-HT2CR mRNA editing has been observed in the frontal cortex of depressed suicide victims (Gurevich et al., 2002; Niswender et al., 2001). Specifically, in suicide victims with a history of major depression, C′ site editing was significantly increased while D site editing was significantly decreased, and these changes could be reversed by fluoxetine treatment (Niswender et. al., 2001). Preclinically, C57BL/6J and BALB/c, two inbred mouse strains, exhibit differences in basal editing rates of 5-HT2CR in the prefrontal cortex (Englander et al., 2005). Further, exposure to certain types of stress during development or adulthood increases 5-HT2CR mRNA editing in the prefrontal cortex of BALB/c mice, and this alteration can be counteracted by antidepressant treatment (Bhansali et al., 2007). These data confirm a potential role of 5-HT2CR mRNA editing in the susceptibility to stress and antidepressant response. Of interest, BALB/c mice demonstrate higher levels of baseline anxiety-like behaviors compared to C57BL/6J mice (Griebel, et. al., 2000; Lepicard et. al., 2000), which may be a reflection of differences in 5-HT2CR mRNA editing seen between the two strains.
Recently, mice expressing either the non-edited (5-HT2CR-INI) or the completely-edited form (5-HT2CR –VGV) of the 5-HT2C receptor were generated (Kawahara et al., 2008). Characterizing these mice in behavioral paradigms that evaluate anxiety- and antidepressant-like behavior will enable us to evaluate the functional significance of receptor mRNA editing in the context of anxiety disorders and depression.
2. Materials and Methods
2.1 Animals
5-HT2CR-VGV and 5-HT2CR-INI expressing mice were generated and backcrossed for at least 10 generations into both C57BL/6J and BALB/c genetic backgrounds (Kawahara et al., 2008). Mice were matched for both age (3 to 8 months) as well as sex (both M and F) across groups. There was no effect of sex on behaviors observed. Mice were group-housed with food and water available ad libitum. All animals were housed in a temperature- and humidity-controlled animal care facility with a 12 hr light/dark cycle (lights on a 6:00 A.M.). All procedures were approved by the Institutional Care and Use Committee of the Wistar Institute.
2.2 Elevated-plus maze (EPM)
Animals are placed in the center of a maze that consists of two perpendicular, intersecting runways, each 7.6 cm wide and 60 cm long. One runway has 15-cm-tall black walls (closed), while the other has no walls except for a 1-cm-tall lip at the edge (open). The runways are positioned 30 cm above the floor on a pedestal. Behavior of mice is scored by an observer blinded to genotype. Mice are scored on several anxiety-related variables such as time spent and numbers of entries in open arms, as well as latency to enter open arms. In addition, ethologically-relevant behaviors such as stretch-and-attend posture, rearings, grooming, and head dips were quantified in both open and closed arms.
2.3 Locomotor activity
Animals were placed in a new cage for 30 minutes after injection with desipramine or vehicle, and the distance traveled was measured using video tracking, performed by the Noldus Ethovision program (Noldus Information Technology, Leesburg, VA).
2.4 Forced Swim Test (FST)
Mice were placed individually into plexiglass cylinders (23 cm tall and 14 cm in diameter) filled with water (22–24 degrees C) to a depth of 15 cm. All test sessions were recorded by a video camera positioned directly above the cylinders. Total time spent immobile (making only slight movements necessary to remain afloat vs. active escape-oriented behaviors such as swimming or climbing) during the last four minutes of the six-minute test period was quantified by a blinded observer.
2.5 Tail Suspension Test (TST)
Mice were individually suspended by the tail from a metal rod (35 cm above the floor) using adhesive tape. Mice demonstrated several escape-oriented behaviors interspersed with temporally increasing bouts of immobility. Duration of immobility during the entire six-minute test period was scored from videotapes by a blinded observer.
2.6 Drugs
Desipramine (DMI) was obtained from Sigma (St. Louis, MO) and was dissolved in 0.9% saline immediately prior to use. Drugs were administered intraperitoneally by injection.
2.7 Statistics
All data were analyzed using Student’s t-test (EZM) or a two-way analysis of variance (ANOVA), followed by Bonferonni post-hoc tests (locomotor activity, FST and TST, body weights). All statistical analyses are summarized in Tables 1 and 2. The level of significance was set at p < 0.05.
Table 1.
| Elevated-plus maze: | ||||
|---|---|---|---|---|
| BALB/C | ||||
| INI | VGV | |||
| p= | t= | p= | t= | |
| Latency to enter in the open arm: | 0.0413* | t25=2.152 | 0.0454* | t22=2.121 |
| Time spent in the open arm: | 0.0013** | t27=3.579 | 0.0457* | t21=2.124 |
| Number of entries in open arm: | 0.0016** | t27=3.509 | 0.0015** | t20=3.664 |
| Number of Head dips: | 0.0033** | t28=3.208 | <0.0001*** | t21=6.028 |
| Number of Rearings: | 0.0499* | t28=2.049 | 0.0027** | t21=3.402 |
| C57BL6 | ||||
| INI | VGV | |||
| p= | t= | p= | t= | |
| Latency to enter in the open arm: | 0.2367 | t24=1.214 | 0.2814 | t22=1.104 |
| Time spent in the open arm: | 0.1597 | t24=1.451 | 0.1486 | t20=1.503 |
| Number of entries in open arm: | 0.1086 | t24=1.666 | 0.0745 | t20=1.882 |
| Number of Head dips: | 0.0438* | t24=2.128 | 0.0016** | t21=3.629 |
| Number of Rearings: | 0.41 | t24=0.83 | <0.0001*** | t21=5.085 |
| FST: | ||||
| INI | VGV | |||
| F | p= | F | p= | |
| interaction | F1,35=0.002 | 0.96 | F1,44=2,571 | 0.116 |
| genotype | F1,35=23.76 | <0.0001*** | F1,44=103.7 | <0.0001*** |
| treatment | F1,35=16.57 | 0.0003*** | F1,44=15.16 | 0.0003*** |
| TST: | ||||
| INI | VGV | |||
| F | p= | F | p= | |
| interaction | F1,45=4.199 | 0.0463* | F1,49=4.691 | 0.0352* |
| genotype | F1,45=9.117 | 0.0042** | F1,49=77.29 | <0.0001*** |
| treatment | F1,45=51.59 | <0.0001*** | F1,49=116.3 | <0.0001*** |
| Locomotor activity: | ||||
| INI | VGV | |||
| F | p= | F | p= | |
| interaction | F1,17=0.322 | 0.57 | F1,20=3.2856 | 0.084 |
| genotype | F1,17=0.031 | 0.86 | F1,20=3.14 | 0.09 |
| treatment | F1,17=9.072 | 0.0079** | F1,20=6.78 | 0.0169* |
Table 2.
| FST: | Body weight (g) | ||
|---|---|---|---|
| INI | Mean | SEM | |
| Wildtype | Saline | 23.8 | 1.13 |
| Wildtype | DMI | 23.6 | 0.92 |
| 5-HT2C-INI | Saline | 23.1 | 0.86 |
| 5-HT2C-INI | DMI | 24.3 | 0.78 |
| VGV | |||
| Wildtype | Saline | 23.9 | 0.95 |
| Wildtype | DMI | 22.8 | 0.79 |
| 5-HT2C-VGV | Saline | 15.9a | 0.41 |
| 5-HT2C-VGV | DMI | 16.3a | 0.51 |
| TST: | |||
| INI | |||
| Wildtype | Saline | 22.9 | 0.92 |
| Wildtype | DMI | 25.1 | 0.85 |
| 5-HT2C-INI | Saline | 24.8 | 0.91 |
| 5-HT2C-INI | DMI | 23.8 | 1.00 |
| VGV | |||
| Wildtype | Saline | 24.9 | 0.74 |
| Wildtype | DMI | 26.1 | 1.15 |
| 5-HT2C-VGV | Saline | 18.5a | 0.55 |
| 5-HT2C-VGV | DMI | 18.1a | 1.04 |
| Locomotor activity: | |||
| INI | |||
| Wildtype | Saline | 24.0 | 0.93 |
| Wildtype | DMI | 23.5 | 0.99 |
| 5-HT2C-INI | Saline | 25.7 | 1.26 |
| 5-HT2C-INI | DMI | 24.8 | 0.83 |
| VGV | |||
| Wildtype | Saline | 24.7 | 0.68 |
| Wildtype | DMI | 25.4 | 0.87 |
| 5-HT2C-VGV | Saline | 18.5a | 0.72 |
| 5-HT2C-VGV | DMI | 18.8a | 0.91 |
P < 0.0001 for main effect of genotype (FST: F1,42=92.98; TST: F1,49=66.68; Locomotor activity: F1,20=64.45). No significant main effect of genotype in INI cohorts. No significant effect of DMI or interaction in any cohort.
3. Results
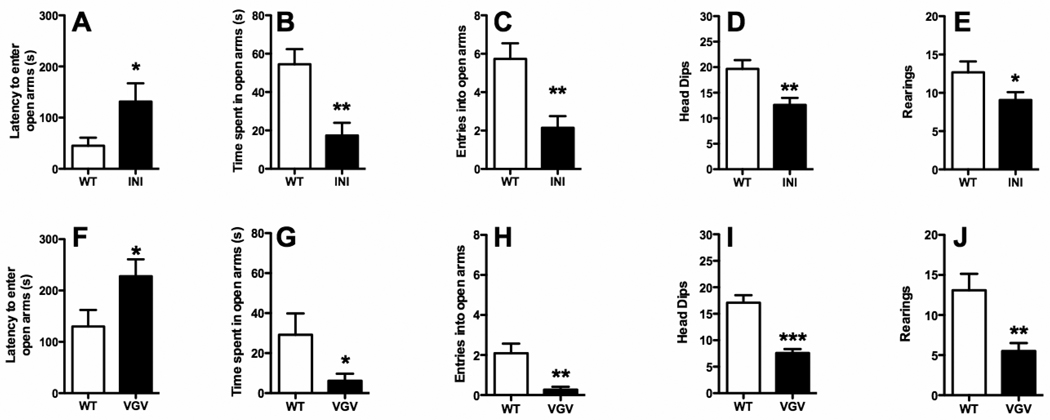
BALB/c and C57BL/6J mice exhibit different 5-HT2CR editing profiles (Englander et al., 2005). These mice vary in baseline response in anxiety- and stress-related paradigms as well as their response to antidepressant drugs (Griebel, et. al., 2000; Lepicard et. al., 2000). To determine if 5-HT2CR editing contributes to these differences, we assessed anxiety-related behavior using the elevated plus maze (EPM) in both 5-HT2CR-INI and 5-HT2CR-VGV mice in these two genetic backgrounds. As shown in Figure 1, mice expressing only the non-edited form of 5-HT2CRs (5-HT2CR-INI mice) spent significantly less time in the open arms (Figure 1B), displayed significantly fewer entries into open arms (Figure 1C), as well as significantly fewer head-dips (Figure 1D) and rearings (Figure 1E); the direction of change in all of these behaviors is associated with increased anxiety-like behavior (all statistical analyses can be found in Table 1). Further, latency to enter the open arms was also significantly increased in 5-HT2CR-INI mice (Figure 1A), an additional change associated with increased anxiety-like behavior. Of interest, BALB/c animals harboring the totally edited form of 5-HT2CRs (5-HT2CR-VGV mice) also showed significantly increased latency to enter the open arms (Figure 1F), significantly decreased entries into and time spent in the open arms (Figures 1 G and H), as well as significantly decreased head dips and rearings (Figures 1 I and J) compared to their wild-type counterparts, again showing increased anxiety-like behavior.
Figure 1. Alterations in 5-HT2CR mRNA editing in BALB/c background result in increased anxiety-like behavior in the elevated plus maze.
Both 5-HT2CR-INI (A–E) and 5-HT2CR-VGV mice (F–J) exhibit increases in anxiety-like behaviors, affecting latency to enter in open arms (A, F), time spent in open arms (B, G), the number of entries into open arms(C, H), head-dips (D, I) and rearings (E, J). Values are means +/−SEM. * p<0.05, ** p<0.01, and *** p<0.001 for significant difference from wild-type animals. Full statistical analysis can be found in Table 1.
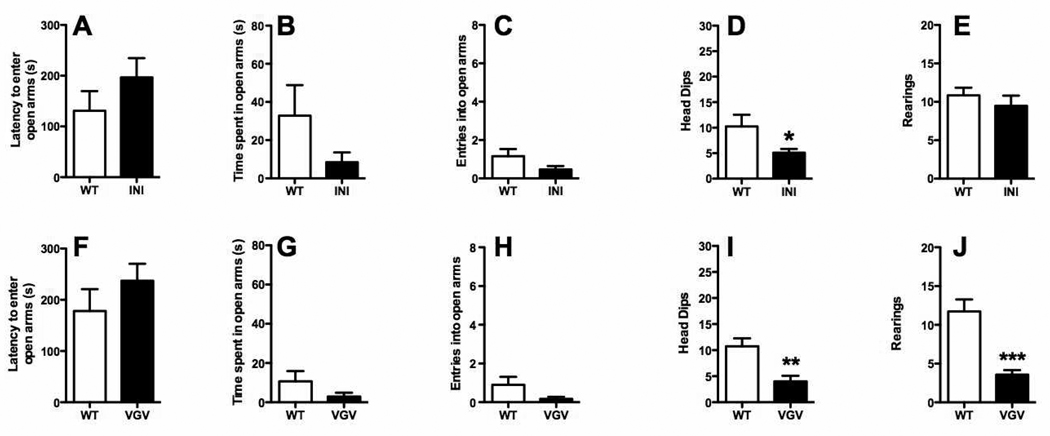
In the C57BL/6J background, we observed behavioral changes in the same direction as the BALB/c mice in both 5-HT2CR-INI (Figure 2A–E) and 5-HT2CR-VGV (Figure 2F–J) mice, but these alterations were less marked, failing to reach significance except in the case of the ethologically-relevant behaviors (rearings and head-dips), in which there were significant decreases (Figures 2D, I and J). It is important to note that wildtype animals of this genetic background exhibited increased latency to enter the open arms, as well as fewer entries into open arms, less time spent in open arms, and fewer rearings and head dips compared to their wildtype BALB/c counterparts (Figures 1 and 2), indicative of increased anxiety-like behaviors at baseline in the C57BL/6J strain.
Figure 2. The anxiety-related phenotype of 5HT2CR-INI and 5-HT2CR-VGV mice is attenuated in C57BL/6J genetic background.
Both 5-HT2CR-INI (A–E) and 5-HT2CR-VGV mice (F–J) exhibit increases in anxiety-like behaviors, with significant decreases in head dips in both transgenic lines (D, I) and significantly decreased rearings in VGV mice (J) as compared to wildtype animals. Latency to enter open arms (A, F), time spent in open arms (B, G), and entries into open arms (C, H) were not significantly changed in INI or VGV mice as compared to wildtype animals. Values are means +/−SEM. * p < 0.05, ** p < 0.001, and *** p < 0.001 for significant difference from wild-type animals. Full statistical analysis can be found in Table 1.
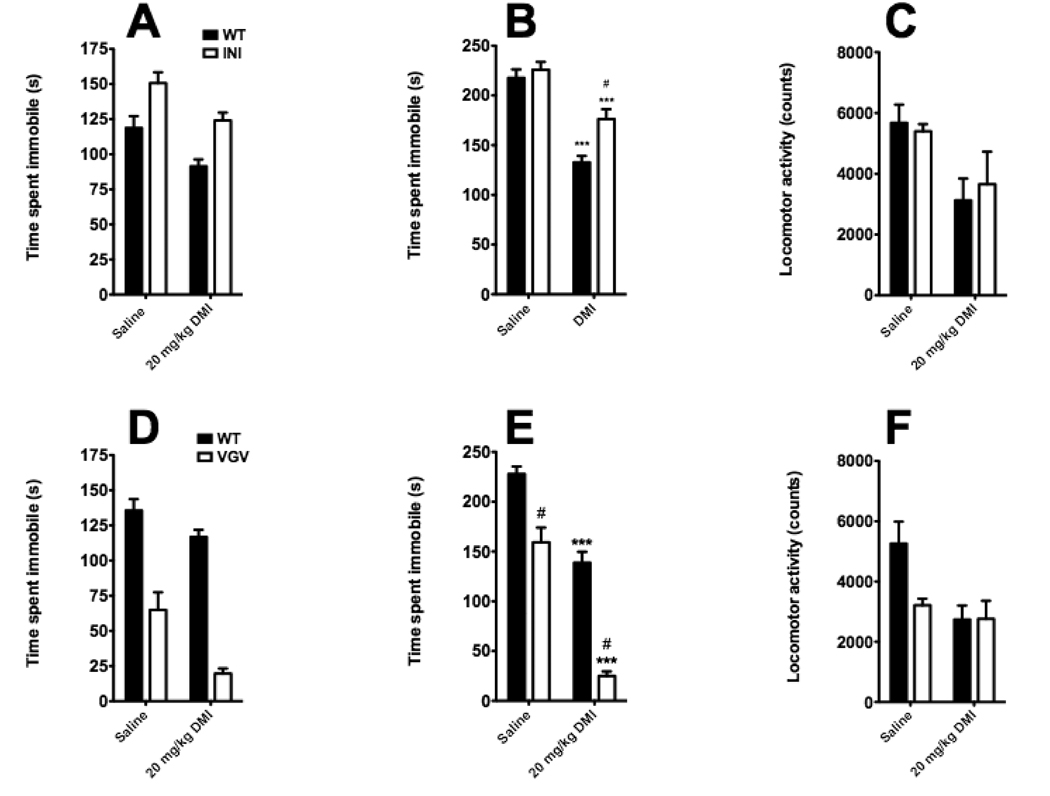
As the differences in anxiety-like behavior between transgenic lines were more pronounced in mice on the BALB/c background, we focused our investigation of the role of 5-HT2CR mRNA editing on antidepressant response in the BALB/c strain. Saline-treated 5-HT2CR-INI mice showed a significant increase in the time spent immobile (a pro-depressant effect) in the FST (Figure 3A) but not in the TST (Figure 3B). In contrast, we observed that 5-HT2CR-VGV mice displayed a robust and significant decrease in time spent immobile in both the FST (Figure 3D) and TST Figure 3E), an antidepressant-like phenotype. Desipramine (DMI) decreased immobility in both transgenic lines in both the FST (Figures 3A and D) and TST (Figures 3B and E), the expected antidepressant-like effect. However, the effects of DMI were attenuated in 5-HT2CR-INI mice and potentiated in 5-HT2CR-VGV mice in the TST paradigm (Figure 3B, 3E).
Figure 3. Impact of alterations of 5-HT2CR mRNA editing on despair behavior and antidepressant response in FST, TST and locomotor activity.
5-HT2CR-INI mice exhibit a pro-depressant phenotype in the FST (A) but not in the TST (B). Conversely, 5-HT2CR-VGV mice exhibit an antidepressant-like phenotype in both the FST (D) and TST (E). Both lines showed significant decreases in immobility in both tests in response to DMI (A, B, D, and E), and the effect of DMI was potentiated in 5-HT2CR-VGV in the TST but not the FST. DMI significantly decreased locomotor activity in both lines (C and F). There was no significant difference in locomotor activity in either line after vehicle injection (C and F), although there was a trend toward a decrease in the VGV mice (p = 0.09)(F). Values are means +/−SEM. * p < 0.05 and *** p < 0.001 for significant difference from respective saline-treated animals. # p < 0.05 for significant difference from respective wild-type animals. Full statistical analysis can be found in Table 1.
As has been previously reported (Kawahara et al., 2008), the 5-HT2CR-VGV mice used in behavioral experiments weighed significantly less than their wildtype littermates, while body weights of 5-HT2CR-INI mice were comparable to wildtype controls (Table 2). However, we did not observe any significant alterations of locomotor activity in either transgenic line that might confound interpretation of results in the FST or TST (Figure 3C, 3F).
4. Discussion
This study is the first to examine the effects of 5-HT2CR mRNA editing on behaviors related to psychiatric disease. Our results in the EPM indicate that both complete editing of 5-HT2CR mRNA (5-HT2CR –VGV) and lack of editing of the 5-HT2CR mRNA (5-HT2CR –INI) induced an anxiogenic-like effect in the BALB/c strain (Figure 1), and a trend toward increased anxiety-like behavior in the C57BL/6J strain (Figure 2). In the BALB/c strain, complete editing of 5-HT2CR mRNA (5-HT2CR –VGV) elicited an antidepressant-like phenotype in both the FST and TST, and accentuated the effects of DMI in the TST (Figures 3D and E). In contrast, BALB/c mice expressing only the un-edited form of 5-HT2CR mRNA (5-HT2CR –INI) showed a pro-depressant effect in the FST only and an attenuation of the effect of DMI in the TST (Figures 3A and B). The lack of effect in the TST at baseline in the 5-HT2CR –INI mice may be due to a ceiling effect.
We observed a significantly lower average body weight in the 5-HT2CR –VGV mice (Table 2), as has been reported previously (Kawahara et al., 2008). However, this difference in body weight does not appear to affect overall locomotor activity in these mice, which was not significantly different from wildtype (Figure 3F). Although there is a non-significant trend towards reduced locomotor activity in the 5-HT2CR –VGV mice, this does not confound the results of the FST and TST experiments, as these mice showed a decrease in immobility in these tests, which is opposite in direction to any potential decrease in general activity levels. Decreased body mass and adiposity might affect behavior specifically in the FST in that leaner mice might be forced to swim more due to an inability to float. However, the 5-HT2CR –VGV mice showed an identical phenotype in the TST, an independent measure of antidepressant response that does not involve swimming or floating behavior and thus should not be affected by body weight or adiposity. Of note, the 5-HT2CR –INI mice did not show alterations in locomotor activity (Figure 3C) or body weight (Table 2) as compared to their wildtype littermates, removing either of these confounds from interpretation of their behavioral phenotype.
5-HT2CR-null mice were the first genetically-altered mice studied to investigate the role of this receptor in psychiatric disorders. Mice lacking the 5-HT2CR exhibit a dramatic hyperphagia and late-onset obesity (Tecott et al., 1995), but their phenotypes in anxiety- and depression-like paradigms are somewhat ambiguous. 5-HT2CR-null mice have decreases in anxiety-like behavior (Heisler et al., 2007) but also increased obsessive compulsive-like behaviors (Chou-Green et al., 2003). These mice display no baseline difference in depression-related behaviors, but their response to antidepressants is potentiated (Cremers et al., 2004). Here, the results of the EPM indicate that complete editing of 5-HT2CR mRNA induces an anxiogenic-like effect. In the FST and TST, this manipulation elicits an antidepressant-like phenotype and accentuates the effects of desipramine. 5-HT2CR-VGV mice have 5-HT2CR mRNA and protein levels comparable to wild-type mice, but 5-HT2CR binding and 5-HT2CR-mediated neurotransmission are increased in these animals (Kawahara et al., 2008). This observed gain of function might contribute to the behavioral alterations seen here in 5-HT2CR-VGV mice. Indeed, pharmacological studies demonstrate that activation of 5-HT2CRs induce anxiety-like behaviors in rodents and humans (Griebel, 1995; Millan, 2005). Further, selective 5-HT2CR agonists, such as WAY 163909, have been shown to exert antidepressant-like effects in a range of rodent assays (Cryan and Lucki, 2000; Rosenzweig-Lipson et al., 2007). The present data are consistent with genetic ablation studies where the loss of 5-HT2CRs induced an anxiolytic-like effect. Thus, complete editing of 5-HT2CR mRNA likely alters basal anxiety-like behaviors and antidepressant-like behavior via an increase in 5-HT2CR function.
Further emphasizing the importance of mRNA editing for behavioral phenotypes, 5-HT2CR-VGV mice share some behavioral alterations with the recently-developed mice overexpressing the RNA editing enzyme ADAR2 (Singh et al., 2007; Singh et al., 2009). Indeed, ADAR2 transgenic animals displayed an increase in basal anxiety-like behaviors, analogous to 5-HT2CR-VGV mice. However, ADAR2 transgenic mice exhibit an increase in immobility in the FST, whereas we observed decreased immobility in the 5-HT2CR-VGV mice. One reason for this discrepancy could be that ADAR2 transgenic mice also show alterations in editing of other mRNAs, such as AMPA glutamate receptor B subunit, α3 subunit of the GABAA receptor, ADAR2 itself and microRNAs (Higuchi et al., 1993; Nishikura, 2006; Ohlson et al., 2007; Palladino et al., 2000), some of which could contribute to behavioral phenotypes. Moreover, ADAR2 regulates the editing of only the C and D sites (not A and B), suggesting that the overexpression of ADAR2 might result in partially-edited 5-HT2CR mRNA rather than the fully-edited VGV form expressed in the 5-HT2CR-VGV mice (Wang et al., 2004; Yang et al., 2004).
In the present study, we showed that absence of editing in 5-HT2CR-INI mice induced anxiety-like behaviors in the EPM and a pro-depressive phenotype in the FST. At the molecular level, INI receptor isoforms are spontaneously internalized in an agonist-independent manner through a GRK/βarrestin-dependent mechanism (Marion et al., 2004), suggesting that this genetic manipulation might induce a decrease in 5-HT2CR function. However, these assertions can only be confirmed using appropriate in vivo studies. Although our data suggest that the absence of editing affects anxiety- and depression-like behavior in mice, further investigation of the functional consequences of lack of mRNA editing on receptor expression, affinity, and activity is necessary.
Previous studies have demonstrated that BALB/c and C57BL/6J exhibit dramatically different 5-HT2CR mRNA editing profiles (Englander et al., 2005). In addition, BALB/c are generally thought to be more anxious-like than C57BL/6J (Griebel et al., 2000; Lepicard et al., 2000) but see (Trullas and Skolnick, 1993). Thus, our study was designed to evaluate whether these differences in levels of mRNA editing between the strains might be involved in determining their differential behavioral phenotypes. In the present studies, we observed a more pronounced phenotype in the BALB/c background than in C57BL/6J animals. We hypothesize that this is due to the higher baseline level of anxiety-like behaviors in the C57BL/6J that we observed. This baseline difference in anxiety-like behavior is surprising given that most of the literature finds BALB/c mice to be the more anxious-like strain, as mentioned above. Further, it may appear counterintuitive that both genetic manipulations induced a more marked effect in one strain than the other. Indeed, we would anticipate that the VGV manipulation might alter behavior more significantly in the BALB/c strain, which normally exhibits less editing, and that the INI manipulation might induce more significant alterations in the C57BL/6J strain, which exhibits a higher level of editing. Further studies are required to clarify this point and to understand how editing of 5-HT2CR mRNA might participate in the behavioral characteristics of these two strains. It is clear that other genetic factors contribute to the differential phenotypes of these two strains, as differences in behavior are seen between the two strains even when levels of editing are experimentally controlled, as they are in this study.
Thus, the present studies provide the first in vivo demonstration of the involvement of mRNA editing of 5-HT2CR mRNA in anxiety- and depression-like behaviors, taking advantage of the recently generated 5-HT2CR-INI and 5-HT2CR-VGV mice. These animals offer a new opportunity to investigate the relevance of 5-HT2CR mRNA editing in the context of neuropsychiatric disorders.
Acknowledgements
We thank S. Liu for her technical assistance. This work was supported in part by grants from the National Institutes of Health (CA010815, GM040536 and HL099342), the Ellison Medical Foundation (AG-SS-2281-09), and the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bhansali P, Dunning J, Singer SE, David L, Schmauss C. Early life stress alters adult serotonin 2C receptor pre-mRNA editing and expression of the alpha subunit of the heterotrimeric G-protein G q. J Neurosci. 2007;27:1467–1473. doi: 10.1523/JNEUROSCI.4632-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Chou-Green JM, Holscher TD, Dallman MF, Akana SF. Compulsive behavior in the 5-HT2C receptor knockout mouse. Physiol Behav. 2003;78:641–649. doi: 10.1016/s0031-9384(03)00047-7. [DOI] [PubMed] [Google Scholar]
- Cremers TI, Giorgetti M, Bosker FJ, Hogg S, Arnt J, Mork A, Honig G, Bogeso KP, Westerink BH, den Boer H, Wikstrom HV, Tecott LH. Inactivation of 5-HT(2C) receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacology. 2004;29:1782–1789. doi: 10.1038/sj.npp.1300474. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Lucki I. Antidepressant-like behavioral effects mediated by 5-Hydroxytryptamine(2C) receptors. J Pharmacol Exp Ther. 2000;295:1120–1126. [PubMed] [Google Scholar]
- Englander MT, Dulawa SC, Bhansali P, Schmauss C. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci. 2005;25:648–651. doi: 10.1523/JNEUROSCI.3895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G. 5-Hydroxytryptamine-interacting drugs in animal models of anxiety disorders: more than 30 years of research. Pharmacol Ther. 1995;65:319–395. doi: 10.1016/0163-7258(95)98597-j. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Gurevich I, Tamir H, Arango V, Dwork AJ, Mann JJ, Schmauss C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002;34:349–356. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH. Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav. 2007;6:491–496. doi: 10.1111/j.1601-183X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Single FN, Kohler M, Sommer B, Sprengel R, Seeburg PH. RNA editing of AMPA receptor subunit GluR-B: a base-paired intron-exon structure determines position and efficiency. Cell. 1993;75:1361–1370. doi: 10.1016/0092-8674(93)90622-w. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Grimberg A, Teegarden S, Mombereau C, Liu S, Bale TL, Blendy JA, Nishikura K. Dysregulated editing of serotonin 2C receptor mRNAs results in energy dissipation and loss of fat mass. J Neurosci. 2008;28:12834–12844. doi: 10.1523/JNEUROSCI.3896-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepicard EM, Joubert C, Hagneau I, Perez-Diaz F, Chapouthier G. Differences in anxiety-related behavior and response to diazepam in BALB/cByJ and C57BL/6J strains of mice. Pharmacol Biochem Behav. 2000;67:739–748. doi: 10.1016/s0091-3057(00)00419-6. [DOI] [PubMed] [Google Scholar]
- Marion S, Weiner DM, Caron MG. RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. J Biol Chem. 2004;279:2945–2954. doi: 10.1074/jbc.M308742200. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie. 2005;60:441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- Nishikura K. Editor meets silencer: crosstalk between RNA editing and RNA interference. Nat Rev Mol Cell Biol. 2006;7:919–931. doi: 10.1038/nrm2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Herrick-Davis K, Dilley GE, Meltzer HY, Overholser JC, Stockmeier CA, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-HT2C receptor. alterations in suicide and implications for serotonergic pharmacotherapy. Neuropsychopharmacology. 2001;24:478–491. doi: 10.1016/S0893-133X(00)00223-2. [DOI] [PubMed] [Google Scholar]
- Ohlson J, Pedersen JS, Haussler D, Ohman M. Editing modifies the GABA(A) receptor subunit alpha3. RNA. 2007;13:698–703. doi: 10.1261/rna.349107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MJ, Keegan LP, O'Connell MA, Reenan RA. dADAR, a Drosophila double-stranded RNA-specific adenosine deaminase is highly developmentally regulated and is itself a target for RNA editing. RNA. 2000;6:1004–1018. doi: 10.1017/s1355838200000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Sabb A, Stack G, Mitchell P, Lucki I, Malberg JE, Grauer S, Brennan J, Cryan JF, Sukoff Rizzo SJ, Dunlop J, Barrett JE, Marquis KL. Antidepressant-like effects of the novel, selective, 5-HT2C receptor agonist WAY-163909 in rodents. Psychopharmacology (Berl) 2007;192:159–170. doi: 10.1007/s00213-007-0710-6. [DOI] [PubMed] [Google Scholar]
- Singh M, Kesterson RA, Jacobs MM, Joers JM, Gore JC, Emeson RB. Hyperphagia-mediated obesity in transgenic mice misexpressing the RNA-editing enzyme ADAR2. J Biol Chem. 2007;282:22448–22459. doi: 10.1074/jbc.M700265200. [DOI] [PubMed] [Google Scholar]
- Singh M, Zimmerman MB, Beltz TG, Johnson AK. Affect-related behaviors in mice misexpressing the RNA editing enzyme ADAR2. Physiol Behav. 2009;97:446–454. doi: 10.1016/j.physbeh.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berl) 1993;111:323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, Weiss MJ, Nishikura K. Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene. J Biol Chem. 2004;279:4952–4961. doi: 10.1074/jbc.M310162200. [DOI] [PubMed] [Google Scholar]
- Werry TD, Loiacono R, Sexton PM, Christopoulos A. RNA editing of the serotonin 5HT2C receptor and its effects on cell signalling, pharmacology and brain function. Pharmacol Ther. 2008;119:7–23. doi: 10.1016/j.pharmthera.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Yang W, Wang Q, Kanes SJ, Murray JM, Nishikura K. Altered RNA editing of serotonin 5-HT2C receptor induced by interferon: implications for depression associated with cytokine therapy. Brain Res Mol Brain Res. 2004;124:70–78. doi: 10.1016/j.molbrainres.2004.02.010. [DOI] [PubMed] [Google Scholar]