Abstract
Purpose
To estimate the rate of late grade 3 or greater genitourinary (GU) and gastrointestinal (GI) adverse events (AEs) following treatment with external beam radiation therapy and prostate high dose rate (HDR) brachytherapy.
Methods and Materials
Each participating institution submitted CT based HDR brachytherapy dosimetry data electronically for credentialing and for each study patient. Patients with locally confined T1c-T3b prostate cancer were eligible for this study. All patients were treated with 45 Gy in 25 fractions from external beam radiotherapy and one HDR implant delivering 19 Gy in 2 fractions. All AEs were graded according to CTCAEv3.0. Late GU/ GI AEs were defined as those occurring more than nine months from the start of the protocol treatment, in patients with at least 18 months of potential follow-up.
Results
A total of 129 patients from 14 institutions were enrolled in this study. 125 patients were eligible and AE data was available for 112 patients. The pretreatment characteristics of the patients were as follows: T1c-T2c 91%, T3a-T3b 9%, PSA ≤ 10 70%, PSA >10-≤20 30%, GS 2-6 10%, GS 7 72%, and GS 8-10 18%. At a median follow-up time of 29.6 months, 3 acute and 4 late grade 3 GU/GI AEs were reported. The estimated rate of late grade 3-5 GU and GI AE at 18 months was 2.56%.
Conclusion
This is the first prospective, multi-institutional trial of CT based HDR brachytherapy and external beam radiotherapy. The technique and doses used in this study resulted in acceptable levels of adverse events.
Keywords: Prostate cancer, High Dose Rate, Brachytherapy, Prospective multi-institutional clinical trial
INTRODUCTION
Studies have shown that dose escalation improved the clinical result of external beam radiotherapy. Patients with intermediate and high-risk prostate cancer may benefit from dose escalation. Brachytherapy is an alternative method of delivering highly conformal radiotherapy for treatment of prostate cancer. Dosimetry comparison of conformal external beam radiotherapy with high dose rate (HDR) brachytherapy showed significantly less rectal and bladder volume irradiated using HDR brachytherapy boost compared with conformal external beam radiotherapy.(1) Brenner et al estimated the alpha-beta ratio for prostate carcinoma based on mature clinical data and has shown that prostate cancer has an exceptionally low alpha-beta ratio of 1.5 Gy.(2) If this is the case, then prostate cancer’s alpha-beta ratio is lower than the alpha-beta ratio of dose limiting structures around the prostate (3-5 Gy). This suggests there may a potential gain for treating prostate cancer using hypofractionated radiotherapy such as HDR brachytherapy.(3-5)
The technique of HDR prostate brachytherapy has been in clinical practice since the 1980’s.(6-10) Kovacs et al reported one of the earliest experiences using HDR brachytherapy boost at University of Kiel.(10-12) Patients treated were mostly T2b-T3 tumors and high grade tumors. They used a combination of split course external beam radiotherapy and two 15 Gy HDR treatments. At 10 years 78 percent of 171 patients remained free of disease. Mate et al at Swedish Medical Center reported their experience with HDR brachytherapy.(13) They use a more moderate hypofractionated schema with four treatments of 3-4 Gy fractions of HDR treatments combined with 45-50 Gy of external beam radiotherapy. They recommended routine cystoscopy at the end of the implant procedure to ensure the catheters were placed at the proper depth and to avoid injuring the urethra. Martinez et al at the William Beaumont Hospital reported the ongoing prospective dose escalation trial using HDR brachytherapy as a boost. There have been multiple updates of their results.(8, 14-18) They have continued to dose escalate using increasingly larger fractions of HDR treatment ranging from 5.5-6.5 Gy × 3 to 8.25-11.5 Gy × 2 combined with 46 Gy of external beam radiotherapy. The most recent update showed the treatment is well tolerated with favorable biochemical outcomes.
Given the physics, radiobiological rationales and promising clinical results, the Radiation Therapy Oncology Group (RTOG) has developed this prospective multi-institutional phase II trial to study the role of HDR brachytherapy boost for prostate cancer. The goal of the study is to evaluate the safety and feasibility of this treatment approach and develop the quality assurance process for future phase III studies.
METHODS AND MATERIALS
Patient Population
All patients were staged based on the AJCC staging 6th edition. The patients eligible for this study must have the following combinations of factors: Clinical stage T1c-T2c, Gleason score 2-6 and PSA >10 but ≤ 20; clinical stage T3a-T3b, Gleason score 2-6 and PSA ≤ 20; or clinical stage T1c-T3b, Gleason score 7-10 and PSA ≤ 20. All patients must be clinically N0, M0 based on CT or MR within 90 days of registration. A Zubrod performance status of 0-1 was required. Patients were ineligible for this study if they had prior TURP, radical surgery for prostate cancer, hip prosthesis, prior pelvic or prostate radiation, or chemotherapy for prostate cancer. Previous hormonal therapy beginning less or equal to 120 days prior to registration and concurrent and adjuvant hormonal therapy were allowed. The initial follow-up visit was 3 months from the start of treatment. After initial follow-up visit, follow-ups were done at 6, 9, and 12 months post therapy, then every six months until five years post-implant, then annually thereafter. At each follow-up, PSA and testosterone levels, digital rectal exam and assessment of toxicity were done and recorded.
Radiation Therapy
Since this was the first RTOG prostate HDR brachytherapy trial, the goal was also to develop a quality assurance process for HDR prostate brachytherapy. Each participating institution was required to submit CT based HDR brachytherapy dosimetry data electronically for credentialing and for each study patient. All credentialing requirements were reviewed by Radiological Physics Center (RPC). Upon successful completion, the RPC notified both the registering institution and RTOG Headquarters that the institution is eligible to enter patients onto this study. As part of the credentialing process, all institutions must demonstrate the ability to perform electronic data submission to the Image-Guided Therapy Center (ITC) prior to enrolling patients. To ensure general feasibility and applicability of the study results, a maximum of 20 patients from each institution may be registered.
The clinical target volume (CTV) for the external beam portion of the treatment was defined as the prostate and seminal vesicles or whole pelvis depending on the lymphatic risk. If 2/3 PSA + [(GS-6) × 10] is > 15%, whole pelvis radiation was required. The whole pelvis volume included internal and external iliac lymph nodes inferior to L5 and posterior to the anterior boarder of the pubic symphysis and anterior to S2. Prostate and seminal vesicles target volumes must be obtained based on a pretreatment CT. The Planning Target Volume (PTV) is a 1-1.5 cm margin around the prostate gland and the seminal vesicles (CTV). Daily doses of 1.8 Gy given five times per week for a total dose of 45 Gy was prescribed to the central axis at the projected center of the target volume for conventional (non-CT based) treatment planning and to the highest isodose line which encompasses the PTV for conformal (CT based) treatment planning. Intensity modulation radiation therapy (IMRT) was not allowed in this study.
The overall treatment course was limited to less than 8 weeks. HDR brachytherapy was performed either before or after the external beam radiotherapy. All HDR afterloading catheters had to be CT compatible and placed with transrectal ultrasound (TRUS) guidance. No fewer than 14 catheters had to be in the CTV for adequate coverage without excessive hot spots. Flexible cystoscopy was done to ensure that no implant catheter was left in the bladder or urethra. To facilitate with identification of target volume, fiducial marker seeds had to be placed under TRUS guidance at the base and the apex of the prostate. Three-dimensional CT based brachytherapy treatment planning was required. The treatment planning CT scan were performed with the patient in the supine position with a Foley catheter in place. Metallic obturators or non-CT compatible dummy ribbons had to be removed prior to the CT scan. Dwell time in dwell positions located outside of the target volume turned down or off to minimize normal tissue irradiated. The definition of volumes was in accordance with ICRU Report 58: Dose and Volume Specification for reporting interstitial therapy.(19) The CTV was defined by the physician based on the treatment planning CT scan. For T1c-T2b, the brachytherapy CTV included the prostate only and for T3a-T3b, the brachytherapy CTV included the prostate and extra-capsular extension. The brachytherapy PTV was identical to the CTV. Critical structures contoured included the bladder, rectum, and urethra. When contouring the bladder and rectum, the outer most border of the mucosa was contoured. For the urethra, the outer surface of the Foley catheter was contoured. The prescription dose of 19 Gy in two fractions was prescribed to the periphery of the PTV. The goal was to deliver the prescription dose to at least 90% of the PTV. However, the dose to critical normal structures was kept to a minimum. The volume of bladder and rectum receiving 75% of the prescription dose had to be kept to less than 1 cc (V75 rectum and V75 bladder < 1 cc) and the volume of urethra receiving 125% of the prescription dose had to be kept to less than 1 cc (V125 urethra < 1 cc).
The first HDR treatment was delivered on the day of the catheter placement. The second treatment was delivered within 24 hours after the first treatment with no less than 6 hours between fractions. The physician could adjust the catheters if catheter displacement was identified prior to the second treatment. If the physician felt the catheters could not be satisfactorily repositioned and dosimetry could not be corrected by re-planning, then the treatment was postponed until a satisfactory implant could be done. After treatment was completed all catheters were removed. CT image based HDR treatment plan including the overall treatment time, source activity, and dwell times of each fraction delivered were submitted electronically to ITC. All plans were reviewed by the principal investigator for quality assurance.
Study Design and Endpoints
The study was designed to test whether the 18-month late genitourinary (GU) and GI (gastrointestinal) adverse events (AE) from the start of protocol treatment was above 10% (0.012/month). The sample size was determined so that the probability of rejecting the treatment because of excessive late AE was 90% if the true late AE rate was 20% (0.025/month). Ninety-eight patients were required to be accrued within a year and be followed for an additional 18 months to have a statistical power of 90% with one-sided significance level of 0.05. Assuming 10% of the cases were ineligible cases or lack-of-data cases, the total sample size of the study was determined to 110 patients.
The analysis of the rate of late grade 3 or greater GU and GI AE following protocol treatment was done when each patient had at least 18 months of follow-up. The secondary objectives include an estimation of acute grade 3 or greater GU and GI AE, freedom from biochemical failure, overall survival, disease-specific survival, and clinical relapse including local/regional and distant relapse. For the secondary endpoints except acute AE endpoint, additional two years of follow-up were needed to estimate the 3-year failure rate (November 2010). All adverse events were graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Acute AE period was defined as 9 months from the start of protocol treatment and late AE period started from the end of 9 month from the start of protocol treatment.
Statistical Methods
The time to the occurrence of severe late GU/GI AE was defined as the time interval from the tenth month after start of protocol treatment to the date of onset of grade 3-5 GU/GI AE. If no such AE was observed till the time of the analysis, the patient was censored at the time of the analysis. The hazard rate was estimated by life table approach with time span of 9 months, between study months 10 and 18. Then the one-sided Z-test was performed to test the significance of the difference between the logarithm of the observed hazard rate and the logarithm of the hypothesized hazard rate of 0.012/month with the variance equal to the reciprocal of the number of cases with late grade 3-5 GU/GI AE observed at the significance level of 0.05. Because of the lead time of 9 months for the acute period, the late toxicity at study month 18 was estimated by the 9 month toxicity rate using the cumulative incidence approach to the defined time to severe late GU/GI AE.(20)
RESULTS
This study was opened on July 30, 2004 and closed on May 26, 2006, with a total of 129 patients enrolled in the study. The median number of patients accrued per institution was 8 (range 1-20), and the average was 9 patients. The average monthly accrual for the study was 5.9 patients. This initial primary analysis includes all the information received by the RTOG headquarter as of November 18, 2008. Two cases were ineligible. One was ineligible due to hip surgery prior to protocol treatment and the other was ineligible due to risk category based on Gleason, PSA and T-stage. Two other cases withdrew consent. At the time of this analysis, of the 125 eligible patients, follow-up information was available for 124 patients and adverse event information was available from 112 patients. The mean, median and range of follow-up time were 29.1 months, 29.6 months, and 2.89-44.61 months respectively. Six patients were censored before month 18 with respect to the primary endpoint. Table 1 shows pretreatment characteristics for 125 eligible patients. The median age of the group was 68 years old range 49-81. Majority of the patient had T1 (54%) and Gleason Score 7 (72%) disease. The median pretreatment PSA was 7.54, range 0.85-19.3. Sixty percent of patient did not receive any adjuvant hormonal therapy. Fourteen institutions enrolled patients to this trial.
Table 1.
Pretreatment characteristics of all eligible patients (n=125)
| Age (years) | |
| Median | 68 |
| Range | 49-81 |
| Race | |
| White | 86 |
| African American | 32 |
| Asian | 2 |
| American Indian/Alaska Native | 1 |
| More than one race | 2 |
| Unknown or not reported | 2 |
| Ethnic Category | |
| Hispanic or Latino | 3 |
| Not Hispanic or Latino | 99 |
| Unknown or not reported | 23 |
| Zubrod performance status | |
| 0 | 121 (97%) |
| 1 | 4 (3%) |
| Gleason Score | |
| 2-6 | 13 (10%) |
| 7 | 90 (72%) |
| 8-10 | 22 (18%) |
| PSA | |
| ≤ 10 | 87 (70%) |
| >10 - ≤ 20 | 38 (30%) |
| Median | 7.54 |
| Range | 0.85-19.30 |
| T-Stage | |
| T1 | 67 (54%) |
| T2 | 47 (38%) |
| T3 | 11 (8%) |
| Hormonal Therapy | |
| No | 75 (60%) |
| Yes | 50 (40%) |
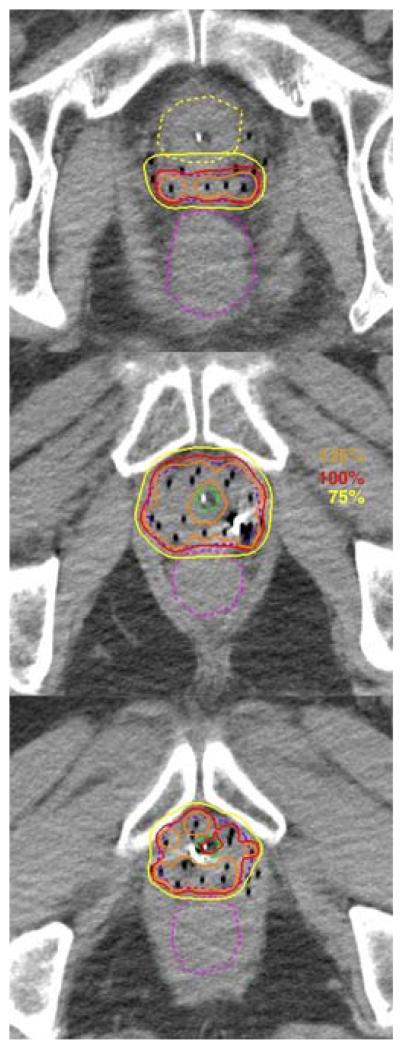
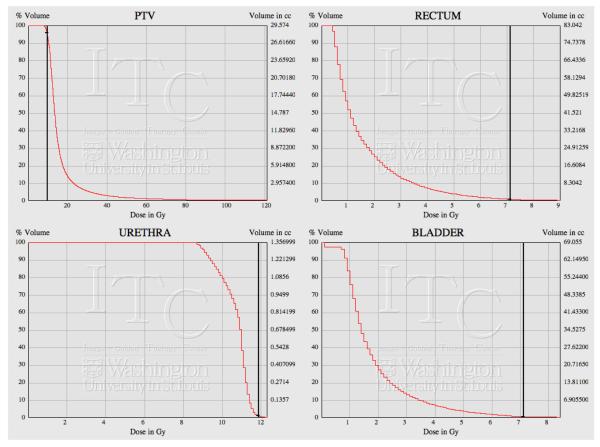
All quality assessment of the implants were done using the Image-Guided Therapy Center (ITC) remote review tool. Figure 1 and 2 show an example implant and its dose-volume histogram from the remote review tool. Out of 125 eligible patients, 4 patients’ data were not available for review. The dosimetry parameters of the reminding 121 patients were calculated based on submitted contours and listed in Table 2. The mean V100PTV was 94.7% (87.6-98.9%). There were 2 cases with V100PTV <90% (87.6%, 89.7%). The mean V75Rectum was 0.34 cc (0-1.59 cc). There were 8 cases with V75Rectum ≥ 1cc (1-1.59 cc). The mean V75Bladder was 0.64 cc (0-4.24 cc). There were 18 cases with V75Bladder ≥ 1 cc (1.01-4.24 cc). The mean V75Urethra was 0.15 cc (0-0.94 cc). No patient had V125Urethra ≥ 1 cc. There were other non-dosimetric protocol variations including: 1 case submitted without a Foley catheter, 4 cases submitted with metallic markers in the catheters, and 2 cases with bladder contours excluding the Foley balloon. Although there was an option of re-adjusting the catheter and re-planning if there was significant catheter migration detected, no patient underwent re-planning prior to the second HDR fraction in this study.
Figure 1.
Implant images displayed on the ITC remote viewing tool. The PTV (dark blue), Rectum (fuchsia), Bladder (yellow) and urethra (green) are shown in dotted lines. Three isodose lines, 125% (orange), 100% (red), and 75% (yellow) are shown in solid lines.
Figure 2.
Dose-Volume Histogram as displayed on the ITC remote viewing tool. The black vertical line represents the reference dose for each structure. (V100PTV = 9.5 Gy, V125Urethra = 11.875 Gy, V75Rectum, Bladder = 7.125 Gy)
Table 2.
Quality assessment of the implants (n = 112 )
| PTV V100 (percent) | |
| Mean | 94.7% |
| Range | 87.6-98.9% |
| Number of cases with V100 <90% | 2 |
| Rectal V75 (cc) | |
| Mean | 0.34 cc |
| Range | 0-1.59 cc |
| Number of cases with V75 ≥1 cc | 8 |
| Bladder V75 (cc) | |
| Mean | 0.64 cc |
| Range | 0-4.24 |
| Number of cases with V75 ≥1 cc | 18 |
| Urethral V125 (cc) | |
| Mean | 0.15 cc |
| Range | 0-0.94 |
| Number of cases with V75 ≥1cc | 0 |
The estimated rate of acute grade 3-5 GU and GI adverse events was 2.43%. The results are summarized in Table 3. There were three ≥3 acute GU/GI AEs. Two patients had grade 3 urinary frequency and one patient had grade 3 urinary retention. The other non-GU/GI grade 3 AEs included one kidney infection, and 2 patients with erectile dysfunction. The median time to acute GU/GI AE was 1.15 month (range 0.89-2.20).
Table 3.
Acute adverse events (≤9 month) by category (n = 112 )
| Grade | ||||
|---|---|---|---|---|
| GU/GI Adverse Events | 2 | 3 | 4 | 5 |
| Frequency | 0 | 2 | 0 | 0 |
| Urinary retention | 8 | 1 | 0 | 0 |
| Non-GU/GI Adverse Events | ||||
| Kidney infection | 0 | 1 | 0 | 0 |
| Erectile dysfunction | 17 | 2 | ||
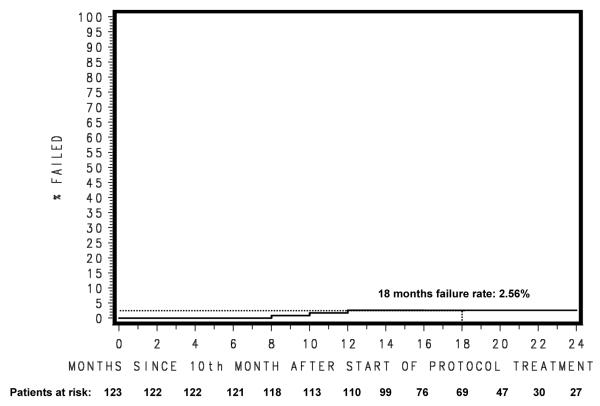
The estimated rate of late grade 3-5 GU and GI adverse events at 18 months was 2.56%, which is translated into hazard rate of 0.0014/month. (Figure 3) Test statistics show that late grade 3-5 GU and GI adverse events at 18 months were less than 10% at the significance level of 0.05 (Z-statistic= −3.7212, p-value ≤ 0.0001). There were four ≥ 3 late GU/GI AEs. The results are summarized in Table 4. The grade 3 GU AEs reported were one of each of the following: urinary retention, cystitis, and urinary incontinence. The only case of grade 3 GI AEs was proctitis. One patient had multiple grade 3 AEs including urinary retention from a stricture and hemorrhage from cystitis requiring a Foley catheter. Although not listed as GI AE, one patient did have grade 3 proctalgia, rectal hemorrhage and low hemoglobin that require transfusion. The other non-GU/GI AE included one grade 3 kidney infection; this was the same patient with the acute kidney infection. (Table 4) There were 5 patients with grade 3 sexual dysfunction. The time to grade ≥ 3 GI/GU adverse event were 19.38, 17.94, 21.98, and 26.87 months from the start of protocol treatment.
Figure 3.
Late (>9 month after treatment start) Grade 3+ GU/GI Adverse Event
Table 4.
Late adverse events (>9 month) by category (n = 112 )
| Grade | ||||
|---|---|---|---|---|
| GU/GI Adverse Events | 2 | 3 | 4 | 5 |
| Urinary retention | 0 | 1 | 0 | 0 |
| Cystitis | 4 | 1 | 0 | 0 |
| Urinary incontinence | 1 | 1 | 0 | 0 |
| Proctitis | 2 | 1 | 0 | 0 |
| Non-GU/GI Adverse Events | ||||
| Proctalgia | 0 | 1 | 0 | 0 |
| Urogenital hemorrhage | 3 | 1 | 0 | 0 |
| Rectal hemorrhage | 0 | 1 | 0 | 0 |
| Anemia | 0 | 1 | 0 | 0 |
| Kidney infection | 0 | 1 | 0 | 0 |
| Ejaculatory disorder | 3 | |||
| Erectile dysfunction | 26 | 5 | ||
DISCUSSION
With the successful completion of this trial, the RTOG has achieved several important landmarks. This is the first perspective multi-institutional study using a HDR prostate brachytherapy. This is the first brachytherapy trial based completely on three-dimensional electronic image data. By prescribing dose base on volume instead of points, this trial marks the arrival of image-guided brachytherapy for the treatment of prostate cancer. The application of HDR prostate brachytherapy in combination with external radiotherapy is well documented in the literature. Multiple single institutions data using a wide range of fractionations and implant schedules have been published in the literature. This study has been designed to study the safety of combining external beam radiotherapy with HDR brachytherapy. The technique and doses used in this study resulted acceptable level of toxicity.
Given the variety of different techniques of HDR brachytherapy, one of the initial challenges is to give a written description of the procedure that is acceptable by all potential participants, yet uniform enough to be scientific. It was reassuring that despite variation in techniques (number of catheters, catheter patterns, different templates), the acute toxicity from the treatment combination was low. The reported acute toxicity was mostly genitourinary. This is consistent with results from other studies using HDR boost. It is also consistent with the dosimetry. Quality assessment showed the most common structure that received dose above its constraint was the bladder. The organ at risk (OAR) with the highest dose constraint was the urethra. Both the dose to bladder and urethra may have contributed to the genitourinary toxicity, and these OARs may be the dose limiting structures. Avoiding injury and lower doses to the bladder and urethra may further reduce treatment toxicity.
So far, the late toxicity from this study shows a variety of single events without clustering. The most common category of adverse event is genitourinary. However, one patient has had multiple grade 3 AEs involved pain and bleeding from the rectum. It is reassuring the overall frequency and severity of the reported AE was low, and it are comparable to other prospective brachytherapy studies.(6, 21-23) This is also comparable to other prospective HDR brachytherapy studies. Borghede et al reported on the results of 50 patients treated with 50 Gy of external beam radiotherapy combined with 2 HDR brachytherapy implants delivering a total dose of 20 Gy (2 fractions). With a median follow-up time of 45 months, they reported 8% RTOG grade 3 urinary toxicity. There were no grade ≥ 3 gastrointestinal complications.(6) Martinez et al reported results of a prospective dose escalation trial of HDR boost. Patients were treated with 46 Gy combined with 2-3 HDR implants delivering between 5.5 Gy × 3 to 11.5 Gy × 2. With 207 patients at a median follow-up time of 3.8 years, the 5 year RTOG GU grade 3 complication rates were 8%, 0.5% for GI grade 3, and 0.5% for GI grade 4 complications.(17) Finally, from the only reported prospective randomized HDR trial, Hoskin et al reported on 109 patients randomized to external radiotherapy of 35.75 Gy/13 fractions and HDR boost of 17 Gy/2 fractions. At a median follow up of 30 months, the rate of grade 2 or worse bladder and bowel reactions were approximately 37% and 10% respectively. (24)
The overall quality of implant dosimetry calculated based on submitted contours was very good. Ninety-eight percent of the V100PTV were above 90%. However, the protocol treatment planning philosophy was not always followed. The goal of the protocol was to maximize the PTV coverage without sacrificing normal tissue sparing. Seven percent of V75Rectum, and 15% of V75Bladder received doses higher than the accepted values. Longer follow-up is needed to see if these and other dosimetry indices correlate with toxicity. Catheter migration between HDR fractions is another important quality issue that has been documented in the literature.(25-28) If a “significant” catheter displacement occurs, the corrective actions including: adjusting the catheter and re-plan before delivering the second HDR fraction, or repeat the whole implant procedure. These options were written in the protocol, but no one chose this approach. Longer follow-up will tell if a more rigorous evaluation is needed. Finally, the dosimetry is only as accurate as the contours. Metallic markers, tubes, or even undiluted contrast can generate significant artifacts on CT and makes contouring and evaluation more challenging. The protocol forbids the use of metallic dummy markers. The use of other tubes or contrast should also be limited in future studies.
One limitation of this study is that it did not use a validated quality of life (QOL) instrument to study the effects of treatment on patients’ sexual function. Without QOL measurement taken before and after the treatment, it is difficult to make any conclusion about the effect of treatments on sexual function. The RTOG’s strategy is to include a quality of life measures in future phase III protocols. As expected the number of patients with grade 3 events, which is defined as decrease sexual function not helped by erectile aids, increased with longer follow-up.
This is a preliminary report of this study after it reached its primary end point. At the time of this report, the follow up time is too short to make any assessment on the efficacy of this treatment. Future updates of this study will include analysis efficacy and dosimetry.
CONCLUSIONS
The result of this trial demonstrated that image guided HDR boost for prostate cancer is feasible in a multi-institutional setting. The technique and doses used in this study resulted in acceptable level of adverse events. Longer follow-up is needed to evaluate the efficacy of this treatment combination.
Acknowledgments
Supported by RTOG U10 CA21661, CCOP U10 CA37422, Stat U10 CA32115 grants and U24 CA81647 from the NCI. This manuscript’s contents are the sole responsibility of the authors and do not necessarily represent the official views of the NCI.
Footnotes
CONFLICTS OF INTEREST NOTIFICATION None for all authors
REFERENCES
- 1.Hsu I-C, Pickett B, Shinohara K, et al. Normal tissue dosimetric comparison between HDR prostate implant boost and conformal external beam radiotherapy boost: potential for dose excalation. International Journal of Radiation Oncology Biology Physics. 2000;46:851–858. doi: 10.1016/s0360-3016(99)00501-5. [DOI] [PubMed] [Google Scholar]
- 2.Brenner D, Hall E. Fractionation and protraction for radiotherapy of prostate carcinoma. International Journal of Radiation Oncology Biology Physics. 1999;43:1095–1101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 3.D’Souza WD, Thames HD. Is the alpha/beta ratio for prostate cancer low? [Comment On: Int J Radiat Oncol Biol Phys. 2001 Sep 1;51(1):213-4 UI: 21407968] International Journal of Radiation Oncology, Biology, Physics. 2001;51:1–3. doi: 10.1016/s0360-3016(01)01650-9. [DOI] [PubMed] [Google Scholar]
- 4.Duchesne GM, Peters LJ. What is the alpha/beta ratio for prostate cancer? Rationale for hypofractionated high-dose-rate brachytherapy [editorial] International Journal of Radiation Oncology, Biology, Physics. 1999;44:747–748. doi: 10.1016/s0360-3016(99)00024-3. [DOI] [PubMed] [Google Scholar]
- 5.Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? International Journal of Radiation Oncology, Biology, Physics. 2001;50:1021–1031. doi: 10.1016/s0360-3016(01)01607-8. [DOI] [PubMed] [Google Scholar]
- 6.Borghede G, Hedelin H, Holmäng S, et al. Combined treatment with temporary short-term high dose rate iridium-192 brachytherapy and external beam radiotherapy for irradiation of localized prostatic carcinoma. Radiotherapy and Oncology. 1997;44:237–244. doi: 10.1016/s0167-8140(97)00121-7. [DOI] [PubMed] [Google Scholar]
- 7.Dinges S, Deger S, Koswig S, et al. High-dose rate interstitial with external beam irradiation for localized prostate cancer--results of a prospective trial. Radiotherapy and Oncology. 1998;48:197–202. doi: 10.1016/s0167-8140(98)00054-1. [DOI] [PubMed] [Google Scholar]
- 8.Martinez A, Gonzalez J, Stromberg J, et al. Conformal prostate brachytherapy: initial experience of a phase I/II dose-escalating trial. International Journal of Radiation Oncology, Biology, Physics. 1995;33:1019–1027. doi: 10.1016/0360-3016(95)00254-5. [DOI] [PubMed] [Google Scholar]
- 9.Mate T, Gottesman J. Fractionated HDR Conformal Prostate Brachytherapy. 8th International Brachytherapy Conference.1995. pp. 75–78. [Google Scholar]
- 10.Kovács G, Galalae R, Loch T, et al. Prostate preservation by combined external beam and HDR brachytherapy in nodal negative prostate cancer. Strahlentherapie und Onkologie. 1999;175(Suppl 2):87–88. doi: 10.1007/BF03038899. [DOI] [PubMed] [Google Scholar]
- 11.Galalae RM, Kovacs G, Schultze J, et al. Long-term outcome after elective irradiation of the pelvic lymphatics and local dose escalation using high-dose-rate brachytherapy for locally advanced prostate cancer. International Journal of Radiation Oncology, Biology, Physics. 2002;52:81–90. doi: 10.1016/s0360-3016(01)01758-8. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs G, Wirth B, Bertermann H, et al. Prostate Preservation by Combined External Beam and HDR Brachytherapy at Nodal Negative Prostate Cancer Patients - An Intermediate Analysis After Ten Years Experience. Int. J. Radiation Oncology Biol. Phys. 1996;36:198. [Google Scholar]
- 13.Mate TP, Gottesman JE, Hatton J, et al. High dose-rate afterloading 192Iridium prostate brachytherapy: feasibility report. International Journal of Radiation Oncology, Biology, Physics. 1998;41:525–533. doi: 10.1016/s0360-3016(98)00097-2. [DOI] [PubMed] [Google Scholar]
- 14.Martinez AA, Pataki I, Edmundson G, et al. Phase II prospective study of the use of conformal high-dose-rate brachytherapy as monotherapy for the treatment of favorable stage prostate cancer: A feasibility report. International Journal of Radiation Oncology Biology Physics. 2001;49:61–69. doi: 10.1016/s0360-3016(00)01463-2. [DOI] [PubMed] [Google Scholar]
- 15.Vargas CE, Martinez AA, Boike TP, et al. High-dose irradiation for prostate cancer via a high-dose-rate brachytherapy boost: results of a phase I to II study. Int J Radiat Oncol Biol Phys. 2006;66:416–423. doi: 10.1016/j.ijrobp.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 16.Martinez AA, Kestin LL, Stromberg JS, et al. Interim report of image-guided conformal high-dose-rate brachytherapy for patients with unfavorable prostate cancer: The William Beaumont Phase II dose-escalating trial. International Journal of Radiation Oncology Biology Physics. 2000;47:343–352. doi: 10.1016/s0360-3016(00)00436-3. [DOI] [PubMed] [Google Scholar]
- 17.Martinez A, Gonzalez J, Spencer W, et al. Conformal high dose rate brachytherapy improves biochemical control and cause specific survival in patients with prostate cancer and poor prognostic factors. J Urol. 2003;169:974–979. doi: 10.1097/01.ju.0000052720.62999.a9. discussion 979-980. [DOI] [PubMed] [Google Scholar]
- 18.Vargas C, Martinez A, Galalae R, et al. High-dose radiation employing external beam radiotherapy and high-dose rate brachytherapy with and without neoadjuvant androgen deprivation for prostate cancer patients with intermediate- and high-risk features. Prostate Cancer Prostatic Dis. 2006;9:245–253. doi: 10.1038/sj.pcan.4500882. [DOI] [PubMed] [Google Scholar]
- 19.(ICRU Report 58) Dose and volume specification for reporting interstitial therapy. International Commission on Radiation Units and Measurements; Bethesda, MD: 1997. [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J. Amer. Statist. Assoc. 1958;53:457–481. [Google Scholar]
- 21.Lee WR, DeSilvio M, Lawton C, et al. A phase II study of external beam radiotherapy combined with permanent source brachytherapy for intermediate-risk, clinically localized adenocarcinoma of the prostate: preliminary results of RTOG P-0019. Int J Radiat Oncol Biol Phys. 2006;64:804–809. doi: 10.1016/j.ijrobp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Lee WR, Bae K, Lawton C, et al. Late toxicity and biochemical recurrence after external-beam radiotherapy combined with permanent-source prostate brachytherapy: analysis of Radiation Therapy Oncology Group study 0019. Cancer. 2007;109:1506–1512. doi: 10.1002/cncr.22560. [DOI] [PubMed] [Google Scholar]
- 23.Hurwitz MD, Halabi S, Ou SS, et al. Combination external beam radiation and brachytherapy boost with androgen suppression for treatment of intermediate-risk prostate cancer: an initial report of CALGB 99809. Int J Radiat Oncol Biol Phys. 2008;72:814–819. doi: 10.1016/j.ijrobp.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Hoskin PJ, Motohashi K, Bownes P, et al. High dose rate brachytherapy in combination with external beam radiotherapy in the radical treatment of prostate cancer: initial results of a randomised phase three trial. Radiother Oncol. 2007;84:114–120. doi: 10.1016/j.radonc.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Damore S, Syed N, Puthawala A, et al. Needle displacement during HDR brachytherapy in the treatment of prostate cancer. International Journal of Radiation Oncology Biology Physics. 2000;46:1205–1211. doi: 10.1016/s0360-3016(99)00477-0. [DOI] [PubMed] [Google Scholar]
- 26.Hoskin PJ, Bownes PJ, Ostler P, et al. High dose rate afterloading brachytherapy for prostate cancer: catheter and gland movement between fractions. Radiother Oncol. 2003;68:285–288. doi: 10.1016/s0167-8140(03)00203-2. [DOI] [PubMed] [Google Scholar]
- 27.Mullokandov E, Gejerman G. Analysis of serial CT scans to assess template and catheter movement in prostate HDR brachytherapy. Int J Radiat Oncol Biol Phys. 2004;58:1063–1071. doi: 10.1016/j.ijrobp.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Kim Y, Hsu IC, Pouliot J. Measurement of craniocaudal catheter displacement between fractions in computed tomography-based high dose rate brachytherapy of prostate cancer. J Appl Clin Med Phys. 2007;8:2415. doi: 10.1120/jacmp.v8i4.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]