Abstract
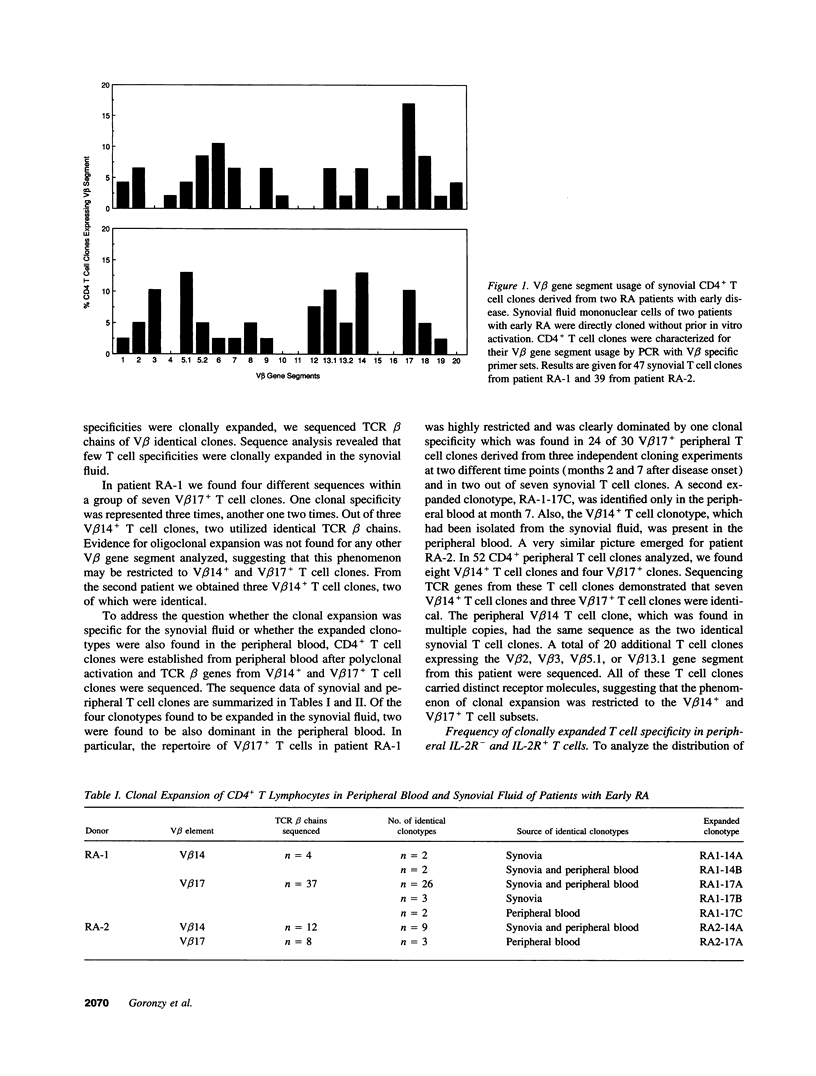
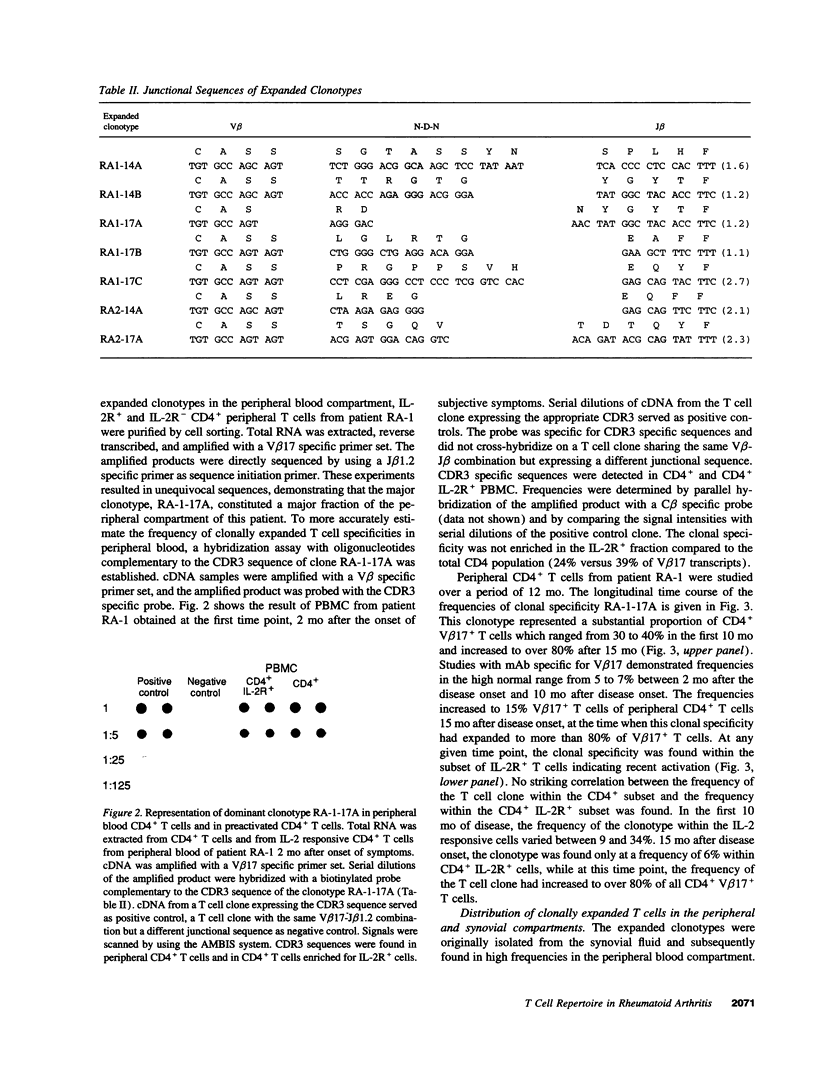
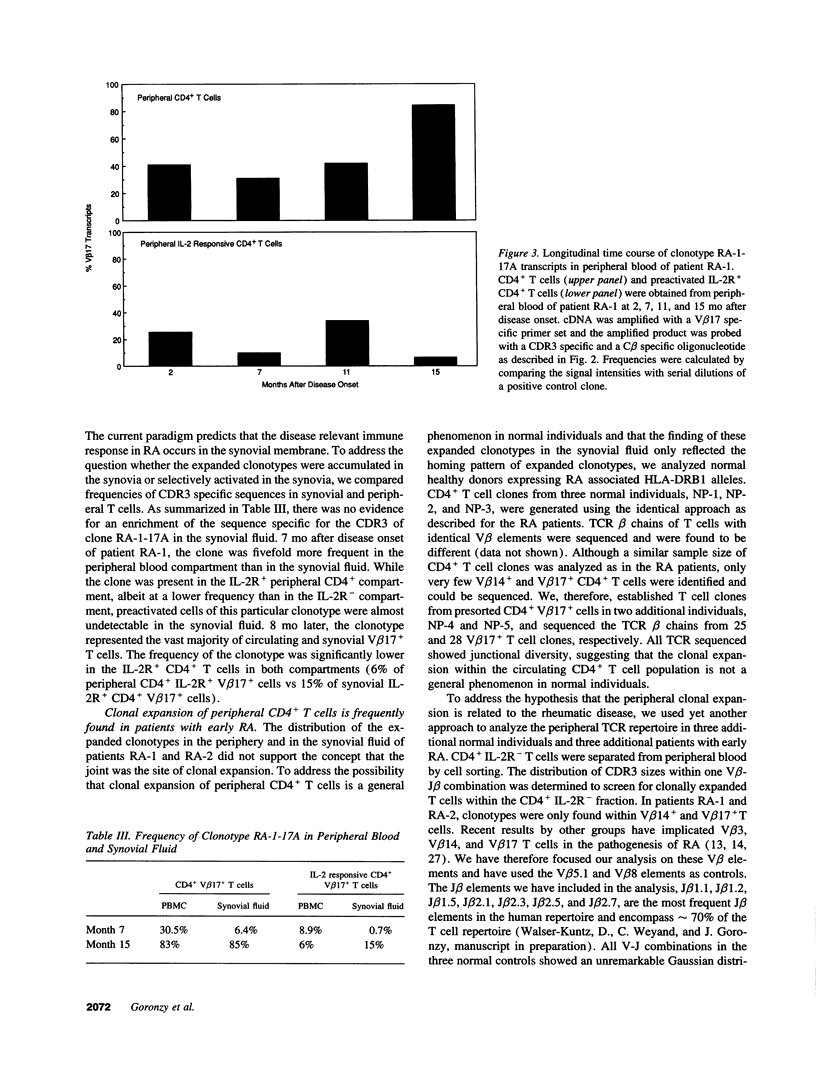
Clonal expansion of T cell specificities in the synovial fluid of patients has been taken as evidence for a local stimulation of T cells. By studying the T cell receptor (TCR) repertoire of CD4+ T cells in the synovial and peripheral blood compartments of patients with early rheumatoid arthritis (RA), we have identified clonally expanded CD4+ populations. Expanded clonotypes were present in the peripheral blood and the synovial fluid but were not preferentially accumulated in the joint. Dominant single clonotypes could not be isolated from CD4+ cells of HLA-DRB1*04+ normal individuals. Clonal expansion involved several distinct clonotypes with a preference for V beta 3+, V beta 14+, and V beta 17+CD4+ T cells. A fraction of clonally related T cells expressed IL-2 receptors, indicating recent activation. The frequencies of clonally expanded V beta 17+CD4+ T cells fluctuated widely over a period of one year. Independent variations in the frequencies of two distinct clonotypes in the same patient indicated that different mechanisms, and not stimulation by a single arthritogenic antigen, were involved in clonal proliferation. These data support the concept that RA patients have a grossly imbalanced TCR repertoire. Clonal expansion may result from intrinsic defects in T cell generation and regulation. The dominance of expanded clonotypes in the periphery emphasizes the systemic nature of RA and suggests that T cell proliferation occurs outside of the joint.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan W., Tabi Z., Cleary A., Doherty P. C. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990 May 15;144(10):3980–3986. [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Barton J. C., Prasthofer E. F., Egan M. L., Heck L. W., Jr, Koopman W. J., Grossi C. E. Rheumatoid arthritis associated with expanded populations of granular lymphocytes. Ann Intern Med. 1986 Mar;104(3):314–323. doi: 10.7326/0003-4819-104-3-314. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T. S., Gorga J. C., Stern L. J., Urban R. G., Strominger J. L., Wiley D. C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993 Jul 1;364(6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Allan J. E., Tabi Z., Lynch F., Doherty P. C. Phenotypic analysis of the inflammatory exudate in murine lymphocytic choriomeningitis. J Exp Med. 1987 Jun 1;165(6):1539–1551. doi: 10.1084/jem.165.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. W., Herman A., DiGiusto D., Wade T., Marrack P., Kappler J. Residues of the variable region of the T-cell-receptor beta-chain that interact with S. aureus toxin superantigens. Nature. 1990 Aug 2;346(6283):471–473. doi: 10.1038/346471a0. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Lafferty J. A., Clements J. R., Todd J. K., Gelfand E. W., Kappler J., Marrack P., Kotzin B. L. Selective expansion of T cells expressing V beta 2 in toxic shock syndrome. J Exp Med. 1990 Sep 1;172(3):981–984. doi: 10.1084/jem.172.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M. T cell receptor gene diversity and selection. Annu Rev Biochem. 1990;59:475–496. doi: 10.1146/annurev.bi.59.070190.002355. [DOI] [PubMed] [Google Scholar]
- DerSimonian H., Sugita M., Glass D. N., Maier A. L., Weinblatt M. E., Rème T., Brenner M. B. Clonal V alpha 12.1+ T cell expansions in the peripheral blood of rheumatoid arthritis patients. J Exp Med. 1993 Jun 1;177(6):1623–1631. doi: 10.1084/jem.177.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duby A. D., Sinclair A. K., Osborne-Lawrence S. L., Zeldes W., Kan L., Fox D. A. Clonal heterogeneity of synovial fluid T lymphocytes from patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6206–6210. doi: 10.1073/pnas.86.16.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales-Chambers R., Przepiorka D., Winkelstein A., Agarwal A., Starz T. W., Kline W. E., Hawk H. Lymphocyte subsets associated with T cell receptor beta-chain gene rearrangement in patients with rheumatoid arthritis and neutropenia. Arthritis Rheum. 1992 May;35(5):516–520. doi: 10.1002/art.1780350505. [DOI] [PubMed] [Google Scholar]
- Goronzy J. J., Oppitz U., Weyand C. M. Clonal heterogeneity of superantigen reactivity in human V beta 6+ T cell clones. Limited contributions of V beta sequence polymorphisms. J Immunol. 1992 Jan 15;148(2):604–611. [PubMed] [Google Scholar]
- Goronzy J. J., Weyand C. M. Interplay of T lymphocytes and HLA-DR molecules in rheumatoid arthritis. Curr Opin Rheumatol. 1993 Mar;5(2):169–177. doi: 10.1097/00002281-199305020-00008. [DOI] [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Grom A. A., Thompson S. D., Luyrink L., Passo M., Choi E., Glass D. N. Dominant T-cell-receptor beta chain variable region V beta 14+ clones in juvenile rheumatoid arthritis. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11104–11108. doi: 10.1073/pnas.90.23.11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Hingorani R., Choi I. H., Akolkar P., Gulwani-Akolkar B., Pergolizzi R., Silver J., Gregersen P. K. Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. J Immunol. 1993 Nov 15;151(10):5762–5769. [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Programmed cell death and extrathymic reduction of Vbeta8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991 Jan 17;349(6306):245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- Keystone E. C., Minden M., Klock R., Poplonski L., Zalcberg J., Takadera T., Mak T. W. Structure of T cell antigen receptor beta chain in synovial fluid cells from patients with rheumatoid arthritis. Arthritis Rheum. 1988 Dec;31(12):1555–1557. doi: 10.1002/art.1780311213. [DOI] [PubMed] [Google Scholar]
- Kurosaka M., Ziff M. Immunoelectron microscopic study of the distribution of T cell subsets in rheumatoid synovium. J Exp Med. 1983 Oct 1;158(4):1191–1210. doi: 10.1084/jem.158.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanchbury J. S., Jaeger E. E., Sansom D. M., Hall M. A., Wordsworth P., Stedeford J., Bell J. I., Panayi G. S. Strong primary selection for the Dw4 subtype of DR4 accounts for the HLA-DQw7 association with Felty's syndrome. Hum Immunol. 1991 Sep;32(1):56–64. doi: 10.1016/0198-8859(91)90117-r. [DOI] [PubMed] [Google Scholar]
- Loughran T. P., Jr, Starkebaum G., Kidd P., Neiman P. Clonal proliferation of large granular lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1988 Jan;31(1):31–36. doi: 10.1002/art.1780310105. [DOI] [PubMed] [Google Scholar]
- Nepom G. T., Seyfried C. E., Holbeck S. L., Wilske K. R., Nepom B. S. Identification of HLA-Dw14 genes in DR4+ rheumatoid arthritis. Lancet. 1986 Nov 1;2(8514):1002–1005. doi: 10.1016/s0140-6736(86)92614-0. [DOI] [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Haskard D., Panayi G. The preferential accumulation of helper-inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol. 1988 Sep;18(9):1397–1404. doi: 10.1002/eji.1830180915. [DOI] [PubMed] [Google Scholar]
- Posnett D. N., Sinha R., Kabak S., Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to "benign monoclonal gammapathy". J Exp Med. 1994 Feb 1;179(2):609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen A. M., Wade T., Marrack P., Kappler J. W. Identification of the region of T cell receptor beta chain that interacts with the self-superantigen MIs-1a. Cell. 1990 Jun 29;61(7):1365–1374. doi: 10.1016/0092-8674(90)90700-o. [DOI] [PubMed] [Google Scholar]
- Rellahan B. L., Jones L. A., Kruisbeek A. M., Fry A. M., Matis L. A. In vivo induction of anergy in peripheral V beta 8+ T cells by staphylococcal enterotoxin B. J Exp Med. 1990 Oct 1;172(4):1091–1100. doi: 10.1084/jem.172.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I., Stegagno M., Wright K. A., Krane S. M., Amento E. P., Colvin R. B., Duquesnoy R. J., Kurnick J. T. Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1179–1183. doi: 10.1073/pnas.85.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978 Apr 20;298(16):869–871. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- Stoflet E. S., Koeberl D. D., Sarkar G., Sommer S. S. Genomic amplification with transcript sequencing. Science. 1988 Jan 29;239(4839):491–494. doi: 10.1126/science.3340835. [DOI] [PubMed] [Google Scholar]
- Uematsu Y., Wege H., Straus A., Ott M., Bannwarth W., Lanchbury J., Panayi G., Steinmetz M. The T-cell-receptor repertoire in the synovial fluid of a patient with rheumatoid arthritis is polyclonal. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8534–8538. doi: 10.1073/pnas.88.19.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boxel J. A., Paget S. A. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975 Sep 11;293(11):517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J. J. Disease-associated human histocompatibility leukocyte antigen determinants in patients with seropositive rheumatoid arthritis. Functional role in antigen-specific and allogeneic T cell recognition. J Clin Invest. 1990 Apr;85(4):1051–1057. doi: 10.1172/JCI114535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J., Swarztrauber K., Fathman C. G. Immunosuppression by anti-CD4 treatment in vivo. Persistence of secondary antiviral immune responses. Transplantation. 1989 Jun;47(6):1034–1038. doi: 10.1097/00007890-198906000-00023. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Hicok K. C., Conn D. L., Goronzy J. J. The influence of HLA-DRB1 genes on disease severity in rheumatoid arthritis. Ann Intern Med. 1992 Nov 15;117(10):801–806. doi: 10.7326/0003-4819-117-10-801. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Hicok K. C., Goronzy J. J. Nonrandom selection of T cell specificities in anti-HLA-DR responses. Sequence motifs of the responder HLA-DR allele influence T cell recruitment. J Immunol. 1991 Jul 1;147(1):70–78. [PubMed] [Google Scholar]
- Weyand C. M., Oppitz U., Hicok K., Goronzy J. J. Selection of T cell receptor V beta elements by HLA-DR determinants predisposing to rheumatoid arthritis. Arthritis Rheum. 1992 Sep;35(9):990–998. doi: 10.1002/art.1780350903. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Xie C., Goronzy J. J. Homozygosity for the HLA-DRB1 allele selects for extraarticular manifestations in rheumatoid arthritis. J Clin Invest. 1992 Jun;89(6):2033–2039. doi: 10.1172/JCI115814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W. V., Fang Q., Demarco D., VonFeldt J., Zurier R. B., Weiner D. B. Restricted heterogeneity of T cell receptor transcripts in rheumatoid synovium. J Clin Invest. 1992 Aug;90(2):326–333. doi: 10.1172/JCI115866. [DOI] [PMC free article] [PubMed] [Google Scholar]