Abstract
The coordination-driven self-assembly of supramolecular hexagonal prisms has been achieved upon mixing hexa(4-(4-pyridyl)phenyl)benzene donor ligand and carboxylate donor ligands such as sodium terephthalate, sodium(1,1′-biphenyl)-4,4′-dicarboxylate, sodium 4,4′-(diazene-1,2-diyl)dibenzoate, and 4,4′-dipyridyl with cis-Pt(PEt3)2(OTf)2 in a 1:3:6 ratio. Four assembled hexagonal prisms have been characterized by 31P and 1H multinuclear NMR spectroscopy as well as electrospray ionization mass spectrometry (ESI-MS). Molecular force field simulations provide the possible conformation and size of each structure.
During the past two decades, coordination-driven metal-mediated self-assembly has attracted considerable attention and proven to be a powerful tool in the synthesis of well-defined multimetallic architectures with increasing structural complexity.1-6 This was often accomplished by the combination of an organic donor with a metal acceptor, where both reagents possessed well-defined bonding directionality leading to a discrete, highly symmetrical product. A more complex situation in self-assembly arises when more than two starting materials are mixed together in one vessel. Will an ordered discrete supramolecule or an oligomeric product mixture be the result? In fact, the successful achievement of the self-assembly of multiple components into discrete structures is less documented because of the increased number of numerous reaction pathways and products.7 In general, a template molecule or an ion is often necessary to control such multicomponent coordination self-assembly outcome.8
Furthermore, in an attempt to emulate nature's own complexity, synthetic chemists have become more adept at the construction of three-dimensional (3-D) metallo-supramolecular assemblies i.e. cubes,9 double squares,10 cuboctahedra,11 adamantanoids,12 dodecahedra,13 and a sphere.14 Such 3-D species are capable of functions such as reaction catalysis, guest encapsulation, and act as a micro-reaction vessel.15 However, until now the construction of 3-D hexagonal prisms via multicomponent coordination-driven transition-metal-mediated self-assembly is rarely reported.16 Herein, we report the multicomponent template-free synthesis of discrete hexagonal prisms upon self-assembly of a 90° platinum acceptor with a hexapyridyl donor and a carboxylate or dipyridyl ligand. Despite the possibility of forming a myriad of oligomeric structures, discrete supramolecular hexagonal prisms are generated as the predominant products.
The formation of 3D hexagonal prisms is shown in Scheme 1. The linear carboxylate and dipyridyl donors 3a–d are designed as pillars, whereas hexa-(4-(4-pyridyl)phenyl) benzene 1 containing six pyridyl groups and cis-Pt(PEt3)2(OTf)2 2 are selected as faces and corners of the hexagonal prisms, respectively. When the three building blocks are mixed and reacted in a ratio of 1:3:6, discrete supramolecular hexagonal prisms can be formed. Their structures were characterized by 31P and 1H multinuclear NMR spectroscopy and electrospray ionization mass spectrometry (ESI-MS).
Scheme 1.
Graphical Representation of the [2+6+12] Multicomponent Self-Assembly of Hexagonal Prisms 4a–d.
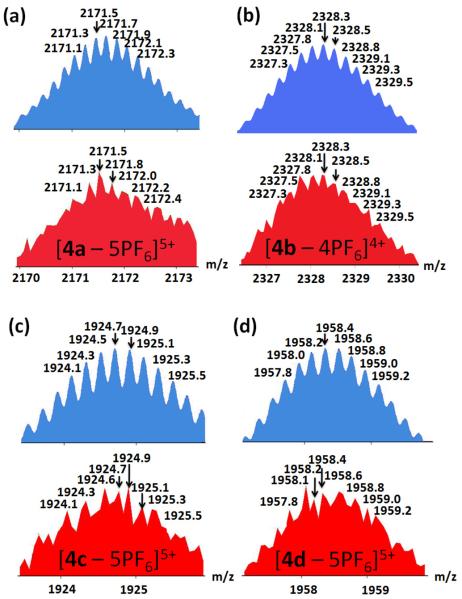
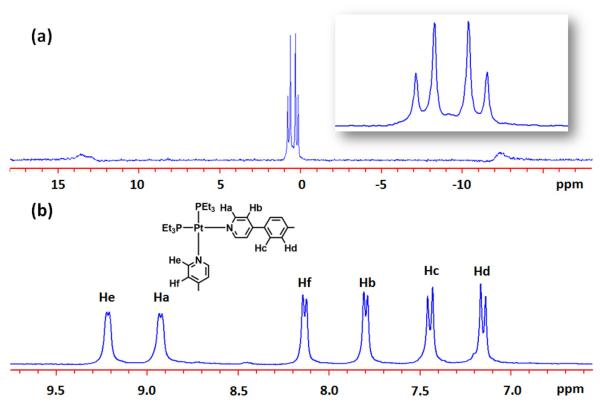
After heating at 50–56 °C for 6 h, the 1:3:6 mixture of 1, 3a, and 2 in acetone-d6 and D2O (v/v 9:1) yielded a clear solution. The 31P{1H}NMR spectrum displayed two coupled doublets at 0.68 and 0.37 ppm with concomitant 195Pt satellites. The signals are upfield shifted by about 12 ppm compared to that of the starting Pt(II) acceptor 2 due to formation of the Pt-N coordination bond. The two different doublets in the 31P{1H}NMR demonstrate that the phosphorus nuclei connected to the platinum atom in 4a are chemically nonequivalent, which can only be rationalized by the coordination of donor 1 and 3a with each platinum center in the hexagonal prism structure 4a.17 In the corresponding 1H NMR spectrum of supramolecule 4a , the α- and β-pyridyl protons of donors 1 and 3a experience downfield shifts (Hα-Py, 0.41-0.48 ppm , Hβ-Py, 0.46-0.59 ppm) because of the loss of electron density that occurs upon coordination. The formation of hexagonal prism structure 4a is further confirmed by ESI-MS. As listed in Figure 2 and Figure S3 in the Supporting Information, the isotopically resolved peaks corresponding to the hexagonal prism with loss of PF6− anions are found at m/z = 2751.2 [4a-4PF6]4+ and 2171.5 [4a- 5PF6]5+.
Figure 2.
Theoretical (top, blue) and experimental (bottom, red) ESIMS results for 4a–d hexagonal prisms.
To further extend the variety of supramolecular hexagonal prisms constructed, we next utilized carboxylate ligands 3b-d as pillars to react with the hexapyridyl donor 1 and the 90° Pt(II) acceptor 2.
Hexapyridyl donor 1 and carboxylate ligands 3b, 3c, or 3d, respectively, were mixed with acceptor cis-Pt(PEt3)2(OTf)2 2 in a 1:3:6 ratio and heated at 50–56 °C for 6 h in aqueous acetone. Upon ion exchange with KPF6, hexagonal prisms 4b–d were obtained in high yields (91%-96%). The 31P{1H}NMR spectra (Figure 3a and Figure S1A and Figure S2A in the Supporting Information) of prisms 4b–d showed two coupled doublets peaks (4b: 6.19 and 1.06 ppm, 2Jp-p = 21.30 Hz; 4c: 6.56 and 1.10 ppm, 2Jp-p = 21.30 Hz; 4d: 6.99 and 2.13 ppm, 2Jp-p = 21.30 Hz) of approximately equal intensity with concomitant 195Pt satellites, and these signals were upfield shifted for 4b: 6.31 and 11.44 ppm; 4c: 5.77 and 11.22 ppm; 4d: 5.34 and 10.19 ppm, compared with the 90° Pt(II) acceptor 2 (δ = 12.50 ppm) upon coordination with the pyridine and carboxylate groups. This result agrees with the heteroleptic coordination motif of 4b–d.18 In the 1H NMR spectra (Figure 3b and Figure S1B and Figure S2B in the Supporting Information) of 4b–d, the α- and β- pyridyl hydrogen signals both experience downfield shifts (4b: 0.25 and 0.46 ppm; 4c: 0.45 and 0.68 ppm; 4d: 0.44 and 0.65 ppm) compared with their chemical shifts in the precursor building block 1, which are associated with the loss of electron density upon coordination to the platinum metal centers. The sharp NMR spectral signals together with the solubility of the assemblies indicate that a self-assembly of high symmetry was formed predominantly in each reaction, and the formation of homoleptic species and oligomers can be ruled out.
Figure 3.
31P{1H} (a) and partial 1H NMR (b) spectra of hexagonal prism 4b.
Further evidence for the formation of assemblies 4b–d was obtained by ESI-MS (Figure 2b–c and Figure S3 in the Supporting Information). The ESI-MS of peaks for [4b exhibited peaks for [4b-4PF6]4+ and [4b-5PF6]5+ at m/z = 2328.3 and 1834.1, respectively. Likewise, peaks attributable to 4c were found at m/z = 1924.7 [4c-5PF6]5+, as were those corresponding to 4d: m/z = 1958.4 [4d-5PF6]5+ and m/z = 1607.9 [4d-6PF6]6+. All ESI-MS signals are in good agreement with their theoretical distributions.
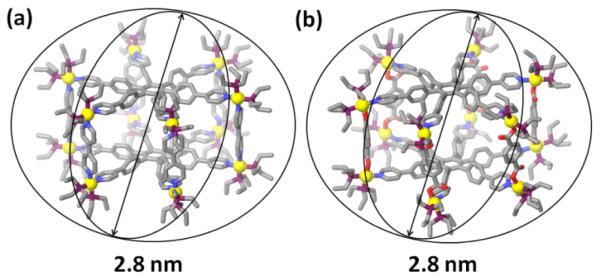
All attempts to crystallize the above four hexagonal prism structures have so far been unsuccessful. We have therefore used molecular force field simulation to investigate the structural characteristics of supramolecular architectures 4a–d. A 1.0 ns molecular dynamics simulation (MMFF force field, 300K, gas phase) was used to equilibrate each supramolecule, and the output of the simulation was then minimized to full convergence. As shown in Figure 4 and Figure S4 (see Supporting Information), models of assemblies 4a–d are in the shape of hexagonal prism with a diameter of 2.8 nm (4a), 2.8 nm (4b), 3.4 nm (4c), and 3.8 nm (4d), respectively.
Figure 4.
Molecular modeling of supramolecular hexagonal prisms 4a (a) and 4b (b) and their computational sizes.
In conclusion, by combining hexapyridyl donor 1 and 90° organoplatinum acceptor 2 with dipyridyl and different size carboxylate donors 3a–d in appropriate stoichiometric ratio, we have successfully constructed discrete, nanoscopic 3-D hexagonal prism of variable size via muticomponent coordination-driven self-assembly without use of any template or ion. In addition, molecular force field modeling has shown that these supramolecule hexagonal prisms have different sizes, which may have applications in host-guest chemistry and function as new micro-reactors.
Supplementary Material
Figure 1.
31P{1H} (a) and partial 1H NMR (b) spectra of hexagonal prism 4a.
Acknowledgments
P.J.S. thanks the NIH (GM-057052) for financial support.
Footnotes
Supporting Information Available: General procedure of self-assembly experiment, detailed characterization of assemblies 4a–d, NMR and ESI-MS data as well as computational simulations for assemblies 4c and 4d. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Stang PJ, Olenyuk B. Acc. Chem. Res. 1997;30:502. [Google Scholar]; (b) Seidel SR, Stang PJ. Acc. Chem. Res. 2002;35:972. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]; (c) Leininger S, Olenyuk B, Stang PJ. Chem. Rev. 2000;100:853. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (d) Northrop BH, Zheng Y-R, Chi K-W, Stang PJ. Acc. Chem. Res. 2009;42:1554. doi: 10.1021/ar900077c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Yoshizawa M, Klosterman JK, Fujita M. Angew. Chem. Int. Ed. 2009;48:3418. doi: 10.1002/anie.200805340. [DOI] [PubMed] [Google Scholar]; (b) Fujita M, Tominaga M, Hori A, Therrien B. Acc. Chem. Res. 2005;38:369. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; (c) Pitt MA, Johnson DW. Chem. Soc. Rev. 2007;36:1441. doi: 10.1039/b610405n. [DOI] [PubMed] [Google Scholar]; (d) Cotton FA, Lin C, Murillo CA. Acc. Chem. Res. 2001;34:759. doi: 10.1021/ar010062+. [DOI] [PubMed] [Google Scholar]; (e) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc. Chem. Res. 2005;38:349. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]; (f) Holliday BJ, Mirkin CA. Angew. Chem. Int. Ed. 2001;40:2022. [PubMed] [Google Scholar]; (g) Ruben M, Rojo J, Romero-Salguero FJ, Uppadine LH, Lehn J-M. Angew. Chem. Int. Ed. 2004;43:3644. doi: 10.1002/anie.200300636. [DOI] [PubMed] [Google Scholar]; (h) Nitschke JR. Acc. Chem. Res. 2007;40:103. doi: 10.1021/ar068185n. [DOI] [PubMed] [Google Scholar]; (i) Oliver CG, Ulman PA, Wiester MJ, Mirkin CA. Acc. Chem. Res. 2008;41:1618. doi: 10.1021/ar800025w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Raymond KN. Nature. 2009;460:585. [Google Scholar]; (k) De S, Mahata K, Schmittel M. Chem. Soc. Rev. 2010;39:1555. doi: 10.1039/b922293f. [DOI] [PubMed] [Google Scholar]; (l) Seeber G, Tiedemann BEF, Raymond KN. Top. Curr. Chem. 2006;265:147. [Google Scholar]
- 3.(a) Pentecost CD, Chichak KS, Peters AJ, Cave GWV, Cantrill SJ, Stoddart JF. Angew. Chem. Int. Ed. 2007;46:218. doi: 10.1002/anie.200603521. [DOI] [PubMed] [Google Scholar]; (b) Pentecost CD, Peters AJ, Chichak KS, Cave GWV, Cantrill SJ, Stoddart JF. Angew. Chem. Int. Ed. 2006;45:4099. doi: 10.1002/anie.200600817. [DOI] [PubMed] [Google Scholar]; (c) Chichak KS, Cantrill SJ, Pease AR, Chiu SH, Cave GWV, Atwood JL, Stoddart JF. Science. 2004;304:1308. doi: 10.1126/science.1096914. [DOI] [PubMed] [Google Scholar]; (d) Cantrill SJ, Chichak KS, Peters AJ, Stoddart JF. Acc. Chem. Res. 2005;38:1. doi: 10.1021/ar040226x. [DOI] [PubMed] [Google Scholar]
- 4.(a) Collin J-P, Dietrich-Buchecker C, Gaviña P, Jimenez-Molero MC, Sauvage J-P. Acc. Chem. Res. 2001;34:477. doi: 10.1021/ar0001766. [DOI] [PubMed] [Google Scholar]; (b) Dietrich-Buchecker C, Colasson BX, Sauvage J-P. Top. Curr. Chem. 2005;249:261. [Google Scholar]; (c) Sauvage J-P. Acc. Chem. Res. 1998;31:611. [Google Scholar]; (d) Beyler M, Heitz V, Sauvage J-P. J. Am. Chem. Soc. 2010;132:4409. doi: 10.1021/ja910747h. [DOI] [PubMed] [Google Scholar]
- 5.(a) Crowley JD, Goldup SM, Lee AL, Leigh DA, McBurney RT. Chem. Soc. Rev. 2009;38:1530. doi: 10.1039/b804243h. [DOI] [PubMed] [Google Scholar]; (b) Leigh DA, Lusby PJ, McBurney RT, Symes MD. Chem. Commun. 2010;46:2382. doi: 10.1039/c001697g. [DOI] [PubMed] [Google Scholar]; (c) Crowley JD, Hänni KD, Leigh DA, Slawin AMZ. J. Am. Chem. Soc. 2010;132:5309. doi: 10.1021/ja101029u. [DOI] [PubMed] [Google Scholar]; (d) Fuller A-M, Leigh DA, Lusby PJ. J. Am. Chem. Soc. 2010;132:4954. doi: 10.1021/ja1006838. [DOI] [PubMed] [Google Scholar]
- 6.(a) Peinador C, Blanco V, Quintela JM. J. Am. Chem. Soc. 2009;131:920. doi: 10.1021/ja8088372. [DOI] [PubMed] [Google Scholar]; (b) Blanco V, Chas M, Abella D, Peinador C, Quintela JM. J. Am. Chem. Soc. 2007;129:13978. doi: 10.1021/ja074721a. [DOI] [PubMed] [Google Scholar]; (c) Blanco V, Abella D, Pía E, Platas-lglesias C, Peinador C, Quintela JM. Inorg. Chem. 2009;48:4098. doi: 10.1021/ic8022425. [DOI] [PubMed] [Google Scholar]; (d) Peinador C, Pía E, Blanco V, García MD, Quintela JM. Org. Lett. 2010;12:1380. doi: 10.1021/ol1004577. [DOI] [PubMed] [Google Scholar]
- 7.(a) Lehn JM, Eliseev AV. Science. 2001;291:2331. doi: 10.1126/science.1060066. [DOI] [PubMed] [Google Scholar]; (b) Lehn JM. Chem. Eur. J. 1999;5:2455. [Google Scholar]; (c) Zheng Y-R, Yang H-B, Ghosh K, Zhao L, Stang PJ. Chem.-Eur. J. 2009;15:7203. doi: 10.1002/chem.200900230. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Northrop BH, Yang H-B, Stang PJ. Inorg. Chem. 2008;47:11257. doi: 10.1021/ic801711q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zheng Y-R, Yang H-B, Northrop BH, Ghosh K, Stang PJ. Inorg. Chem. 2008;47:4706. doi: 10.1021/ic800038j. [DOI] [PubMed] [Google Scholar]; (f) Zheng Y-R, Northrop BH, Yang H-B, Zhao L, Stang PJ. J. Org. Chem. 2009;74:3554. doi: 10.1021/jo9002932. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Lee J, Ghosh K, Stang PJ. J. Am. Chem. Soc. 2009;131:12028. doi: 10.1021/ja903330j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Schmittel M, Mahata K. Inorg. Chem. 2009;48:822. doi: 10.1021/ic8021084. [DOI] [PubMed] [Google Scholar]; (i) Kishore RSK, Paululat T, Schmittel M. Chem.- Eur. J. 2006;12:8136. doi: 10.1002/chem.200600463. [DOI] [PubMed] [Google Scholar]
- 8.(a) Fujita M, Nagao S, Ogura K. J. Am. Chem. Soc. 1995;117:1649. doi: 10.1021/ja00110a026. [DOI] [PubMed] [Google Scholar]; (b) Hiraoka S, Fujita M. J. Am. Chem. Soc. 1999;121:10239. [Google Scholar]; (c) Hiraoka S, Kubota Y, Fujita M. Chem. Commun. 2000:1509. [Google Scholar]; (d) Liu H-K, Sun W-Y, Ma D-J, Yu K-B, Tang W-X. Chem. Commun. 2000:591. [Google Scholar]; (e) Ikeda A, Udzu H, Zhong Z, Shinkai S, Sakamoto S, Yamaguchi K. J. Am. Chem. Soc. 2001;123:3872. doi: 10.1021/ja003269r. [DOI] [PubMed] [Google Scholar]
- 9.(a) Johannessen SC, Brisbois RG, Fischer JP, Grieco PA, Counterman AE, Clemmer DE. J. Am. Chem. Soc. 2001;123:3818. doi: 10.1021/ja004007s. [DOI] [PubMed] [Google Scholar]; (b) Roche S, Haslam C, Adams H, Heath SL, Thomas JA. Chem. Commun. 1998:1681. [Google Scholar]
- 10.Fujita M, Yu S-Y, Kusukawa T, Funaki H, Ogura K, Yamaguchi K. Angew, Chem. Int. Ed. Engl. 1998;37:2082. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2082::AID-ANIE2082>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Olenyuk B, Whiteford JA, Fechtenkotter A, Stang PJ. Nature. 1999;398:796. doi: 10.1038/19740. [DOI] [PubMed] [Google Scholar]
- 12.Schweiger M, Seidel SR, Schmitz M, Stang PJ. Org. Lett. 2000;2:1255. doi: 10.1021/ol005781n. [DOI] [PubMed] [Google Scholar]
- 13.Olenyuk B, Levin MD, Whiteford JA, Shield JE, Stang PJ. J. Am. Chem. Soc. 1999;121:10434. [Google Scholar]
- 14.Suzuki K, Kawano M, Sato S, Fujita M. J. Am. Chem. Soc. 2007;129:10652. doi: 10.1021/ja073629b. [DOI] [PubMed] [Google Scholar]
- 15.(a) Pluth MD, Bergman RG, Raymond KN. Acc. Chem. Res. 2009;42:1650. doi: 10.1021/ar900118t. [DOI] [PubMed] [Google Scholar]; (b) Vriezema DM, Aragones MC, Elemans JAAW, Cornelissen JJLM, Rowan AE, Nolte RJM. Chem. Rev. 2005;105:1445. doi: 10.1021/cr0300688. [DOI] [PubMed] [Google Scholar]; (c) Pluth MD, Fiedler D, Mugridge JS, Bergman RG, Raymond KN. Prod. Nat. Acad. Sci. USA. 2009;106:10438. doi: 10.1073/pnas.0809806106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yamauchi Y, Yoshizawa M, Akita M, Fujita M. J. Am. Chem. Soc. 2010;132:960. doi: 10.1021/ja904063r. [DOI] [PubMed] [Google Scholar]; (e) Pluth MD, Bergman RG, Raymond KN. Science. 2007;316:85. doi: 10.1126/science.1138748. [DOI] [PubMed] [Google Scholar]
- 16.Bar AK, Chakrabarty R, Mostafa G, Mukherjee PS. Angew. Chem., Int. Ed. 2008;47:8455. doi: 10.1002/anie.200803543. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Zheng Y-R, Ghosh K, Stang PJ. J. Am. Chem. Soc. 2010;132:6282. doi: 10.1021/ja100889h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Chi K-W, Addicott C, Arif AM, Stang PJ. J. Am. Chem. Soc. 2004;126:16569. doi: 10.1021/ja045542l. [DOI] [PubMed] [Google Scholar]; (b) Chi K-W, Addicott C, Moon M-E, Lee HJ, Yoon SC, Stang PJ. J. Org. Chem. 2006;71:6662. doi: 10.1021/jo060971i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.