Abstract
Macrocyclic trichothecenes, which have potent cytotoxicity, have been isolated from many different fungal species. These compounds were evaluated clinically by the U.S. National Cancer Institute in the 1970's and 1980's. However, they have yet to be advanced into viable drugs due to severe side effects. Our team is investigating a diverse library of filamentous fungi for new anticancer leads. To avoid re-isolating macrocyclic trichothecenes via bioactivity-directed fractionation studies, a protocol for their facile dereplication was developed. The method uses readily available photodiode array detectors to identify one of two types of characteristic UV spectra for these compounds. Also, diagnostic signals can be observed in the 1H-NMR spectra, particularly for the epoxide and conjugated diene moieties, even at the level of a crude extract. Using these techniques in a complementary fashion, macrocyclic trichothecenes can be dereplicated rapidly.
Keywords: dereplication, filamentous fungi, macrocyclic trichothecenes
Introduction
Macrocyclic trichothecenes (MTs) are a class of sesquiterpene lactones that are reported to have a wide array of biological activities, including: antimalarial,1,2,3 antileukemic,4 antibiotic,5 antifungal,6 antiviral,7 and anticancer.8,9,10,11 According to Grove,12 they were first described in 1946 and isolated in pure form in 1962, and their potent biological activity and interesting chemistry stimulated a large amount of research throughout the 1970s and 1980s. Grove's comprehensive review12 of the literature on MTs through 1991 should be referenced for more complete details on the history, chemistry, and structural variants of MTs, which at that time were noted at over 60 distinct compounds and today probably represent over 100 compounds.1,13 Moreover, Jarvis and Mazzola4 reviewed both the biological activity of these compounds, which are reported to effect cell growth via inhibition of protein synthesis, and their evaluation against in vivo models of cancer by the U.S. National Cancer Institute (NCI). Jarvis and colleagues conducted medicinal chemistry studies on analogs of the MTs as well.14,15 Yet, despite evaluating over 90 natural and synthetic analogs of MTs in in vitro and in vivo models of cancer, only two MTs were evaluated clinically by the NCI, and both were dropped due to severe toxicity (D.J. Newman, National Cancer Institute, Frederick, MD, USA, personal communication).
In the course of our early research on filamentous fungi, a project that targets the isolation of structurally unique compounds with potent anticancer activity,16,17 several macrocyclic trichothecenes [e.g. verrucarin A (1) and roridin E (2)] were isolated via bioactivity-directed fractionation based on cytotoxic activity vs a human tumor panel consisting of three cell lines. Not surprisingly based on the literature, these MTs and related analogs were quite cytotoxic, being on the same order of potency as the positive control, camptothecin, even when the MTs were present at a low concentration in a crude extract. As such, the exciting cytotoxicity results skewed the chemistry efforts towards the isolation and elucidation of known MTs and/or closely related analogs, and in turn, pulled resources away from the discovery of bioactive leads with new structural types.
What was needed to circumvent this problem was a procedure to dereplicate MTs, particularly at the level of the crude extract and using techniques and tools that were readily available at the bench. Since most of the filamentous fungi under examination for this project, sampled from a library of over 50,000 isolates, were of unknown taxonomy at the time of initial evaluation,16 genera well known to produce MTs (e.g., Fusarium spp., Myrothecium spp., Stachybotrys spp., and Trichothecium spp.)4 could not be eliminated indiscriminately; other authors have noted similar challenges when relying upon the taxonomy of fungi for dereplication.18 Moreover, with three notable exceptions,13,18,19 the literature on analytical dereplication procedures for MTs was scant, probably because, as Grove observed,12 MTs have not been implicated as key mycotoxin contaminants of grain or grain products, and thus, there has not been a demand to develop analytical methods for their rapid identification/quantification. The exceptions were promising and comprehensive, however, two relied upon techniques and tools that may not be available in all laboratories, namely HRMS18 and capillary flow NMR.19 The third was more accessible, using photodiode array detection, although it focused on toxins of Stachybotrys sp.;13 methods that could be applied to all structural variants of MTs were needed.
Thus, a dereplication strategy was developed and implemented that capitalized upon the UV (via photodiode array detection) and 1H NMR spectra. The procedure discerns readily samples that contain MTs, and the pattern recognition afforded by the UV spectra makes the strategy straightforward to implement.
Results and Discussion
Key structural features of macrocyclic trichothecenes
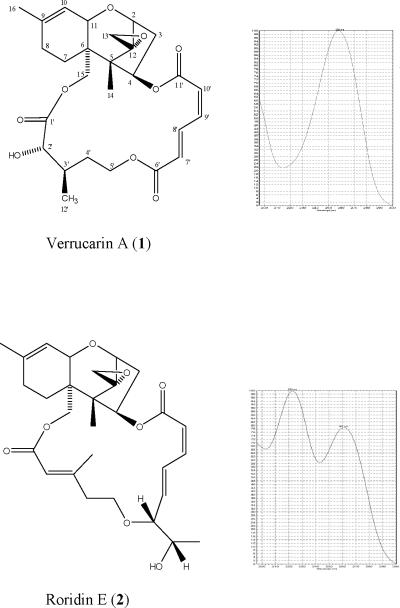
Macrocyclic trichothecenes (MTs) are comprised of several structural features that are discernable in the 1H NMR and/or UV spectra (Table 1). When coupled together, and supported by potent cytotoxicity, these features permitted the facile dereplication of even crude extracts for MTs. The most telling feature in the 1H NMR spectrum may be the epoxide ring across positions C-12 to C-13, which is present in all but three MTs,12 and the cytotoxicity of this class of compounds was reported to decrease substantially when this moiety was absent.20 The two protons of the epoxide, H-13a and H-13b, displayed characteristic doublets (J=4 Hz) in the 1H NMR spectrum at approximately δ 3.10 and 2.78. The sesquiterpene core of the molecule has a mono-substituted double bond across positions C-9 to C-10, and this rendered two more distinguishing features in the 1H NMR spectrum. The only proton of this double bond, H-10, displayed a broad doublet at approximately δ 5.45 (J=6 Hz). Moreover, the methyl substituent at position C-9 (i.e. methyl C-16) displayed a singlet for H3-16 at approximately δ 1.73; at higher field, allylic coupling of ~1 Hz may be observed between H3-16 and H-10. Finally, the macrocycle moiety, which loops from C-4 to C-15 via a carbon chain of variable lengths (typically C11 or C12 chains), includes α,β-unsaturated lactone moieties that provided key spectroscopic features, particularly in UV spectra.18 In the case of compounds like verrucarin A (1), there was a single UV maximum at approximately 260 nm. Whereas, in the case of compounds like roridin E, the additional unsaturated carbonyl accounts for a second UV peak at approximately 230 nm. These dienes also produced key signals in the olefinic region of the 1H NMR (i.e. δ 5.72 – 8.01), but the UV spectrum provides a tool that is readily discernable. In summary, when an active extract was identified, if these key structural features were noted in the 1H NMR data and/or the UV spectrum (Table 1), then the activity of the fungus was ascribed with great confidence to MTs.
Table 1.
Key structural features and spectroscopic properties of macrocyclic trichothecenes.
| Structural Feature | Spectroscopic Properties |
|---|---|
| Epoxide across positions C-12 to C-13 | Two prominent doublets (H-13a and H-13b) in the 1H NMR spectrum (e.g. δ 3.10 and 2.78; J = 4 Hz). |
| Double bond across positions C-9 to C-10 | Prominent broad doublet (with possible allylic coupling at higher field) in 1H NMR spectrum for H-10 (e.g. δ 5.45). |
| Vinyl methyl group at position C-16 | Prominent singlet (with possible allylic coupling at higher field) in 1H NMR spectrum (e.g. δ 1.73). |
| Two α,β-unsaturated lactone moieties at positions C-6' to C-11' [e.g. compounds similar to verrucarin A (1)]. | Prominent UV spectrum with a single maximum (λ ~ 260 nm). |
| Additional α,β-unsaturated lactone moieties at positions C-1' to C-3' [e.g. compounds similar to roridin E (2)]. | Prominent UV spectrum with two maxima (λ ~ 260 and 230 nm). |
Development of the dereplication methodology
Initial screening of 154 organic extracts of filamentous fungi for cytotoxicity against a human tumor panel resulted in the identification of eight active samples, two of which were the most potent. One of these two was carried through a bioactivity-directed fractionation. After several rounds of chromatography followed by assessment of cytotoxicity, the activity could be ascribed to two known compounds, the macrocyclic trichothecenes verrucarin A (1) and roridin E (2), among other similar compounds; the 1H and 13C NMR data for 1 and 2 were in excellent agreement with the literature.1,6,21,22 The taxonomy of the fungus (MSX 28737) was evaluated and found to be closely related to Myrothecium roridum, a known producer of MTs.23 However, at the time of initial examination, the taxonomy of the fungus was unknown.
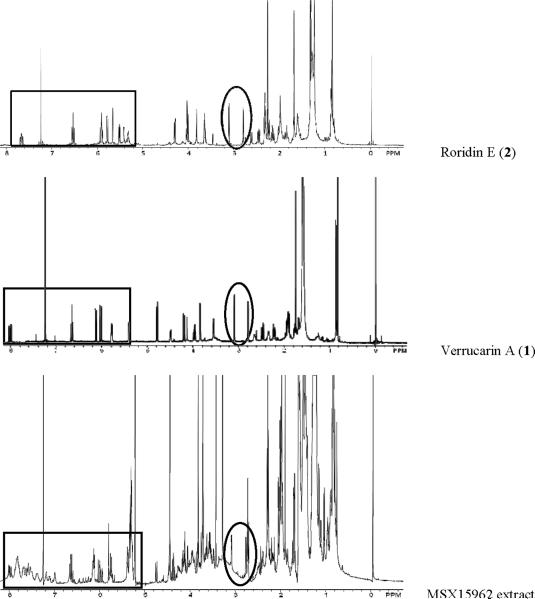
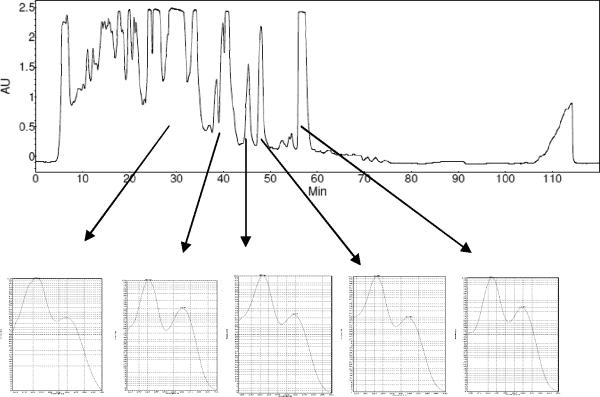
The isolation of these two known MTs was somewhat disappointing, and the team feared that the potent activity of other extracts could be due to similar MTs. Thus, the MTs isolated from MSX 28737 were used as reference standards to develop the dereplication methodology. Figures 1 and 2 display the structures of compounds 1 and 2 and their corresponding UV and 1H NMR spectra. Prominent signals in the NMR spectra (Fig. 2) are notated as they relate to the key signals outlined in Table 1. Specifically, the epoxide protons (H2-13), the olefinic proton (H-10), and the vinylic methyl group (H3-16) were observed in both compounds 1 and 2. If there were major differences between the two with respect to their 1H NMR spectra, it was only the larger number of olefinic signals observed in compound 2. However, the key differences between the verrucarin-like compounds (based on 1) and the roridin-like compounds (based on 2) was discernable in the UV spectra (Fig. 1). The macrocyclic portion of 1 resulted in a single UV maximum (~260 nm), whereas the added unsaturation in the macrocycle of 2 resulted in two UV maxima (~260 and 230 nm). In essence, these two pieces of spectroscopic data (1H NMR and UV), both of which can be acquired readily on relatively crude extracts, work in a complementary fashion to dereplicate MTs. The 1H NMR data identified key features of the sesquiterpene core (e.g., epoxide, monosubstituted olefin, vinylic methyl group), while the UV data identified aspects of the macrocycle (e.g., more or less unsaturation). In particular, the visual nature of the UV spectrum may be the most straightforward to recognize. For example, Figure 3 displays a prep-HPLC chromatogram of an active fraction, and several peaks have a UV profile based on the roridin-like compounds, further justifying the dereplication of this sample.
Figure 1.
Structures and UV profiles of verrucarin A (1) and roridin E (2).
Figure 2.
1H NMR spectra of the MTs, roridin E (2) and verrucarin A (1), and a crude extract that was dereplicated for MTs. The oval highlights the typical region for the chemical shifts of epoxide protons. The rectangle highlights the typical region for the chemical shift of olefinic protons.
Figure 3.
Prep-HPLC chromatogram (top) and UV spectra (bottom) of several potential MTs with a roridin-type macrocycle from another fraction of Myrothecium roridum (MSX 28737).
Use of the dereplication methodology
Over the past year, this dereplication methodology has been used routinely on scores of cytotoxic extracts of filamentous fungi. For this, the goal was either 1) to determine that the cytotoxicity could be ascribed to macrocyclic trichothecenes (MTs), in which case samples were dropped, or 2) to determine that the cytotoxicity cannot be ascribed to MTs, in which case samples were prioritized for bioactivity-directed fractionation.
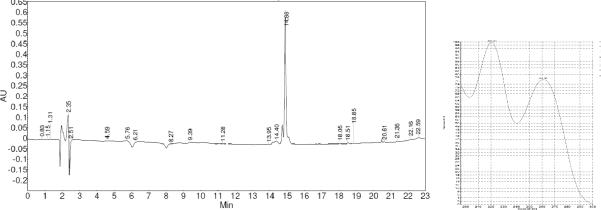
The case of fungal strain MSX 15962 illustrates the use of these spectral techniques for dereplication (Table 1), especially when the taxonomy of the fungus did not suggest MTs. For example, based on the taxonomy of MSX 15962, which was found to be closely related to Gliocladium viride, one would not expect MTs. However, the 1H NMR data (Figure 2) displayed some of the key signals of MTs. The methylene epoxide protons (encircled in Figure 2) were in the expected region, even when partially obscured at the level of the crude extract. Moreover, the downfield region (from ~6 to 8 ppm; Figure 2) had signals typical of the MT olefinic protons. After initial rounds of bioactivity-directed fractionation, the activity concentrated in an HPLC fraction where the UV spectrum was diagnostic for roridin-type compounds (Figure 4). In short, the spectroscopic signatures of MTs, noted in both 1H NMR and UV spectra of active fractions, permitted dereplication of this sample.
Figure 4.
HPLC chromatogram (left) and UV spectrum (right) of an active fraction of MSX 15962.
Conclusion
A method to dereplicate extracts of filamentous fungi for macrocyclic trichothecenes (MTs) was developed and implemented. The method utilized tools and instrumentation common to most laboratories of natural products, particularly the 1H NMR and UV spectra. The pattern recognition afforded by these spectral techniques does not require sophisticated training of laboratory staff, and the methods were quite versatile, being applicable to crude extracts, partially purified mixtures, and pure compounds.
EXPERIMENTAL SECTION
General
All 1H NMR experiments were performed in CDCl3 with TMS as an internal standard and were acquired on either a Varian Unity INOVA-500 or a JEOL ECA-500. Flash chromatography was conducted using a CombiFlash Rf system using RediSep Rf Si-gel columns (both from Teledyne-Isco; Lincoln, NE, USA). HPLC was carried out on Varian Prostar HPLC systems (Walnut Creek, CA, USA) equipped with Prostar 210 pumps and a Prostar 335 photodiode array detector (PDA), with data collected and analyzed using Galaxie Chromatography Workstation software (version 1.9.3.2). For preparative HPLC, YMC ODS-A (5 μm; 250 × 20 mm) columns were used with a 10 mL/min flow rate, while for analytical HPLC, YMC ODS-A (5 μm; 150 × 4.6 mm) columns were used with a 1 mL/min flow rate (both from Waters; Milford, MA, USA). For analytical HPLC, MetaTherm HPLC column temperature controllers (Varian) maintained these columns at 30°C.
Producing Organisms
Both producing fungi were from the Mycosynthetix culture collection. MSX 28737 was isolated from a sample of leaf litter, and the closest match using DNA analysis suggested it was Myrothecium roridum. MSX 15962 was isolated from aerial leaves in a varzea community from an area which was occasionally flooded, and the closest match using DNA analysis suggested it was Gliocladium viride. DNA analyses were performed by MIDI Labs Inc (Newark, DE, USA), and the D2 variable region of the Large Subunit (LSU) rRNA was sequenced and compared to the MIDI Labs database.
Fermentations
The cultures were grown on solid medium prepared using 12 g of rice to which was added 24 mL of YESD medium prepared by adding yeast extract (2% w/v), soy peptone (2%), dextrose (2%), and malt extract (1%) in water in 250 mL Erlenmeyer flasks. This was sterilized at 121°C for 20 min. For large scale cultures, similar conditions were used, scaling to 150 g of rice and 300 mL of YESD medium in 2.8 L Fernback flasks. Cultures were inoculated either directly from a slant (small flasks), or for larger fermentations, by using an inoculum prepared by growing the fungus in a 50 mL polypropylene centrifuge tube containing 7 mL of YESD medium incubated for 7 d at 22°C with agitation. The cultures were incubated at 22°C for 7 to 14 d at which time they were extracted.
Extraction and Isolation
A solid phase fungal culture of MSX 28737 was scored with a spatula and submerged with 60 mL of 1:1 CHCl3:MeOH and shaken overnight at 135 rpm in a Lab-line Environ-shaker (Thermo Scientific, Waltham, MA). The resultant solution was filtered on a Buchner funnel using Whatman #1 filter paper, and 90 mL of CHCl3 and 150 mL of H2O were added to the filtrate to generate a biphasic solution of 4:1:5 (CHCl3:MeOH:H2O). This solution was stirred for 2 hrs, transferred to a separatory funnel, and the organic layer (lower) was removed. This organic extract was dried in vacuo (127 mg) and then defatted via partitioning between hexane and CH3CN. The CH3CN sample was dried (41 mg) and subjected to reversed-phase HPLC (60:40 to 100:0 CH3CN:H2O over 120 min) to afford nine fractions. Fractions 3 and 5, both active in the cytotoxicity assay, were identified as verrucarin A (1, 1 mg) and roridin E (2, 1 mg); both compounds were >96% pure by analytical RP-HPLC.
A generalized methodology for dereplication of MTs begins by using essentially the same procedures outlined above, resulting in a defatted CH3CN-soluble sample. This organic extract is then subjected to flash column chromatography using a RediSep Si-gel column and a two stage gradient (100:0 to 0:100 Hexane:CHCl3 then 100:0 to 75:25 CHCl3:MeOH) over 70 column volumes total. Active pools from the initial column are then profiled by obtaining a 1H NMR spectrum and performing analytical reversed-phase HPLC on a system equipped with a PDA detector.
Cytotoxicity Assays
The cytotoxicity measurements were carried out as described previously24 and with the recently noted modification.25
ACKNOWLEDGMENTS
This research was supported by P01 CA125066 from the National Cancer Institute/National Institutes of Health, Bethesda, MD, USA. The Golden LEAF Foundation (Rocky Mount, NC) provided partial support to D. J. K. Mycology technical support was provided by Blaise Darveaux and Maurica Lawrence. The authors thank Drs. A. D. Kinghorn and D. S. Ayers for helpful discussions.
References
- 1).Cole RJ, Jarvis BB, Schweikert MA. Handbook of Secondary Fungal Metabolites. Academic Press; Amsterdam: Boston: 2003. p. xi.p. 672. [Google Scholar]
- 2).Zhang HJ, et al. Antimalarial agents from plants. III. Trichothecenes from Ficus fistulosa and Rhaphidophora decursiva. Planta Med. 2002;68:1088–1091. doi: 10.1055/s-2002-36350. [DOI] [PubMed] [Google Scholar]
- 3).Isaka M, Punya J, Lertwerawat Y, Tanticharoen M, Thebtaranonth Y. Antimalarial activity of macrocyclic trichothecenes isolated from the fungus Myrothecium verrucaria. J. Nat. Prod. 1999;62:329–331. doi: 10.1021/np980323x. [DOI] [PubMed] [Google Scholar]
- 4).Jarvis BB, Mazzola EP. Macrocyclic and other novel trichothecenes - their structure, synthesis, and biological significance. Acc. Chem. Res. 1982;15:388–395. [Google Scholar]
- 5).Matsumoto M, Minato H, Uotani N, Matsumoto K, Kondo E. New antibiotics from Cylindrocarpon sp. J. Antibiot. 1977;30:681–682. doi: 10.7164/antibiotics.30.681. [DOI] [PubMed] [Google Scholar]
- 6).Liu JY, et al. Antifungal and new metabolites of Myrothecium sp. Z16, a fungus associated with white croaker Argyrosomus argentatus. J. Appl. Microbiol. 2006;100:195–202. doi: 10.1111/j.1365-2672.2005.02760.x. [DOI] [PubMed] [Google Scholar]
- 7).Garcia CC, Rosso ML, Bertoni MD, Maier MS, Damonte EB. Evaluation of the antiviral activity against Junin virus of macrocyclic trichothecenes produced by the hypocrealean epibiont of Baccharis coridifolia. Planta Med. 2002;68:209–212. doi: 10.1055/s-2002-23134. [DOI] [PubMed] [Google Scholar]
- 8).Bloem RJ, Smitka TA, Bunge RH, French JC, Mazzola EP. Roridin-L-2, a new trichothecene. Tetrahedron Lett. 1983;24:249–252. [Google Scholar]
- 9).Smitka TA, Bunge RH, Bloem RJ, French JC. Two new trichothecenes, PD 113,325 and PD 113,326. J. Antibiot. 1984;37:823–828. doi: 10.7164/antibiotics.37.823. [DOI] [PubMed] [Google Scholar]
- 10).Wagenaar MM, Clardy J. Two new roridins isolated from Myrothecium sp. J. Antibiot. 2001;54:517–520. doi: 10.7164/antibiotics.54.517. [DOI] [PubMed] [Google Scholar]
- 11).Yu NJ, Guo SX, Lu HY. Cytotoxic macrocyclic trichothecenes from the mycelia of Calcarisporium arbuscula Preuss. J. Asian Nat. Prod. Res. 2002;4:179–183. doi: 10.1080/10286020290011387. [DOI] [PubMed] [Google Scholar]
- 12).Grove JF. Macrocyclic trichothecenes. Nat. Prod. Rep. 1993;10:429–448. doi: 10.1039/np9880500187. [DOI] [PubMed] [Google Scholar]
- 13).Hinkley SF, Jarvis BB. Chromatographic method for Stachybotrys toxins. Methods Mol. Biol. 2001;157:173–194. [PubMed] [Google Scholar]
- 14).Jarvis BB, Midiwo JO, Mazzola EP. Antileukemic compounds derived by chemical modification of macrocyclic trichothecenes. 2. Derivatives of roridin-A and roridin-H and verrucarin-A and verrucarin-J. J. Med. Chem. 1984;27:239–244. doi: 10.1021/jm00368a025. [DOI] [PubMed] [Google Scholar]
- 15).Jarvis BB, Stahly GP, Pavanasasivam G, Mazzola EP. Anti-leukemic compounds derived from the chemical modification of macrocyclic trichothecenes. 1. Derivatives of verrucarin-A. J. Med. Chem. 1980;23:1054–1058. doi: 10.1021/jm00183a018. [DOI] [PubMed] [Google Scholar]
- 16).Kinghorn AD, et al. Discovery of anticancer agents of diverse natural origin. Pure Appl. Chem. 2009;81:1051–1063. doi: 10.1351/PAC-CON-08-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Orjala J, Oberlies NH, Pearce CJ, Swanson SM, Kinghorn AD. Discovery of potential anticancer agents from aquatic cyanobacteria, filamentous fungi, and tropical plants. In: Tringali C, editor. Bioactive Compounds from Natural Sources. Second Edn. Taylor & Francis; London: submitted. [Google Scholar]
- 18).Nielsen KF, Smedsgaard J. Fungal metabolite screening: database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography-UV-mass spectrometry methodology. J. Chromatogr. A. 2003;1002:111–136. doi: 10.1016/s0021-9673(03)00490-4. [DOI] [PubMed] [Google Scholar]
- 19).Lang G, et al. Evolving trends in the dereplication of natural product extracts: new methodology for rapid, small-scale investigation of natural product extracts. J. Nat. Prod. 2008;71:1595–1599. doi: 10.1021/np8002222. [DOI] [PubMed] [Google Scholar]
- 20).Grove JF. Non-macrocyclic trichothecenes. Nat. Prod. Rep. 1988;5:187–209. doi: 10.1039/np9880500187. [DOI] [PubMed] [Google Scholar]
- 21).Namikoshi M, et al. A new macrocyclic trichothecene, 12,13-deoxyroridin E, produced by the marine-derived fungus Myrothecium roridum collected in Palau. J. Nat. Prod. 2001;64:396–398. doi: 10.1021/np000443g. [DOI] [PubMed] [Google Scholar]
- 22).Saikawa Y, et al. Toxic principles of a poisonous mushroom Podostroma cornu-damae. Tetrahedron. 2001;57:8277–8281. [Google Scholar]
- 23).Gutzwiller J, Tamm C. Uber die struktur von verrucarin B. Verrucarine und roridine, 6. Helv. Chim. Acta. 1965;48:177–182. doi: 10.1002/hlca.19650480118. [DOI] [PubMed] [Google Scholar]
- 24).Alali FQ, et al. New colchicinoids from a native Jordanian meadow saffron, Colchicum brachyphyllum: Isolation of the first naturally occurring dextrorotary colchicinoid. J. Nat. Prod. 2005;68:173–178. doi: 10.1021/np0496587. [DOI] [PubMed] [Google Scholar]
- 25).Li C, et al. Bioactive constituents of the stem bark of Mitrephora glabra. J. Nat. Prod. 2009;72:1949–1953. doi: 10.1021/np900572g. [DOI] [PMC free article] [PubMed] [Google Scholar]