Abstract
Mitochondrial bioenergetics and reactive oxygen species (ROS) often play important roles in cellular stress mechanisms. In this study we investigated how these factors are involved in the stress response triggered by resazurin (Alamar Blue) in cultured cancer cells. Resazurin is a redox reactive compound widely used as reporter agent in assays of cell biology (e.g. cell viability and metabolic activity) due to its colorimetric and fluorimetric properties. In order to investigate resazurin-induced stress mechanisms we employed cells affording different metabolic and regulatory phenotypes. In HL-60 and Jurkat leukemia cells resazurin caused mitochondrial disintegration, respiratory dysfunction, reduced proliferation, and cell death. These effects were preceded by a burst of ROS, especially in HL-60 cells which also were more sensitive and contained autophagic vesicles. Studies in Rho0 cells (devoid of mitochondrial DNA) indicated that the stress response does not depend on the rates of mitochondrial respiration. The anti-proliferative effect of resazurin was confirmed in native acute myelogenous leukemia (AML) blasts. In conclusion, the data suggest that resazurin triggers cellular ROS production and thereby initiates a stress response leading to mitochondrial dysfunction, reduced proliferation, autophagy and cell degradation. The ability of cells to tolerate this type of stress may be important in toxicity and chemoresistance.
Keywords: Cellular stress (reactive oxygen species, mitochondrial respiration); Cell fate (autophagy, cell death); Cell proliferation; Resazurin (Alamar Blue)
Introduction
Cellular stress occurs when a cell is exposed to conditions, such as oxidative stress and nutrient starvation, that threaten its survival. The molecular mechanisms of cellular stress range from specific signaling pathways to random reactions of unstable chemical species [Calabrese et al., 2008]. Severe stress leads to the propagation of detrimental cascades involving elements such as macromolecule damage, energy catastrophe, autophagy and cell death. The type of response depends on both the nature and the extent of the stress-related events as well as cell-specific properties. Specifically, the production of reactive oxygen species (ROS) and disturbances in energy metabolism are two common phenomena that may signal cellular stress.
Resazurin is a blue-colored compound used as an oxidation-reduction indicator in assays examining sperm viability [Comhaire and Vermeulen, 1995], bacteria [Benere et al., 2007], cell proliferation [Porter et al., 2005], toxicity [Husain et al., 1997] and mitochondrial metabolism [Abu-Amero and Bosley, 2005; Zhang et al., 2004]. It is also the primary constituent of the Alamar Blue assay for cell viability [O'Brien et al., 2000; Rasmussen, 1999]. In these assays, resazurin is converted to the pink-colored and fluorescent product resorufin. In living cells, this conversion is typically attributed to the reduction of resazurin by different oxidoreductase enzyme systems that use NAD(P)H as the primary electron donor [O'Brien et al., 2000; Zalata et al., 1998].
Exogenous compounds that react with, and disturb, vital cell functions may induce cellular stress. In the present study, we investigated cellular stress responses induced by the xenobiotic compound resazurin, which is known to react with a wide array of biological substances [O'Brien et al., 2000; Prutz, 1995; Prutz et al., 1996]. When present in cells, resazurin reacts with cell components such as NADH, thiols (e.g., glutathione), amino acids and phenols in both non-enzymatic and enzymatic reactions [Prutz et al., 1996] [Villegas et al., 2005]. Importantly, chemical reactions involving resazurin often involve the generation of ROS [Prutz et al., 1996]. Resazurin is known to accept electrons from free radicals and to react with molecular oxygen in a fashion that promotes ROS generation [Prutz, 1995; Prutz et al., 1996]. Additionally, resazurin acts as an electron acceptor in the electron transport chain within the inner mitochondrial membrane [Ahmed et al., 1994] and has also been linked to oxidation-reduction reactions in the cytosol and nucleus [Gonzalez and Tarloff, 2001]. In summary, the presence of resazurin in living cells is likely to affect redox conditions and energy homeostasis.
Although the chemical reactivity of resazurin has been described, there is little information regarding the physiological consequences of resazurin exposure in living cells. It is generally accepted that resazurin exhibits low toxicity within the timeframe of the assays [Ahmed et al., 1994; Fields and Lancaster, 1993], and the compound is well tolerated in rats [Lutty, 1978]. Hence, potential effects of resazurin itself are normally not evaluated in cell culture applications. On the other hand, cytotoxic effects of resazurin have been demonstrated in leukemia cells [Gloeckner et al., 2001] and ovarian cancer cells [Squatrito et al., 1995].
Due to the broad cross-reactivity of resazurin, we speculated that it would provoke a stress response in cells, which was supported by preliminary observations in cell culture. In the present study, resazurin exposure was used as a model of cellular stress. The aim was to investigate the contributions of ROS generation and mitochondrial impairment as two candidate mechanisms known to play important roles in cellular stress. We used two leukemia cell lines, HL-60 and Jurkat, which have distinct differences in terms of their metabolic profiles and survival signaling [Freeley et al., 2007; Jiang et al., 2008]. These cells were also compared with primary acute myelogenous leukemia (AML) cells. Proliferation, viability and morphology were assessed to study tolerance of the treatment and the nature of the stress response. The effects of resazurin on ROS production and mitochondrial respiratory function were investigated in detail. Cells lacking mitochondrial respiration (MDA-MB-435 Rho0 cells) were used to investigate the importance of mitochondrial respiration during resazurin-induced cellular stress.
Materials and Methods
Cell culture
All cells were cultured at 37 °C with 5% CO2 in humidified incubators. The human acute myeloid leukemia cell line HL-60, derived from a French-American-British (FAB)-M2 patient (DSMZ GmbH, Braunschweig, Germany), and the leukemic T-cell line Jurkat (American Type Culture Collection, ATCC) were cultured in HEPES-modified RPMI-1640 medium (GIBCO, Invitrogen). The medium was supplemented with 10% fetal bovine serum gold (PAA laboratories GmbH, Pasching, Germany) along with streptomycin (5 μg/ml), penicillin (5 units/ml) and L-glutamine (2 mM) (all from Sigma Aldrich). The MDA-MB-435 cell line (ATCC) and the derived mitochondrial DNA-depleted MDA-MB-435 Rho0 cell line [Delsite et al., 2002] were maintained in Dulbecco's modified Eagle's medium (Ham's F-12, 50:50 mix, Mediatech, Herndon, VA) also supplemented with 10% fetal bovine serum gold, 2 mM L-glutamine, 1% penicillin/streptomycin and 50 μg/mL uridine (Sigma-Aldrich). The MDA-MB-435 cell line was originally described as a breast cancer cell line, but recent observations suggest that it is of melanoma origin [Rae et al., 2007].
Resazurin conversion
Resazurin conversion in total cell cultures was measured in flat-bottomed 96-well NUNC tissue culture plates in triplicate cultures (5 × 104 cells/well). Resazurin (Sigma-Aldrich), dissolved in PBS (pH 7.4), was added at concentrations corresponding to 5%, 10% or 20% of the commercial Alamar Blue assay, which is equivalent to 22 μM, 44 μM and 88 μM resazurin, respectively [O'Brien et al., 2000]. Fluorescence excitation was measured at 530 nm while emission was recorded at 590 nm (SpectraMAX-GenimiEM, Molecular Devices Corporation, Sunnyvale, CA) at various time points (0-48 h). Reduction of resazurin to resorufin in single cells was measured by flow cytometry. After incubation in the presence of resazurin, cells were washed twice with PBS and kept on ice until flow cytometric analysis was preformed (Ex 488, Em 585/42; BD FACS Calibur™ flow cytometer and BD CellQuest computer software, BD Bioscience). Further data analysis was done using FlowJo flow cytometry software (Tree Star, Inc.).
Proliferation assay
DNA synthesis was determined by [3H]-thymidine incorporation as previously described [Tronstad et al., 2001]. Briefly, 2 × 104 cells/well were grown and treated in 96-well NUNC tissue culture plates before addition of [3H]-thymidine (1 μCi/well; TRA310, Amersham International, Amersham, UK). Following 6 h incubation, the DNA was harvested and radioactivity was assessed by liquid scintillation counting (Packard Microplate Scintillation and Luminescence counter, Perkin Elmer Life and Analytical Science, Inc., Waltham, USA).
ROS measurements
ROS levels were measured using the fluorescent probe 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) according to the manufacturer's recommendations (Invitrogen, Carlsbad, USA). For the earliest time point (0.25 hours of resazurin exposure) the cell cultures received resazurin simultaneously with 5 μM CM-H2DCFDA, whereas the other cultures were pretreated with resazurin for 2, 4 or 24 hours before addition of the probe. Following 15 min (minutes) of incubation (37 °C, 5% CO2) in presence of 5 μM CM-H2DCFDA the cells were washed twice with PBS and kept on ice until flow cytometric analysis was preformed (Ex 488, Em 530/30; BD FACS Calibur™ flow cytometer, BD CellQuest computer software, BD Bioscience; FlowJo flow cytometry analyzing software, Tree Star, Inc.). Cell debris and irregular particles were gated out before the population median fluorescence intensities were determined and used to calculate the mean values of each group of cultures. The interaction between resazurin and CM-H2DCFDA in the absence of cells was measured in RPMI-1640 medium with different concentrations of H2O2. Resazurin (44 μM) and CM-H2DCFDA (5 μM) were added followed by 15 min of incubation (37 °C, 5% CO2). CM-H2DCFDA fluorescence was then detected (Ex 488, Em 535; Cary Eclipse fluorescence spectrophotometer, Varian).
Oxygen consumption rates
Oxygen consumption rates were analyzed using Oxygraph O2K and DatLab software (Oroboros Instruments, Austria). The mitochondrial experiments were conducted in samples of 1-4 × 106 HL-60 cells in RPMI-1640 medium (GIBCO, Invitrogen, Carlsbad, USA) at 37 °C to reflect normal culture conditions, and the measurements were taken after sequential additions of resazurin (22-88 μM), oligomycin (2 μg/ml), carbonylcyanide-4-(trifluoromethoxy)-phenylhydrazone (FCCP) (titrated to maximal activity, titration range 0.1-1.4 μM), rotenone (0.5 μM), antimycin A (2.5 μM) and NaCN (10 μM). Cells were also pretreated with 44 or 88 μM resazurin for 24 h before they were analyzed in the oxygraph using the same chemicals listed above.
Transmission electron microscopy
Cells were fixed in 0.1 M Na-Cadcodylate buffer, pH 7.4, containing 1.5% glutaraldehyde for 15 min. Samples were rinsed with 0.1 M Na-Cacodylate buffer (10 min) and post-fixed in 1% osmium tetraoxide (OsO4) for 60 min. The specimens were dehydrated using graded ethanol and embedded in epoxy resin, and ultra-thin sections were double-stained with uranyl acetate and lead citrate. Specimens were examined with a Jeol JEM-1230 at the Molecular Imaging Centre in Bergen. Pictures were taken and analyzed using the GATAN multiscan camera.
Nuclear morphology – cell death
Nuclear morphology was investigated using the DNA intercalating dye Hoechst 33342 as previously described [Erikstein et al., 2009]. The nuclei were visually classified as normal, when the fluorescence was evenly distributed and of low intensity, or as abnormal, when nuclei were fragmented or condensed (higher fluorescence intensity) compared to normal morphology [Gjertsen et al., 1994]. To determine the fraction of cells with normal/abnormal nuclear morphology, 300-500 cells were counted in each well.
AML blast cells from patients
The study was approved by the local Ethics Committee (Regional Ethics committee III, University of Bergen, Norway), and samples were collected after informed consent was given. AML cells were isolated from blood samples by density gradient separation (Lymphoprep; Axis-Shield, Oslo, Norway). This method separates a high percentage (> 90%) of AML blast among leukocytes [Bruserud et al., 2004]. Separated cells were frozen in 10% DMSO and stored in liquid nitrogen until use. Clinical and molecular characteristics of the four patients are shown in Table 1. Peripheral blood leukocyte samples with more than 80% AML blasts from four patients with de novo disease were cultured for evaluation of resazurin-mediated effects.
Table 1. Characteristics of donor patients with AML.
The four patients embedded in Haukeland University Hospital were characterised with regards to age, sex, French-American-Britisch (FAB) classification, karyotype, FMS like tyrosine kinase (Flt)-3 mutational status, nucleophosmin (NPM)-1 mutational status, relaps of AML and survival. Karyotype were either normal, translocation from chormosone 4 to 20 (t(4:20)) or non-tested (nt). Flt-3 abnormalities were internal tandem duplications (ITD), Asp(D) 835 mutations (D835) or wild type (wt).
| Patient | Age | Sex | FAB | Karyotype | FLT3 | NPM1 | Relaps | Survival |
|---|---|---|---|---|---|---|---|---|
| 1. | 29 | F | M5 | Normal | ITD, D835 | wt | No | >16 mnd |
| 2. | 59 | F | M4 | Normal | ITD | mut. | No | 9 mnd |
| 3. | 62 | M | M2 | t(4:20) | wt | wt | Yes | 1 mnd |
| 4. | 82 | F | M4 | nt | ITD | wt | No | > 16 mnd |
Statistical analysis
Statistical comparisons were made using GraphPad PRISM® (version 3.0 and 5.0, GraphPad Software, Inc., USA) software with one-way analysis of variance and Tukey's multiple comparison post-tests to determine significant differences between several treatment groups. A students unpaired or paired t-test was employed when only two groups were compared. The number of experiments (n) and experimental replicates are given in the figures and legends.
Results
Resazurin reduces leukemia cell proliferation
The effects of resazurin on cell proliferation were investigated in two leukemia cell lines, HL-60 and Jurkat. First, the conversion of resazurin to resorufin (the principle reaction of viability assays such as the Alamar Blue assay) was measured in cell cultures (Fig 1A). Cells were treated with 22 μM, 44 μM or 88 μM resazurin, with 44 μM representing the concentration normally used in the viability assay [Ahmed et al., 1994]. Resorufin fluorescence accumulated in the two cell cultures with time, as expected (Fig 1A), and their fluorescence intensities were generally of similar levels. Apparently, 22 μM resazurin was not sufficient to saturate the capacity for dye conversion since the intensity was lower than those of 44 μM and 88 μM resazurin. In both cell lines, the rate of resazurin conversion was at its highest in the 4-24 h period compared to conversion at the 0-4 and 24-48 h periods. The ability of individual cells to accumulate intracellular resorufin was confirmed by flow cytometry (Fig 1B). These data clearly demonstrate that the cellular level of fluorescence reaches a near maximum level after only four hours. The continuous increase observed in cell cultures (Fig 1A) may thus be explained by the accumulation of extracellular resorufin in the cell culture medium.
Fig 1. Resazurin conversion and inhibition of proliferation in leukemia cells.
(A) HL-60 and Jurkat cells were treated with 22, 44 or 88 μM resazurin. The conversion of resazurin to resorufin in the cultures was measured by fluorometry (1, 4, 24 or 48 h), and the values are presented as the mean ± SEM of three independent experiments performed in triplicate. B) The level of resorufin was measured in single cells by flow cytometry (0, 2, 4 or 24 h). 10,000 cells were analyzed in each experiment, and the data are presented as the mean ± SEM of the median values from four or five individual cultures. (C) HL-60 and Jurkat cell lines were treated with 22, 44, and 88 μM resazurin for 6 or 24 h, followed by incubation (6 h) in the presence of 3H-thymidine to determine proliferation. The amount of incorporated 3H-thymidine was assessed by scintillation counting. The results are presented as the mean ± SEM of the relative values compared to the untreated controls in three independent experiments, each done in triplicate. (D) Primary acute myelogenous leukemic blasts (> 90%) from four patients (P1-P4; see Materials and Methods for details) were exposed to 200 μM resazurin for 24 h. The proliferation of native AML blasts was determined by 3H-thymidine incorporation after treatment with resazurin. After 18 hours, 3H-thymidine was added and the cultures were incubated for an additional 6 h. The results in A-C are presented as the mean ± SEM of the relative values compared to the untreated controls in three independent experiments, each done in triplicate. The levels of incorporated 3H-thymidine (counts per min) are given as the mean ± SD of six technical replicates. (*: p < 0.05; **: p < 0.01; ***: p < 0.001)
To determine if resazurin and/or its metabolites influence cell proliferation, we measured [3H]-thymidine incorporation (i.e., DNA synthesis) in cell cultures treated for 6 or 24 h periods. HL-60 cells were clearly more sensitive, and their proliferation was significantly reduced even after a short time (6 h) in the lowest resazurin concentration (22 μM). After 24 h, a dose-dependent response was observed; 88 μM resazurin gave nearly complete inhibition. Jurkat cells were more resistant, and an effect was only seen after 24 h at the highest dose (88 μM). The attenuating effect of resazurin on [3H]-thymidine incorporation was also observed in cultures of primary AML blasts isolated from patients. Cells from different individuals displayed unique in vitro proliferative capacities (ranging from 359 to 1145 counts per minute (cpm) per well); however, three of four patient samples demonstrated significantly decreased proliferation in the presence of resazurin (200 μM) (Fig 1D). The fourth patient (P4) showed the same tendency, but we only had duplicate determinations of this sample and could therefore not perform a statistical analysis.
Morphology of cellular stress, autophagy and cell death
The stress-related mechanisms induced by resazurin were investigated in more detail by studying cell morphology. Following treatment and Hoechst-staining of the nuclei, nuclear abnormalities such as chromatin condensation and, at times, fragmentation were observed by fluorescence microscopy. These phenotypes are hallmarks of apoptotic cell death [Galluzzi et al., 2009]. The fraction of abnormal nuclei increased after exposure to resazurin (Fig 2A). A low or negligible number of cells with abnormal nuclei were seen after 6 h, but after 24 h, there was a dose-dependent increase in both cell lines (Fig 2A). Consistent with the effect on proliferation (Fig 1C), HL-60 cells were clearly more sensitive than Jurkat cells. Similar observations were also made in primary AML blast cultures in which there was an overall increase in the fraction of abnormal nuclei for the four patients (Fig 2D; p < 0.001).
Fig 2. Resazurin induces morphological features of cellular stress, autophagy and cell death in leukemia cells.

(A) HL-60 and Jurkat cells were incubated for 6 or 24 h in the presence of 22, 44 or 88 μM resazurin prior to staining with Hoechst 33342. The fraction of cells with abnormal nuclei (i.e., chromatin condensation and fragmentation) was assessed by fluorescence microscopy. Values are presented as the mean ± SEM of three independent experiments, each done in triplicate. (B-C) HL-60 and Jurkat cells were incubated for 24 h with 0, 44 or 88 μM resazurin before transmission electron microscopy analysis. The left columns in panels B) and C) display entire cells with representative morphological features (magnitude 10,000×). The right columns show selected details (30,000×). Treatment with 88 μM resazurin in HL-60 cells resulted in a prevalent amount of cells undergoing autophagy with nuclear condensation. Arrows with letters represent: a = autophagosome and d = disrupted mitochondrion. (D) Primary acute myelogenous leukemia blasts (> 90%) from four patients (P1-P4; see Materials and Methods for details) were exposed to 200 μM resazurin for 24 h. Nuclear morphology was visualized by Hoechst 33342 staining, and the fraction of abnormal nuclei with condensed chromatin consistent with cell death was determined. The percentages of cells with abnormal nuclei are given as the mean ± SD of six technical replicates. Images show sections from a typical experiment with blasts from one patient (P3). Arrows with letters: c = condensed and n = normal nuclear morphology. (*: p < 0.05; ***: p < 0.001)
Transmission electron microscopy (TEM) indicated that HL-60 cells were larger and appeared to harbor more mitochondria than Jurkat cells (Fig 2B). Treatment with 44 μM or 88 μM resazurin for 24 h induced distinct morphological changes (Fig 2B) with more pronounced effects at the higher concentration. The major observations in affected cells were the following: 1) nuclear chromatin condensation consistent with the observations in Fig 2A; 2) mitochondrial alterations such as cristae disintegration, swelling and lysis; 3) double-membrane organelles with dense content, classified as autophagic vesicles, within HL-60 cells indicating an induction of autophagy [Degtyarev et al., 2008] and 4) an intact cell membrane without apoptotic blebbing.
These observations suggest that resazurin induces cellular stress that culminates in autophagy and cell death. These findings were more evident in HL-60 cells than in Jurkat cells, a finding that correlates well with the observed effect on cell proliferation (Fig 1).
Resazurin promotes cellular ROS production
Resazurin has various alternative reaction schemes under biological conditions, and several include ROS generation [Prutz, 1995; Prutz et al., 1996], which may trigger autophagy and apoptosis [Ferraro and Cecconi, 2007]. We therefore studied ROS production using the fluorescent indicator CM-H2DCFDA. First, we investigated if resazurin reacts spontaneously with CM-H2DCFDA in the absence of cells and if this disturbs ROS measurements. The signal from CM-H2DCFDA was measured (using a fluorometer) in the presence of increasing concentrations of hydrogen peroxide (in RPMI) in both the presence and absence of resazurin (Fig 3A). Resazurin clearly quenched the hydrogen peroxide-mediated ROS signal. Such interactions would most likely be a problem with alternative ROS probes due to the complex chemistry of resazurin. Therefore, aware that resazurin may result in the underestimation of ROS levels, even when detected in single cells by flow cytometry, we decided to employ the CM-H2DCFDA probe in cell culture studies. Despite interference, the ROS signal significantly increased in cultures exposed to resazurin for 15 min (Fig 3B). Although the fold ROS induction varied to some degree for five different experiments, the ROS level consistently increased to a greater extent in HL-60 cells compared to Jurkat cells. The level declined in HL-60 cells at later stages while it remained at a high level in Jurkat cells. The gradual decline of the ROS-signal at late time points in HL-60 cells was possibly due to leakage of the probe caused by the corresponding decrease in these cells' viability (Fig 2A). The experiment was repeated in HL-60 cells suspended in RPMI medium without phenol red and HEPES to investigate if these two redox reactive medium components were involved [Prutz et al., 1996; Spierenburg et al., 1984]. The fold ROS induction after addition of resazurin was similar under these conditions (data not shown), demonstrating that resazurin does not react with phenol red or HEPES to cause ROS production. The dramatic difference in ROS induction between HL-60 and Jurkat cells underscores resazurin's role in mediating this effect via biological mechanisms. This may also explain why HL-60 cells are more sensitive than Jurkat cells.
Fig 3. Resazurin induces ROS production in leukemia cells.
The level of ROS was measured using the fluorescence probe CM-H2DCFDA. (A) The effect of resazurin on the ROS signal was measured by fluorometry after 15 min of incubation with various levels of H2O2 (in RPMI-1640 medium, no cells) in the absence or presence of resazurin (44 μM). The figure presents one representative experiment (with duplicate samples) out of a series of three yielding comparable results. (B) The effects of resazurin on ROS levels in individual HL-60 and Jurkat cells were assessed by flow cytometry after incubation with resazurin (44 μM) for varying periods of time. Representative histograms for HL-60 cells are shown. Ten-thousand cells were analyzed in each culture, and the data are presented as the mean ± SEM of the median values from 2-4 cultures. The data are representative of five comparable experiments. (**: p < 0.01; ***: p < 0.001).
Resazurin exposure leads to impairment of mitochondrial respiration
Resazurin interacts with oxygen and mitochondrial metabolism [Prutz, 1995; Prutz et al., 1996; Talbot et al., 2008], and it was therefore of interest to measuring oxygen consumption rates in cell culture. Resazurin was first added to cell culture medium (RPMI) in the absence of cells. This induced spontaneous consumption of molecular oxygen present in the chamber (Fig 4A). The effect disappeared when the instrument light source (visual light, broad spectrum) was turned off and when RPMI medium was exchanged with PBS. The oxygen consumption rate was induced to an equal level in RPMI medium lacking or containing phenol red and HEPES (data not shown). These results demonstrate that resazurin participates in oxygen-dependent photo-oxidation reactions that involve RPMI constituents other than phenol red and HEPES.
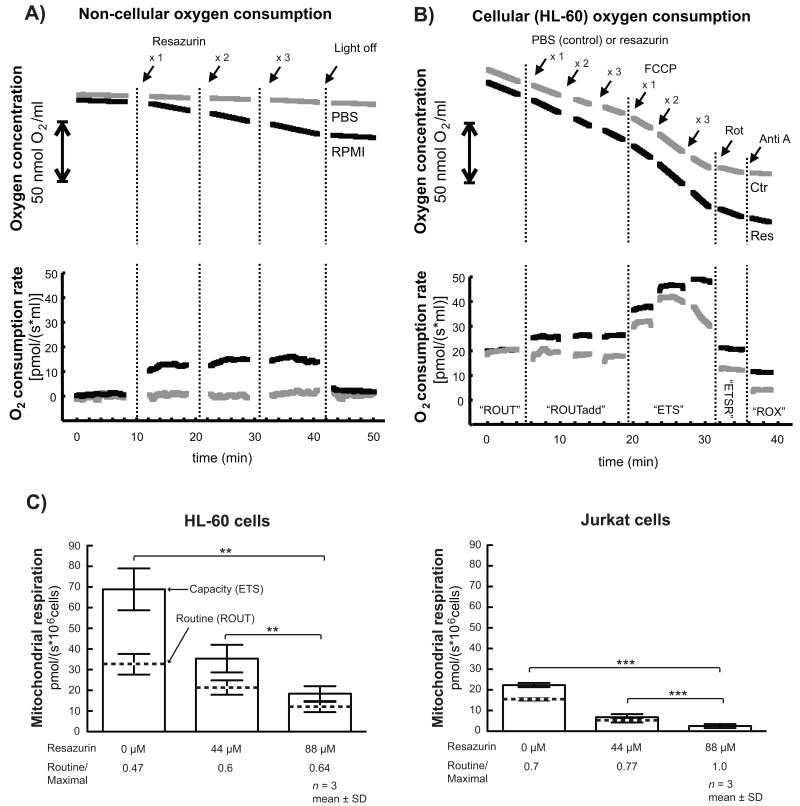
Fig 4. Resazurin does not affect mitochondrial respiration directly, but mitochondrial function is impaired over time.
(A) Non-biological (i.e., non-cellular) oxygen consumption rates in PBS and RPMI-1640 (in the absence of cells) were monitored during three sequential additions of resazurin; final concentrations were 22 μM, 44 μM and 66 μM, respectively. The instrument light source (visual light, broad spectrum) was turned off at the end of the experiment. The scale bar (|↔|) that replaces the y-axis in the upper graph displays a change in oxygen concentration corresponding to 50 nmol O2/ml. The figure shows one representative experiment out of a series of three yielding equivalent results. (B) Oxygen consumption rates were monitored in HL-60 cells during the sequential addition of resazurin (final concentrations: 22 μM, 44 μM and 66 μM), FCCP titrated from 0.5 mM stock, (final concentrations: 0.23, 0.45 and 0.68 mM), 0.5 μM rotenone and 2.5 μM antimycin A. Control cells were treated with PBS instead of resazurin. The figure displays typical traces representative of at least three experiments. ROUT, routine respiration; ROUTadd, routine respiration after addition of PBS (control) or resazurin; ETS, capacity of the electron transport system (the maximum uncoupled rate after FCCP titration); ETSR, rotenone insensitive ETS; ROX, residual oxygen consumption. (C) Routine respiration (ROUT) and electron transport system capacity (ETS) rates were determined in HL-60 or Jurkat cells exposed to 44 or 88 μM resazurin for 24 h. All values are presented as the mean ± SD of 3-4 independent experiments (**: p < 0.01; ***: p < 0.001 double sided paired t-test).
To examine if resazurin interacts directly with mitochondrial respiration, oxygen consumption rates in HL-60 cells were continuously monitored during sequential infusion of resazurin and respiratory modulators. The oxygen consumption rate was spontaneously induced upon addition of resazurin (Fig 4B); however, the induction was equal to the residual activity observed after complete respiratory inhibition with antimycin A and to chemical induction in RPMI (Fig 4A). This suggests that the resazurin-mediated increase in oxygen consumption in cell cultures is solely due to chemical interactions with RPMI constituents and not mitochondrial respiration. Furthermore, resazurin did not alter the maximal uncoupled capacity of the electron transport system (ETS) or rotenone-insensitive activity (Fig 4B).
To investigate if resazurin affects respiratory function over time, we measured oxygen consumption in both HL-60 and Jurkat cells after 24 h of treatment (Fig 4C). Interestingly, untreated HL-60 cells had a significantly higher basal respiratory activity than Jurkat cells. Resazurin exposure caused a loss of mitochondrial function in both cell types, and the effect was more severe with 88 μM than with 44 μM resazurin. The uncoupled respiratory rate (ETS), which is a measure of the total respiratory capacity, was significantly reduced. Although routine respiration also fell, the cells clearly tried to adapt by utilizing a larger fraction of their respiratory capacity. Simultaneously, the respiratory control ratio (ETS:oligomycin insensitive activity) was reduced from 10.6 to 4.5 and 4.4 in HL-60 cells treated with 44 μM and 88 μM, respectively. In Jurkat cells, the ratios were determined to be 6.3 to 3.1 and 1.1, respectively. This loss in respiratory control indicates that there is an attenuation of mitochondrial integrity, which is supported by the TEM images (Fig 2B and C). These data suggest that the bioenergetic function of mitochondria is compromised after resazurin treatment.
Mitochondrial respiration is not required for resazurin-induced cellular stress
One interpretation of the data presented thus far is that a low basal respiratory rate, as seen in Jurkat cells, may confer a protective effect against resazurin-induced stress. To test this possibility, we compared MDA-MB-435 Rho0 cells, which are respiratory deficient, with the original MDA-MB-435 cell line [Delsite et al., 2002]. The MDA-MB-435 cells were extremely sensitive to resazurin, both in terms of abnormal nuclei (Fig 5A) and proliferation (Fig 5B), and the effects were maximal even at the lowest dose of resazurin. Interestingly, MDA-MB-435 Rho0 cells were equally sensitive compared to the wild type MDA-MB-435 cells. These data indicate that there is no correlation between the bioenergetic function of mitochondria and cellular sensitivity to resazurin-induced stress. It cannot be excluded, however, that Rho0 cells undergo additional or alternative stress responses given that their metabolic machinery is amputated compared to the wild type cells.
Fig 5. Resazurin inhibits proliferation and induces cell death in cells lacking mitochondrial respiration.

(A) The effect of resazurin on proliferation and viability was investigated in the MDA-MB-435 cell line and the respiratory deficient daughter line MDA-MB-435 Rho0. Cells were incubated with 22, 44 and 88 μM resazurin for 24 h before fixation. The nuclei were stained with Hoechst 33342, and the fraction of cells with abnormal nuclei (i.e., condensed chromatin) was analyzed by fluorescence microscopy. Values are presented as the mean ± SEM of three independent experiments, each performed in triplicate (***: p < 0.001). The fluorescence images are representative of each experiment. Arrows with letters: c = condensed nuclei and r = rounded cellular morphology. (B) Proliferation was assessed by 3H-thymidine incorporation in MDA-MB-435 and MDA-MB-435 Rho0 cells treated with 22, 44 and 88 μM resazurin. 3H-thymidine was added after 18 h of incubation, followed by an additional 6 h of incubation. Values are presented as the mean ± SEM of three independent experiments, each performed in triplicate (***: p < 0.001). (C) Resazurin conversion was determined by fluorometry in MDA-MB-435 and MDA-MB-435 Rho0 cells treated as described in (A). Values are presented as the mean ± SEM of three independent experiments, each performed in triplicate.
Discussion
The data presented herein demonstrate that resazurin induces cellular stress mechanisms that reduce cell proliferation and ultimately lead to autophagy and cell death. Resazurin clearly causes oxidative stress due to increased ROS generation, and it possibly results in energetic stress due to mitochondrial impairment. Here we suggest a mechanism in which resazurin triggers ROS production via its vigorous cross-reactivity with cellular constituents and metabolites, which initiates a cellular stress response and leads to mitochondrial dysfunction and degradation of the cell.
Resazurin is the viability indicator in the commercial Alamar Blue assay reagent, which also contains ferricyanide/ferrocyanide and methylene blue to stabilize resazurin and prevent reduction [O'Brien et al., 2000; Rasmussen, 1999]. The effects of the Alamar Blue reagent on cell biochemistry and physiology were equal to those observed with pure resazurin (data not shown). We therefore conclude that resazurin is the principle toxic mediator in the Alamar Blue assay reagent. The conversion of resazurin to resorufin, which is detected in the viability assay, depends on metabolic processes in the cells [Fields and Lancaster, 1993]. This is similar to other viability/proliferation detection methods such as the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)-assay [Pagé et al., 1993]. A caveat of these assays is that the readout may also be influenced by changes in metabolic rate that are not directly linked to viability and proliferation [Pagliacci et al., 1993; Tronstad et al., 2001]. Furthermore, as the present study demonstrates, the possible effects of the indicator itself are suppressed in the result of these assays and are difficult to detect. When using such assays for toxicology screening, it should therefore be considered that resazurin may have additional, or even synergistic, effects in combination with the test agents. Thus, resazurin has been a useful reporting agent in biological applications, but the present findings underscores that care should be taken when this agent is employed in living cells, since it affects mechanisms that are crucial for maintaining cellular homeostasis.
3H-thymidine incorporation and nuclear morphology demonstrated that resazurin has antiproliferative effects and that prolonged exposure induces cell death. This was observed in transformed (HL-60 and Jurkat) as well as primary (AML) leukemia cells. AML is a heterogeneous disease with recurrent aberrations in growth factor signal pathways and transcriptional regulation—e.g., FMS-like tyrosine kinase 3 (Flt-3) and nucleophosmin-1 (NPM-1)—with a major impact on relapse of disease after treatment and survival [Lowenberg, 2008]. In our experiments with AML cells from four patients, sensitivity to resazurin did not tend to correlate with Flt-3 and NPM-1 status, patient age, FAB classification or survival.
Although resazurin is converted to resorufin inside cells, we found that the increase in resorufin fluorescence in these cultures was primarily due to extracellular accumulation of the dye, which is consistent with previous reports [O'Brien et al., 2000]. The cell type-specific sensitivity to resazurin did not correlate with resorufin production, the rate of which varied for the different cell types, which was also consistent with others' observations [Gloeckner et al., 2001]. Interestingly, the cellular level (single cells) of resorufin was actually higher in Jurkat cells, which were more resistant than HL-60 cells (Fig 1A). These observations indicate that the antiproliferative effect may not be mediated via resorufin; resazurin-mediated ROS production seems to take place at the expense of resorufin production [Prutz et al., 1996].
There was a transient burst of ROS produced immediately after addition of resazurin to the cell cultures, and the induction was significantly more pronounced in HL-60 cells compared to Jurkat cells. The difference between the two cell lines demonstrates that this is a biological effect and not simply a chemical artifact. Furthermore, HL-60 cells have been reported to be more sensitive to oxidative stress than Jurkat cells [Netto et al., 2009]. These findings support the idea that ROS production is a critical factor in resazurin-induced stress and provide a rational explanation for why HL-60 cells are the most sensitive in terms of viability and proliferation. Supplementation with the antioxidants Tempol and NAC, however, did not prevent the massive ROS production by resazurin and, therefore, did not rescue the cells in our experiments (data not shown).
Morphological analysis of cells exposed to resazurin demonstrated that HL-60 and Jurkat cells undergo severe stress that induced nuclear and mitochondrial alterations and resulted in apoptosis-like cell death. The most striking differences between these cell types were that HL-60 cells seemed to have more mitochondria than Jurkat cells and that HL-60 cells contained autophagic vesicles after treatment with resazurin. Both autophagy and apoptosis have previously been reported to be consequences of oxidative stress and/or energy depletion [Maiuri et al., 2007; Sasnauskiene et al., 2009b], and apoptosis may also be the ultimate outcome of severe autophagy [Sasnauskiene et al., 2009a]. Previous studies have shown that the Jurkat cell line has a phosphatase and tensin homolog (PTEN) mutation that maintains the Akt survival signaling pathway constitutively active [Freeley et al., 2007]. There are several reports suggesting that Akt must be inactive for autophagy to occur [Degenhardt et al., 2006; Degtyarev et al., 2008]. This may be a possible explanation for why Jurkat cells do not seem to undergo autophagy whereas HL-60 cells do.
Mitochondrial respiration was not acutely affected by addition of resazurin, but alterations in respiratory routine activity, total capacity and respiratory control unambiguously demonstrated that the functions of mitochondrial bioenergetics were impaired in both HL-60 cells and Jurkat cells after 24 h exposure (Fig 4C). Untreated HL-60 cells exhibited a significantly higher basal respiratory rate than Jurkat cells (Fig 4C). Some of this can be explained by differences in cell size illustrated in the TEM images (Fig 2B and C), but these images also indicate that the cytoplasmic density of mitochondria is higher in HL-60 cells. We therefore speculated that there is a positive correlation between basal respiratory rate and resazurin sensitivity. This theory was tested by using respiratory-deficient MDA-MB-435 Rho0 cells; yet these cells were as sensitive as wild type MDA-MB-435 cells (Fig 5). Thus, the basal bioenergetic function of mitochondria does not seem to be a critical determinant of resazurin sensitivity. It should be noted, however, that Rho0 cells may already be stressed compared to normal cells with intact mitochondria since they have less metabolic flexibility per se and produce more ROS [Indo et al., 2007].
To summarize, these data indicate that resazurin induces an immediate burst of ROS that leads to oxidative stress and a gradual loss of vital functions such as mitochondrial respiration. It remains an open question whether mitochondrial impairment is simply a consequence of ROS damage or degradation (e.g., mitophagy) or if it mediates downstream effects in the stress response. Both oxidative stress and energy depletion are consistent with autophagy and apoptosis-like cell death. Cellular sensitivity to resazurin may be determined by multiple factors of metabolism, survival signaling and stress response pathways, but ROS tolerance appears to be an important factor. In conclusion, resazurin initiates a cellular stress response by triggering ROS production and a downstream cascade leading to mitochondrial impairment, autophagy and cell death. More detailed studies are necessary to identify determinants of resazurin sensitivity, but factors regulating ROS tolerance are probable candidates.
Acknowledgments
We thank Ingrid Strand and Anne Karin Nyhaug for excellent technical assistance. We are very thankful to Randi Hovland, PhD, at the Center of Medical Genetics and Molecular Medicine, Haukeland University Hospital, who conducted the genetic analysis. We would like to thank the Molecular Imaging Centre (MIC) in Bergen for professional help with the TEM equipment. This work was supported by the Meltzer Foundation, the Norwegian Cancer Society, Oddrun Mjåland Legacy, Agnes Sars Legacy, the University of Bergen, and the National Institute of Health (grant CA121904 and 113655 to KKS). The authors declare no conflict of interest.
Grant Information: Contract grant sponsors: The University of Bergen (the Meltzer Foundation, Oddrun Mjåland Legacy, Agnes Sars Legacy), The Norwegian Cancer Society, and the National Institute of Health (Contract grant number CA121904 and 113655 to KKS). The authors declare no conflict of interest.
References
- Abu-Amero KK, Bosley TM. Detection of mitochondrial respiratory dysfunction in circulating lymphocytes using resazurin. Arch Pathol Lab Med. 2005;129:1295–8. doi: 10.5858/2005-129-1295-DOMRDI. [DOI] [PubMed] [Google Scholar]
- Ahmed SA, Gogal RM, Jr, Walsh JE. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods. 1994;170:211–24. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Benere E, da Luz RA, Vermeersch M, Cos P, Maes L. A new quantitative in vitro microculture method for Giardia duodenalis trophozoites. J Microbiol Methods. 2007;71:101–6. doi: 10.1016/j.mimet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Bruserud O, Ryningen A, Wergeland L, Glenjen NI, Gjertsen BT. Osteoblasts increase proliferation and release of pro-angiogenic interleukin 8 by native human acute myelogenous leukemia blasts. Haematologica. 2004;89:391–402. [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, Bates TE, Giuffrida Stella AM, Schapira T, Dinkova Kostova AT, Rizzarelli E. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33:2444–71. doi: 10.1007/s11064-008-9775-9. [DOI] [PubMed] [Google Scholar]
- Comhaire F, Vermeulen L. Human semen analysis. Hum Reprod Update. 1995;1:343–62. doi: 10.1093/humupd/1.4.343. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gelinas C, Fan Y, Nelson DA, Jin S, White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyarev M, De Maziere A, Orr C, Lin J, Lee BB, Tien JY, Prior WW, van Dijk S, Wu H, Gray DC, Davis DP, Stern HM, Murray LJ, Hoeflich KP, Klumperman J, Friedman LS, Lin K. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol. 2008;183:101–16. doi: 10.1083/jcb.200801099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsite R, Kachhap S, Anbazhagan R, Gabrielson E, Singh KK. Nuclear genes involved in mitochondria-to-nucleus communication in breast cancer cells. Mol Cancer. 2002;1:6. doi: 10.1186/1476-4598-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikstein BS, McCormack E, Tronstad KJ, Schwede F, Berge R, Gjertsen BT. Protein kinase A activators and the pan-PPAR agonist tetradecylthioacetic acid elicit synergistic anti-leukaemic effects in AML through CREB. Leuk Res. 2009 doi: 10.1016/j.leukres.2009.09.005. in press. [DOI] [PubMed] [Google Scholar]
- Ferraro E, Cecconi F. Autophagic and apoptotic response to stress signals in mammalian cells. Arch Biochem Biophys. 2007;462:210–9. doi: 10.1016/j.abb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Fields RD, Lancaster MV. Dual-attribute continuous monitoring of cell proliferation/cytotoxicity. Am Biotechnol Lab. 1993;11:48–50. [PubMed] [Google Scholar]
- Freeley M, Park J, Yang KJ, Wange RL, Volkov Y, Kelleher D, Long A. Loss of PTEN expression does not contribute to PDK-1 activity and PKC activation-loop phosphorylation in Jurkat leukaemic T cells. Cell Signal. 2007;19:2444–57. doi: 10.1016/j.cellsig.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Aaronson SA, Abrams J, Alnemri ES, Andrews DW, Baehrecke EH, Bazan NG, Blagosklonny MV, Blomgren K, Borner C, Bredesen DE, Brenner C, Castedo M, Cidlowski JA, Ciechanover A, Cohen GM, De Laurenzi V, De Maria R, Deshmukh M, Dynlacht BD, El-Deiry WS, Flavell RA, Fulda S, Garrido C, Golstein P, Gougeon ML, Green DR, Gronemeyer H, Hajnoczky G, Hardwick JM, Hengartner MO, Ichijo H, Jaattela M, Kepp O, Kimchi A, Klionsky DJ, Knight RA, Kornbluth S, Kumar S, Levine B, Lipton SA, Lugli E, Madeo F, Malomi W, Marine JC, Martin SJ, Medema JP, Mehlen P, Melino G, Moll UM, Morselli E, Nagata S, Nicholson DW, Nicotera P, Nunez G, Oren M, Penninger J, Pervaiz S, Peter ME, Piacentini M, Prehn JH, Puthalakath H, Rabinovich GA, Rizzuto R, Rodrigues CM, Rubinsztein DC, Rudel T, Scorrano L, Simon HU, Steller H, Tschopp J, Tsujimoto Y, Vandenabeele P, Vitale I, Vousden KH, Youle RJ, Yuan J, Zhivotovsky B, Kroemer G. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 2009;16:1093–107. doi: 10.1038/cdd.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjertsen BT, Cressey LI, Ruchaud S, Houge G, Lanotte M, Doskeland SO. Multiple apoptotic death types triggered through activation of separate pathways by cAMP and inhibitors of protein phosphatases in one (IPC leukemia) cell line. J Cell Sci. 1994;107(Pt 12):3363–77. doi: 10.1242/jcs.107.12.3363. [DOI] [PubMed] [Google Scholar]
- Gloeckner H, Jonuleit T, Lemke HD. Monitoring of cell viability and cell growth in a hollow-fiber bioreactor by use of the dye Alamar Blue. J Immunol Methods. 2001;252:131–8. doi: 10.1016/s0022-1759(01)00347-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez RJ, Tarloff JB. Evaluation of hepatic subcellular fractions for Alamar blue and MTT reductase activity. Toxicol In Vitro. 2001;15:257–9. doi: 10.1016/s0887-2333(01)00014-5. [DOI] [PubMed] [Google Scholar]
- Husain A, Yan XJ, Rosales N, Aghajanian C, Schwartz GK, Spriggs DR. UCN-01 in ovary cancer cells: effective as a single agent and in combination with cis-diamminedichloroplatinum(II)independent of p53 status. Clin Cancer Res. 1997;3:2089–97. [PubMed] [Google Scholar]
- Indo HP, Davidson M, Yen HC, Suenaga S, Tomita K, Nishii T, Higuchi M, Koga Y, Ozawa T, Majima HJ. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion. 2007;7:106–18. doi: 10.1016/j.mito.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Jiang G, Albihn A, Tang T, Tian Z, Henriksson M. Role of Myc in differentiation and apoptosis in HL60 cells after exposure to arsenic trioxide or all-trans retinoic acid. Leuk Res. 2008;32:297–307. doi: 10.1016/j.leukres.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Lowenberg B. Acute myeloid leukemia: the challenge of capturing disease variety. Hematology Am Soc Hematol Educ Program. 2008:1–11. doi: 10.1182/asheducation-2008.1.1. [DOI] [PubMed] [Google Scholar]
- Lutty GA. The acute intravenous toxicity of biological stains, dyes, and other fluorescent substances. Toxicol Appl Pharmacol. 1978;44:225–49. doi: 10.1016/0041-008x(78)90185-0. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Netto CD, Santos ES, Castro CP, da Silva AJ, Rumjanek VM, Costa PR. (+/-)-3,4-Dihydroxy-8,9-methylenedioxypterocarpan and derivatives: cytotoxic effect on human leukemia cell lines. Eur J Med Chem. 2009;44:920–5. doi: 10.1016/j.ejmech.2008.01.027. [DOI] [PubMed] [Google Scholar]
- O'Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–6. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- Pagé B, Pagé M, Noël C. A new fluorometric assay for cytotoxicity measurements in vitro. International Journal of Oncology. 1993;3:473–476. [PubMed] [Google Scholar]
- Pagliacci MC, Spinozzi F, Migliorati G, Fumi G, Smacchia M, Grignani F, Riccardi C, Nicoletti I. Genistein inhibits tumour cell growth in vitro but enhances mitochondrial reduction of tetrazolium salts: a further pitfall in the use of the MTT assay for evaluating cell growth and survival. Eur J Cancer. 1993;29A:1573–7. doi: 10.1016/0959-8049(93)90297-s. [DOI] [PubMed] [Google Scholar]
- Porter JF, Sharma S, Wilson DL, Kappil MA, Hart RP, Denhardt DT. Tissue inhibitor of metalloproteinases-1 stimulates gene expression in MDA-MB-435 human breast cancer cells by means of its ability to inhibit metalloproteinases. Breast Cancer Res Treat. 2005;94:185–93. doi: 10.1007/s10549-005-7728-4. [DOI] [PubMed] [Google Scholar]
- Prutz WA. Glutathione peroxidase-like activity of simple selenium compounds. Peroxides and the heterocyclic N-oxide resazurin acting as O-atom donors. Z Naturforsch C. 1995;50:209–19. [PubMed] [Google Scholar]
- Prutz WA, Butler J, Land EJ. Photocatalytic and free radical interactions of the heterocyclic N-oxide resazurin with NADH, GSH, and Dopa. Arch Biochem Biophys. 1996;327:239–48. doi: 10.1006/abbi.1996.0116. [DOI] [PubMed] [Google Scholar]
- Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD. MDA-MB-435 cells are derived from M14 melanoma cells--a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat. 2007;104:13–9. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- Rasmussen ES. Use of fluorescent redox indicators to evaluate cell proliferation and viability. In vitro and molecular toxicology. 1999;12:47–58. [Google Scholar]
- Sasnauskiene A, Kadziauskas J, Vezelyte N, Jonusiene V, Kirveliene V. Apoptosis, autophagy and cell cycle arrest following photodamage to mitochondrial interior. Apoptosis. 2009a;14:276–86. doi: 10.1007/s10495-008-0292-8. [DOI] [PubMed] [Google Scholar]
- Sasnauskiene A, Kadziauskas J, Vezelyte N, Jonusiene V, Kirveliene V. Damage targeted to the mitochondrial interior induces autophagy, cell cycle arrest and, only at high doses, apoptosis. Autophagy. 2009b;5:743–4. doi: 10.4161/auto.5.5.8701. [DOI] [PubMed] [Google Scholar]
- Spierenburg GT, Oerlemans FT, van Laarhoven JP, de Bruyn CH. Phototoxicity of N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-buffered culture media for human leukemic cell lines. Cancer Res. 1984;44:2253–4. [PubMed] [Google Scholar]
- Squatrito RC, Connor JP, Buller RE. Comparison of a novel redox dye cell growth assay to the ATP bioluminescence assay. Gynecol Oncol. 1995;58:101–5. doi: 10.1006/gyno.1995.1190. [DOI] [PubMed] [Google Scholar]
- Talbot JD, Barrett JN, Barrett EF, David G. Rapid, stimulation-induced reduction of C12-resorufin in motor nerve terminals: linkage to mitochondrial metabolism. J Neurochem. 2008;105:807–19. doi: 10.1111/j.1471-4159.2007.05176.x. [DOI] [PubMed] [Google Scholar]
- Tronstad KJ, Berge K, Flindt EN, Kristiansen K, Berge RK. Optimization of methods and treatment conditions for studying effects of fatty acids on cell growth. Lipids. 2001;36:305–13. doi: 10.1007/s11745-001-0722-8. [DOI] [PubMed] [Google Scholar]
- Villegas ML, Bertolotti SG, Previtali CM, Encinas MV. Reactions of excited states of phenoxazin-3-one dyes with amino acids. Photochem Photobiol. 2005;81:884–90. doi: 10.1562/2004-10-12-RA-342. [DOI] [PubMed] [Google Scholar]
- Zalata AA, Lammertijn N, Christophe A, Comhaire FH. The correlates and alleged biochemical background of the resazurin reduction test in semen. Int J Androl. 1998;21:289–94. doi: 10.1046/j.1365-2605.1998.00126.x. [DOI] [PubMed] [Google Scholar]
- Zhang HX, Du GH, Zhang JT. Assay of mitochondrial functions by resazurin in vitro. Acta Pharmacol Sin. 2004;25:385–9. [PubMed] [Google Scholar]