Abstract
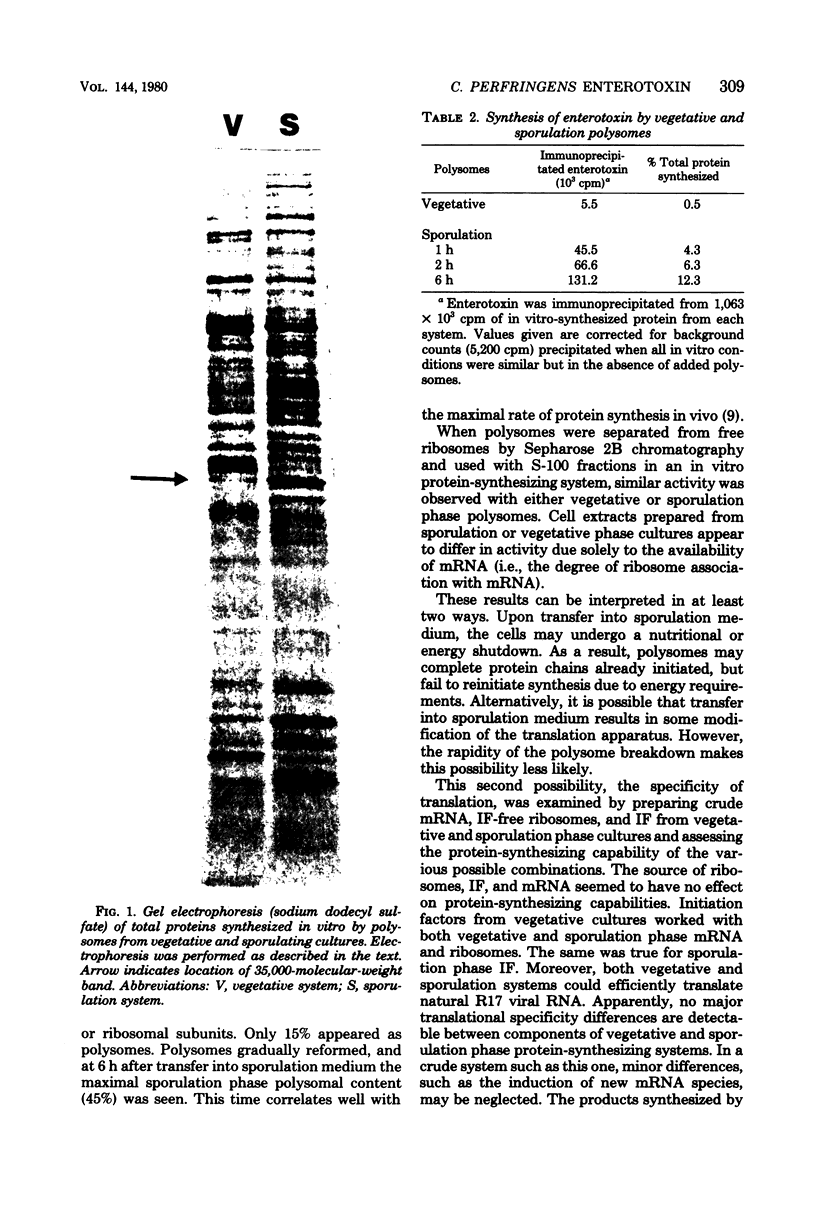
Polysomes were isolated from an enterotoxigenic strain of Clostridium perfringens during vegetative growth and at 1-h intervals after transfer into Duncan-Strong sporulation medium. During vegetative growth, about 67% of the ribosomes were in polysomal complexes. This proportion decreased to about 20% during the first 2 h in sporulation medium and then gradually increased to a maximum of 45% at 6 h. Ribosomes isolated from cells in vegetative or in sporulation phase could equally translate vegetative, sporulation, and natural viral R17 messenger ribonucleic acid with either vegetative or sporulation initiation factors. When polysomes were allowed to complete their nascent chains with labeled amino acids in vitro, most of the polypeptides synthesized by the vegetative phase and by the sporulation phase polysomes appeared to be identical. There were, however, notable differences upon further investigation. Specifically, when antiserum against the enterotoxin was reacted with the completed polypeptides, no counts were precipitated from the vegetative products. On the other hand, up to 12% of the total labeled protein was precipitated from the products obtained with the sporulation phase polysomes. Upon electrophoresis on sodium dodecyl sulfate, the putative enterotoxin synthesized in vitro ran as a major band with a molecular weight of 35,000, and as two minor bands with molecular weights of 17,000 and 52,000, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambliss G. H., Legault-Demare L. Functional modifications of the translational system in Bacillus subtilis during sporulation. J Bacteriol. 1977 Oct;132(1):13–22. doi: 10.1128/jb.132.1.13-22.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCioccio R. A., Strauss N. Patterns of transcription in Bacillus subtilis during sporulation. J Mol Biol. 1973 Jun 25;77(2):325–336. doi: 10.1016/0022-2836(73)90338-0. [DOI] [PubMed] [Google Scholar]
- Doi R. H. Role of ribonucleic acid polymerase in gene selection in procaryotes. Bacteriol Rev. 1977 Sep;41(3):568–594. doi: 10.1128/br.41.3.568-594.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., King G. J., Frieben W. R. A paracrystalline inclusion formed during sporulation of enterotoxin-producing strains of Clostridium perfringens type A. J Bacteriol. 1973 May;114(2):845–859. doi: 10.1128/jb.114.2.845-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Improved medium for sporulation of Clostridium perfringens. Appl Microbiol. 1968 Jan;16(1):82–89. doi: 10.1128/am.16.1.82-89.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H., Sebald M. Sporulation and enterotoxin production by mutants of Clostridium perfringens. J Bacteriol. 1972 Apr;110(1):378–391. doi: 10.1128/jb.110.1.378-391.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L. Time of enterotoxin formation and release during sporulation of Clostridium perfringens type A. J Bacteriol. 1973 Feb;113(2):932–936. doi: 10.1128/jb.113.2.932-936.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders G. L., Jr, Duncan C. L. Anomalous aggregation of Clostridium perfringens enterotoxin under dissociating conditions. Can J Microbiol. 1976 Sep;22(9):1410–1414. doi: 10.1139/m76-209. [DOI] [PubMed] [Google Scholar]
- Frieben W. R., Duncan C. L. Heterogeneity of enterotoxin-like protein extracted from spores fo Clostridium perfringens type A. Eur J Biochem. 1975 Jul 1;55(2):455–463. doi: 10.1111/j.1432-1033.1975.tb02182.x. [DOI] [PubMed] [Google Scholar]
- Fukuda R., Doi R. H. Two polypeptides associated with the ribonucleic acid polymerase core of Bacillus subtilis during sporulation. J Bacteriol. 1977 Jan;129(1):422–432. doi: 10.1128/jb.129.1.422-432.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genigeorgis C. Public health importance of Clostridium perfringens. J Am Vet Med Assoc. 1975 Nov 1;167(9):821–827. [PubMed] [Google Scholar]
- Goldman R. C., Tipper D. J. Bacillus subtilis spore coats: complexity and purification of a unique polypeptide component. J Bacteriol. 1978 Sep;135(3):1091–1106. doi: 10.1128/jb.135.3.1091-1106.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha S., Szulmajster J. Specific alteration of the 30S ribosomal subunits of Bacillus subtilis during sporulation. J Bacteriol. 1977 Sep;131(3):866–871. doi: 10.1128/jb.131.3.866-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild A. H., Hilsheimer R. Purification and characteristics of the enterotoxin of Clostridium perfringens type A. Can J Microbiol. 1971 Nov;17(11):1425–1433. doi: 10.1139/m71-227. [DOI] [PubMed] [Google Scholar]
- Kieras R. M., Preston R. A., Douthit H. A. Isolation of stable ribosomal subunits from spores of Bacillus cereus. J Bacteriol. 1978 Oct;136(1):209–218. doi: 10.1128/jb.136.1.209-218.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe R. G., Duncan C. L. Spore coat protein and enterotoxin synthesis in Clostridium perfringens. J Bacteriol. 1977 Aug;131(2):713–715. doi: 10.1128/jb.131.2.713-715.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau J. V., Smith W. P., Pope D. H. Role of the 30S ribosomal subunit, initiation factors, and specific ion concentration in barotolerant protein synthesis in Pseudomonas bathycetes. J Bacteriol. 1977 Apr;130(1):154–159. doi: 10.1128/jb.130.1.154-159.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Leighton T. Sporulation-specific translational discrimination in Bacillus subtilis. J Mol Biol. 1974 Jul 15;86(4):855–863. doi: 10.1016/0022-2836(74)90358-1. [DOI] [PubMed] [Google Scholar]
- Linn T., Losick R. The program of protein synthesis during sporulation in Bacillus subtilis. Cell. 1976 May;8(1):103–114. doi: 10.1016/0092-8674(76)90191-4. [DOI] [PubMed] [Google Scholar]
- Munoz L., Sadaie Y., Doi R. H. Spore coat protein of Bacillus subtilis. Structure and precursor synthesis. J Biol Chem. 1978 Oct 10;253(19):6694–6701. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Skjelkvålé R., Duncan C. L. Enterotoxin formation by different toxigenic types of Clostridium perfringens. Infect Immun. 1975 Mar;11(3):563–575. doi: 10.1128/iai.11.3.563-575.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Murphy J. R., Davis B. D. Precursor in cotranslational secretion of diphtheria toxin. J Bacteriol. 1980 Jan;141(1):184–189. doi: 10.1128/jb.141.1.184-189.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Wallace B. J., Davis B. D. Selective action of erythromycin on initiating ribosomes. Biochemistry. 1974 Oct 22;13(22):4653–4659. doi: 10.1021/bi00719a029. [DOI] [PubMed] [Google Scholar]




