Abstract
Objective
This study is designed to test the hypothesis that preservation of residual hearing in a pediatric population is possible using standard electrode arrays with full length insertions. Surgical technique during implantation is also described.
Study Design
Retrospective review of patient medical records.
Setting
Academic tertiary care center.
Patients
Thirty-one severely to profoundly hearing impaired pediatric patients with some residual hearing pre-cochlear implantation.
Intervention
Cochlear implantation using a modified “soft surgery” protocol.
Main Outcome Measures
Pre-implant and post-implant pure tone thresholds and pure tone average (PTA) were calculated from unaided pre- and post-operative audiograms from 250, 500, and 1000 Hz. Hearing preservation rates were determined to be either: complete (loss of ≤10 dB), moderate (loss of 11-20 dB), marginal (loss of 21-40 dB), or none (loss of >40 dB or no response at the limits of the audiometer.) Functional residual hearing rates (defined in this study as at least one threshold better than or equal to 75 dB HL for 250, 500, or 1000 Hz) were calculated.
Results
Complete hearing preservation was achieved in 14/31 patients (45.2%), while 28/31 (90.3%) had at least partial hearing preservation (loss of ≤40 dB). The pre- to post-operative low frequency PTA had a mean change of 18.5 dB and median change of 20 dB. Of the patients who had pre-operative functional hearing, 9/18 (50.0%) maintained functional residual hearing post-operatively for at least one pitch.
Conclusion
Preservation of residual hearing is feasible in pediatric cochlear implant patients using standard length electrode arrays with full insertions. These data have implications for cochlear implantation in pediatric patients who are at higher risk of progressive hearing loss than adults.
Keywords: Cochlear implant, Hearing preservation, Hearing conservation, Soft surgery, Pediatric, Hearing loss, Electroacoustic stimulation
Introduction
While residual hearing is usually lost after cochlear implantation, some individuals have retained some acoustic hearing. Residual hearing in implantees has been shown to increase hearing performance in difficult or noisy listening environments, improve music perception and appreciation, and give sound and voices a more natural quality. (1-5)
Possible causes of residual hearing loss after cochlear implantation include acoustic trauma while drilling a cochleostomy, mechanical trauma due to electrode insertion that includes fractures of the osseous spiral lamina, disruption of the basilar membrane, or tearing of the endosteum of the scala tympani. Other possible causes include disturbances of cochlear fluid homeostasis, bacterial infection, osteoneogenesis, or cochlear fibrosis from a foreign body reaction to electrode components, bone dust, or blood. (3,6,7)
Meticulous surgical technique may improve the chance that residual hearing is preserved following implantation. “Soft surgery” techniques proposed by Lehnhardt (8) and other modifications such as shorter insertion depths, off-stylet technique, and changes in the angle of insertion have been described. (9-13) New electrodes have also been developed in an effort to decrease cochlear trauma during insertion. These include “short arrays” which are designed to provide electrical stimulation at the basal end of the cochlea only. Preserved residual hearing may lead to the application of “electro-acoustic” stimulation (EAS), where the lower pitches are aided through acoustic amplification and the mid/high pitches are aided through the cochlear implant within the same ear. (4,12,14-16)
Full insertion of a standard length electrode array may be advantageous over a shortened electrode array because it allows for progression in low frequency hearing loss. A standard length array allows one to activate more electrodes over time. For example, if an acoustic signal is no longer adequate in the low pitches, the standard cochlear implant can be programmed to provide these pitches in addition to the higher pitches. A shortened array does not reach the deeper regions of the cochlea that are tuned to lower pitches, and typically has fewer electrodes to work with. Such an advantage may be particularly important in children, who face many more years of potential progressive hearing loss than adult patients and whose particular physiology and cause for hearing loss may make them more susceptible to progressive loss. (17)
While residual hearing preservation has been observed in adults, it is not as well documented in children. This study is the result of reviewing our pediatric cochlear implant population to quantify residual hearing preservation using a standard length array with full insertion.
Materials and Methods
Subjects
Institutional review board approval was obtained for the study. Participants were drawn from a sequential series of patients undergoing cochlear implantation by either of two experienced surgeons between June 2006 and March 2009 (n=148). Patients with a pre-operative unaided audiogram with at least one threshold better than 90 dB HL at 250, 500, or 1000 Hz and at least one post-operative unaided audiogram were included in the study (n=31).
Audiometric Evaluation
All audiometric testing was performed using calibrated audiometers with outputs of 105 dB HL at 250 Hz, 110 dB HL at 500 Hz , and 115 dB HL at 1000 Hz. Testing was performed in a double-walled sound booth using insert earphones for unaided testing. Pre-operative responses to pure tones spanning from 250 to 8000 Hz were measured for each ear in unaided conditions. A pre- and postoperative low-frequency pure tone average (PTA) was calculated by averaging thresholds at 250, 500, and 1000 Hz.
Only children with a pre-operative unaided audiogram with at least one threshold ≤90dB HL in the low frequencies were included. Post-operative audiograms for 30/31 patients (96.8%) had thresholds measured for all three of these frequencies. One patient was missing a value for 1000 Hz, so a value of no response (NR) was inserted to assume that hearing at that frequency was lost.
Data Analysis
We used two methods that have both been described in the literature for measuring outcomes in patients who had no response (NR) at one or more frequencies. In one method, a value of 5 dB over the limit of the audiometer was substituted for NR. (6,7,10) This number was used to calculate mean values of pure-tone thresholds and pure-tone averages. However, this method had the possible disadvantage of underestimating the amount of hearing loss, because the true amount of hearing loss above the maximum limit of the audiometer was unknown. An alternative method was also used in which we substituted a very large value (=999) for NR before calculating median threshold changes for each frequency. (9,18) We also calculated a low-frequency PTA using these median thresholds. The disadvantage of this method was that individual data could not be compared among patients.
To help categorize the change in hearing thresholds after implantation, the following terminology was used: change of ≤10 dB, complete hearing preservation; 11-20 dB, moderate preservation; 21-40 dB, marginal preservation; and >40 dB or NR, no preservation. To futher clarify and for purposes of comparison to other writers on this topic, we defined “partial hearing preservation” as change in PTA between 0 and 40 dB. Functional hearing was defined as a threshold of ≤75 dB HL for at least one of the frequencies of 250, 500, or 1000 Hz.
Surgical Technique
Each child was given a perioperative, weight appropriate dose of intravenous dexamethasone of 0.25 mg/ kg. A standardized soft surgical technique was used. The facial recess was opened between the chorda tympani and Fallopian canal. The bone anterior to the facial nerve was removed to provide wide exposure of the round window niche. (Figure 1A) The tectulum, or round window overhang, was thoroughly removed to allow complete exposure of the round window membrane. (13) (Figure 1B) The bone inferior and anterior to the margin of the round window was then thinned using a 1.0 or 0.5 mm diamond burr. (Figure 1C) A cochleostomy was performed manually using a small right-angle pick or footplate rasp, limiting the diameter of the cochleostomy to the size needed for the electrode array. (Figure 1D and 1E) No suctioning of perilymph was performed and every attempt was made to avoid bone dust or blood entering the cochlea. Sodium hyaluronate (Healon, Advanced Medical, Optics, Uppsala, Sweden) was gently injected into the scala tympani. The electrode array was then inserted to the manufacturer’s recommended depth into the scala tympani with the natural curvature of the array being directed away from the cochlear partition. The cochleostomy was then sealed with a small amount of connective tissue.
Figure 1.
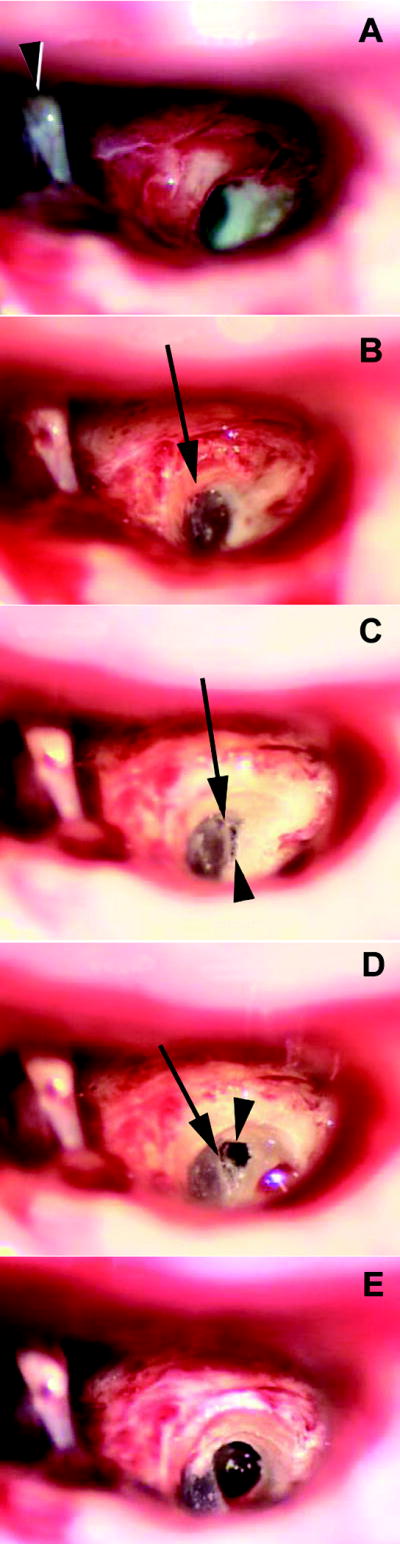
Technique of cochleostomy.
- A wide facial recess approach (right ear). The Fallopian canal is skeletonized and the bone medial to (underneath) the canal is removed to expose the tectulum (bony overhang) of the round window niche. (arrowhead: stapes)
- The tectulum is completely removed (arrow) exposing the entire round window.
- The bone inferior to the round window is thinned to expose the “endosteum” of the scala tympani (arrowhead). The bone removal is just inferior to the annulus (arrow) of the round window membrane.
- The thinned bone of the scala tympani is removed with rasps (arrowhead). Note that the cochleostomy is immediately adjacent to the inferior part of the round window (arrow).
- The completed cochleostomy. The size of the opening into the inferior most part of the scala tympani may vary depending on the size of the electrode array.
Results
Of the 148 pediatric cochlear implants performed between June 2006 and March 2009, 31 patients were eligible for the study because they met the pre-op hearing criteria and they had pre- and post-operative audiograms (Table 1). The mean age was 9.9 years with a range of one to 19 years. Thirteen recipients were female and eighteen were male. Seventeen implants were on the left and fourteen on the right. Patients were implanted either with a HiRes 90K device using a HiFocus Helix or HiFocus 1j electrode, (Advanced Bionics Corp., Sylmar, CA) a Freedom device using a Contour Advance electrode (Cochlear Ltd., Lane Cove NSW, Australia), or with a Med-El Pulsar CI 100 device using a standard electrode array (Med-El, Innsbruck, Austria). The average time between surgery and post-operative audiogram was 10.3 months, with a range of 1-30 months.
Table 1.
Patient characteristics and pre-implant and post-implant pure-tone average with hearing preservation category.
| Patient No. | Sex | Age | Side | Etiology | Progressive | Date of CI | Date of f/u Audio | Implant Type | Pre-op PTA dB HLa | Post-op PTA dB HL | ΔPTA dB SPL | Category of Hearing Preservationb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 15.2 | R | Unknown | Yes | 11/06 | 5/08 | AB 90K Helix | 80 | 90 | 10 | Complete |
| 2 | F | 4.7 | R | EVA | Yes | 11/06 | 3/08 | AB 90K Helix | 80 | 86.7 | 6.7 | Complete |
| 3 | F | 5.2 | L | EVA | Yes | 1/07 | 3/09 | AB 90K Helix | 88.3 | 106.7 | 18.4 | Moderate |
| 4 | F | 7.9 | R | Ototoxic | Yes | 8/06 | 1/07 | Nucleus Freedom | 58.3 | 105 | 46.7 | None |
| 5 | M | 9.3 | L | Neonatal Hypoxia | Yes | 10/06 | 7/08 | Nucleus Freedom | 56.7 | 90 | 33.3 | Marginal |
| 6 | F | 7.4 | R | Unknown | No | 4/07 | 6/08 | AB 90K Helix | 60 | 66.7 | 6.7 | Complete |
| 7 | M | 11.3 | R | Hereditary | Yes | 5/07 | 10/07 | Nucleus Freedom | 90 | 111.7 | 21.7 | Marginal |
| 8 | F | 6.1 | L | Ototoxic | Yes | 6/07 | 2/08 | AB 90K Helix | 56.7 | 100 | 33.3 | Marginal |
| 9 | F | 8.7 | R | Turner syndrome | Yes | 7/07 | 12/07 | Medel Pulsar | 63.3 | 70 | 6.7 | Complete |
| 10 | M | 6.6 | L | EVA | No | 7/07 | 8/07 | Nucleus Freedom | 85 | 90 | 5 | Complete |
| 11 | F | 13.8 | L | Unknown | No | 9/07 | 10/07 | AB 90K 1J | 85 | 101.7 | 16.7 | Moderate |
| 12 | M | 12.4 | L | Unknown | Yes | 9/07 | 8/08 | AB 90K 1J | 46.7 | 56.7 | 10 | Complete |
| 13 | M | 18.2 | R | EVA | Yes | 12/07 | 3/09 | Nucleus Freedom | 78.3 | 116.7 | 38.4 | Marginal |
| 14 | M | 4.7 | R | EVA | Yes | 2/08 | 9/08 | Nucleus Freedom | 75 | 91.7 | 16.7 | Moderate |
| 15 | F | 3.9 | L | Connexin | No | 2/08 | 12/08 | AB 90K 1J | 91.7 | 100 | 8.3 | Complete |
| 16 | M | 15.8 | L | Unknown | No | 7/08 | 7/09 | AB 90K 1J | 90 | 116.7 | 26.7 | Marginal |
| 17 | M | 7.0 | L | Unknown | No | 1/08 | 4/09 | Nucleus Freedom | 80 | 116.7 | 36.7 | Marginal |
| 18 | M | 11.1 | L | Hereditary | Yes | 4/08 | 5/08 | AB 90K 1J | 90 | 96.7 | 6.7 | Complete |
| 19 | M | 10.5 | R | Hereditary | Yes | 5/08 | 10/08 | AB 90K 1J | 80 | 96.7 | 16.7 | Moderate |
| 20 | F | 9.8 | L | Unknown | No | 9/07 | 1/08 | AB 90K 1J | 83.3 | 116.7 | 33.4 | Marginal |
| 21 | M | 8.9 | R | EVA | Yes | 11/08 | 2/09 | AB 90K 1J | 45 | 116.7 | 71.7 | None |
| 22 | F | 1.2 | R | Unknown | Yes | 4/07 | 8/07 | AB 90K Helix | 70 | 75 | 5 | Complete |
| 23 | M | 3.7 | R | Unknown | No | 9/06 | 12/07 | Nucleus Freedom | 80 | 108.3 | 28.3 | Marginal |
| 24 | F | 5.3 | L | Hereditary | Yes | 8/07 | 6/09 | AB 90K | 73.3 | 80 | 6.7 | Complete |
| 25 | F | 19.8 | L | Unknown | No | 8/08 | 7/09 | Nucleus Freedom | 85 | 86.7 | 1.7 | Complete |
| 26 | M | 10.3 | R | Unknown | No | 6/08 | 6/09 | AB 90K 1J | 101.7 | 101.7 | 0 | Complete |
| 27 | M | 13.3 | R | Unknown | Yes | 8/08 | 9/08 | AB 90K | 46.7 | 55 | 8.3 | Complete |
| 28 | M | 16.8 | L | Unknown | No | 11/07 | 7/09 | Nucleus Freedom | 90 | 105 | 15 | Moderate |
| 29 | M | 11.4 | L | Unknown | No | 11/07 | 5/09 | AB 90K | 96.7 | 108.3 | 11.6 | Moderate |
| 30 | M | 11.2 | L | CMV | No | 11/06 | 5/09 | Nucleus Freedom | 91.7 | 111.7 | 20 | Moderate |
| 31 | M | 15.0 | L | Unknown | No | 8/06 | 8/07 | Nucleus Freedom | 98.3 | 95 | -3.3 | Complete |
PTA was calculated as an average of thresholds at 250,500, and 1,000 Hz (maximum audiometer output +5 dB used at frequencies with no response).
Complete hearing preservation refers to post-operative hearing loss of 0 to 10 dB. Moderate refers to post-operative hearing loss of 11-20 dB. Marginal refers to post-operative hearing loss of 21-40 dB. None refers to no residual hearing after implantation or post-operative hearing loss >40 dB.
CI, cochlear implantation. EVA, enlarged vestibular aqueduct. CMV, cytomegalovirus.
Complete residual hearing preservation (change in low-frequency PTA ≤10 dB) was accomplished in 14/31 patients (45.2%). Partial hearing preservation (change in PTA between 0 and 40 dB) was observed in 28/31 patients (90.3%). Moderate hearing preservation (change in PTA between 11 and 20 dB) was observed in 7/31 patients (22.6%). Marginal hearing preservation (change in PTA between 21 and 40 dB) was seen in 7/31 patients (22.6%). Complete loss of residual hearing (change in PTA >40dB or with no response to stimuli), was seen in 3/31 patients (9.7%).
The low frequency PTA across all study patients preoperatively was 77.3 ± 15.9 dB HL (mean ± standard deviation) and postoperatively was 95.8 ± 17.4 dB HL. The change in PTA was 18.5 dB which falls into the moderate hearing preservation for the entire group of 31 patients. The mean change between pre-operative and post-operative thresholds at 250, 500, and 1000 Hz ranged from 14.2 to 21.2 dB HL (Table 2).
Table 2.
Mean changesa in pure-tone thresholds, in dB.
| 250 Hz | 500 Hz | 1000 Hz | PTA (dB HL)b | |
|---|---|---|---|---|
| Pre-op(+/- SDc) | 63.4 (25.6) | 75.2 (20.0) | 95.2 (9.3) | 77.3 (15.85) |
| Post-op(+/-SD) | 81.9 (23.7) | 96.6 (18.9) | 109.4 (11.2) | 95.8 (17.4) |
| Change | 18.9 | 21.2 | 14.2 | 18.5 |
Mean thresholds and PTA were calculated by inserting n +5 dB for no response, where n is the maximum output of the audiometer at a given frequency,
PTA was calculated as the average of 250, 500, and 1000 Hz.
SD is standard deviation
The median low frequency PTA for all study patients preoperatively was 83.3 dB HL. The median post-operative PTA was 103.3 dB HL with a change of 20 dB. This falls within moderate hearing preservation category for the entire group and confirms the values obtained from calculating mean values and assures the results are not underestimating the amount of hearing loss. The median change in hearing thresholds for 250, 500, and 1000 Hz ranged from 17.5 and 22 dB (Table 3).
Table 3.
Median changesa in pure-tone thresholds, in dB.
| 250 Hz | 500 Hz | 1000 Hz | PTAb | |
|---|---|---|---|---|
| Pre-op | 72.5 | 82.5 | 95 | 83.3 |
| Post-op | 90 | 105 | 115 | 103.3 |
| Change | 17.5 | 22.5 | 20 | 20 |
Median thresholds and PTA were calculated by inserting 999 for frequencies where there was no response.
PTA was calculated as the average of 250, 500, and 1000 Hz.
Functional residual hearing after cochlear implantation was defined as having at least one post-operative threshold ≤75 dB HL at 250, 500, or 1000 Hz. Of the patients who had functional hearing preoperatively, 9/18 (50.0%) preserved it following implantation. An additional 3/18 (16.7%) patients had at least one threshold of 80 dB HL or better, which in most cases will also be aidable.
Discussion
This study reports the largest group to date of children with residual pre-operative hearing implanted with full-length arrays. We found that most pediatric patients in our study had some preservation of hearing and nearly half had complete preservation of hearing following cochlear implantation with full-length arrays. This observation raises the possibility that refinements in surgical technique and electrode design may allow reliable residual hearing conservation with full length cochlear implant array insertions.
Experience of hearing preservation with short arrays
Residual hearing preservation has been noted since the inception of CI surgery. An early study used the 3M/House short single-electrode (3M Healthcare Ltd. Loughborough Leicestershire, UK) which was 6-mm long. That study reported 6/9 (66.7%) of children who had measurable pre-implant hearing had measurable post-implant acoustic thresholds.
More recently, Gantz et al. have reported results using the Iowa Nucleus implant (Cochlear Ltd., Lane Cove NSW, Australia) which includes a short 10-mm electrode. This has been given the name of a “hybrid” CI because it can be used in combination with an external hearing aid. They have reported that 85/87 (97.7%) of their CI patients had at least partial immediate hearing preservation, with 79/81 (90.8%) maintaining this hearing preservation for more than 3 months later. (15)
Experience of hearing preservation with full insertion in adults
Several reports have documented hearing preservation in adults with full or partial insertions of electrode arrays that range between 16-32 mm in length, and inserted >360 degrees within the cochlea. In 1997, Hodges et al. reported on fourteen patients that were implanted with a Nucleus device inserted between 22 and 32 mm and four patients that were implanted with a Clarion device at 16 mm. They saw at least partial hearing conservation in 6/18 (33.3%) adult CI patients as defined by measurable hearing in at least one frequency at 500, 1000, or 2000 Hz. (20) Kiefer et al. (6) reported on fourteen patients that were implanted with either a Med-El Combi 40+ or 40+M device at an insertion depth of 19-24mm. They observed at least partial hearing preservation in 12/14 (85.7%) subjects (loss of ≤20 dB) and complete hearing preservation in 9/14 (64.3%) (loss ≤10 dB). Median threshold values worsened by 15 dB at 250 Hz, 17.5 dB at 500 Hz, and 5 dB at 1000 Hz. Gstoettner et al. (11) used a Med-El Combi 40+ implant inserted 18-24mm and reported preservation was achieved in 18/21 (85.7%) patients (any measurable low frequency hearing) and completely preserved in 13/21 (61.9%) (loss ≤ 10 dB). In a multicenter study, James et al. (18) used a Nucleus Contour Advance device inserted 17-19 mm in twelve patients and reported median increases in hearing thresholds of 23 dB at 125 Hz, 27 dB at 250 Hz, and 33 dB at 500 Hz. They also reported that 6/12 (50.0%) of these patients retained sufficient hearing for effective use of an ipsilateral hearing aid to be used in conjunction with the cochlear implant.
Over the last three years, several other studies have also been published on hearing preservation in adults implanted with devices inserted >360 degrees within the cochlea. Balkany et al. (10) implanted 28 patients with a Nucleus Contour Advance electrode inserted 19mm. They cited complete preservation of hearing (change in PTA ≤10 dB) in 9/28 (32.1%) of patients and partial conservation (any measurable hearing) in 16/28 (57.1%) of patients. Median increases of 15 dB were seen at 250, 500, and 1000 Hz. Di Nardo et al. (7) implanted 37 patients with various devices inserted at a depth of >360 degrees within the cochlea. They reported that 14/37 (37.8%) of patients had complete hearing preservation (no change in hearing threshold), with 15/37 (40.5%) additional patients maintaining measurable hearing thresholds. They also reported median increases in hearing threshold levels of 10 dB at 250 Hz, 10 dB at 500 Hz, and 5 dB at 1000 Hz. Skarzynski et al. (21) implanted ten adults with a Med-El Combi 40+ electrode inserted 31 mm using a round window surgical technique. They reported hearing preservation in 9/10 (90.0%) (any measurable low frequency hearing.) Finally, Soda-Merhy, et al. (3) compared results using straight and perimodiolar electrodes and saw no difference in rates of hearing preservation between them, observing that 18/35 (51.4%) of patients had some measurable post-operative hearing.
Experience of hearing preservation in children
In 1997 Hodges et al. (20) reported on 22 children implanted with Nucleus or Clarion devices to a depth between 16 and 32 mm. They noted complete conservation of hearing (loss ≤10 dB) in 15/22 (68%) of children implanted. The pre-operative PTA of those children was 108 dB HL; therefore, forming a very different group than the patients reported in our study who had a mean pre-operative PTA of 77.3 dB HL. More recently in 2007, Skarzynski et al. (4) reported on nine children implanted with Med-El devices that were partially inserted to a depth of 20mm, finding that 9/9 (100.0%) children had preservation of at least some residual hearing and 8/9 were candidates for electro-acoustic stimulation.
A partial insertion in children of 180 degrees would possibly limit damage to the lower frequency regions of the cochlea apically, but a full insertion might be preferable because children have a greater rate of progressive hearing loss than adults. Discussion of how best to preserve residual hearing with cochlear implants must account for this consideration. In the series reported here, 17/31 (54.8%) patients had progressive hearing loss instead of congenital or stable hearing loss (Table 1). In the study that reported the best rates of hearing preservation, over 97% of patients had hearing preservation following implantation with a short electrode and 91% maintained some hearing preservation 3 months later. In addition, 30% exhibited greater than 30 dB mean low-frequency threshold shifts compared to pre-operative thresholds. So even among adults, some showed evidence of progressive hearing loss after implantation of the short (hybrid) electrode. A recent study discussed two patients who had increased hearing benefit after having their hybrid (shorter) array explanted after progressive loss of residual hearing, and being reimplanted with a full-length electrode array. (22)
With a full insertion using a standard length electrode array, low frequency electrodes can be deactivated for an EAS application, or they can be activated to provide electrical stimulation if low frequency residual hearing is lost over time from progressive hearing loss. Such children with functional residual hearing could benefit from EAS without the possibility of a future procedure being needed to change arrays if the hearing loss progresses. This situation was recently reported in a case study of one child from this series who had preservation of functional residual hearing. She has a full-length array cochlear implant with a hearing aid on the same ear, and prefers this combination to the cochlear implant alone. (17)
Further work is needed to determine if full insertion using standard length or redesigned electrodes can preserve hearing in children with only mild to moderate hearing loss in the low frequencies. Of the six patients with a mean pre-operative PTA between 45.0 and 58.3 dB HL (patients 4, 5, 8, 12, 21, and 27 in Table 1), only patients 12 and 27 had complete preservation of residual hearing (loss ≤10 dB). Patients 5 and 8 had marginal hearing preservation (loss between 20 and 40 dB) and patients 4 and 21 had loss of residual hearing (loss ≥40 dB). Further enhancements of electrode array design may result in less traumatic insertions and better residual hearing conservation. Shorter and more delicate electrodes have been designed for this purpose.
The data in this study show that intermediate-term hearing preservation is possible, with the longest follow up being 30 months, and an average follow up time of 10.7 months. It is also important to note that given the progressive nature of hearing loss in over half of the subjects in this study even prior to implantation, residual hearing might have been lost in some patients irrespective of the surgical technique, electrode length, or depth of insertion. Future studies will be necessary to determine if short- and medium- term hearing preservation persists over a longer term and would involve multiple post-operative audiograms.
Techniques in soft surgery have been previously described. (2,4,8) Both implanting surgeons used a documented, consistent surgical technique. Some studies are not as clear or consistent about their techniques and some take place at multiple centers with multiple surgeons. This may account for variability in outcomes. Further advances in surgical technique and understanding of the causes for hearing loss during and after implantation will likely improve our ability to preserve functional hearing.
Acknowledgments
Supported by grants NIH/NIDCD R01 DC000263 (RAC) and NIH/NIDCD K08 DC 006869 (TEH)
References
- 1.Papsin BC, Gordon KA. Cochlear implants for children with severe-to-profound hearing loss. N Engl J Med. 2007;357:2380–7. doi: 10.1056/NEJMct0706268. [DOI] [PubMed] [Google Scholar]
- 2.Fraysse B, Macias AR, Sterkers O, et al. Residual hearing conservation and electroacoustic stimulation with the Nucleus 24 Contour Advance cochlear implant. Otol Neurotol. 2006;27:624–33. doi: 10.1097/01.mao.0000226289.04048.0f. [DOI] [PubMed] [Google Scholar]
- 3.Soda-Merhy A, Gonzalez-Valenzuela L, Tirado-Gutierrez C. Residual hearing preservation after cochlear implantation: Comparison between straight and perimodiolar implants. Otolaryngol Head Neck Surg. 2008;139:399–04. doi: 10.1016/j.otohns.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Skarzynski H, Lorens A, Piotrowska A. Partial deafness cochlear implantation in children. Int J Pediatr Otorhinolaryngol. 2007;71:1407–13. doi: 10.1016/j.ijporl.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Buchner A, Schussler M, Battmer RD. Impact of low-frequency hearing. Audiol Neurotol. 2009;14(suppl 1):8–13. doi: 10.1159/000206490. [DOI] [PubMed] [Google Scholar]
- 6.Kiefer J, Gstoettner W, Baumgartner W, et al. Conservation of low-frequency hearing in cochlear implantation. Acta Otolaryngol. 2004;124:272–80. doi: 10.1080/00016480310000755a. [DOI] [PubMed] [Google Scholar]
- 7.Di Nardo W, Cantore I, Melillo P. Residual hearing in cochlear implant patients. Eur Arch Otorhinolaryngol. 2007;264:855–60. doi: 10.1007/s00405-007-0270-8. [DOI] [PubMed] [Google Scholar]
- 8.Lehnhardt E. Intracochlear placement of cochlear implant electrodes in soft surgery technique. HNO. 1993;41:356–9. [PubMed] [Google Scholar]
- 9.Garcia-Ibanez L, Macias AR, Morera C. An evaluation of the preservation of residual hearing with the Nucleus Contour Advance electrode. Acta Otolaryngol. 2009;129:651–64. doi: 10.1080/00016480802369278. [DOI] [PubMed] [Google Scholar]
- 10.Balkany TJ, Connell SS, Hodges AV, et al. Conservation of residual acoustic hearing after cochlear implantation. Otol Neurotol. 2006;27:1083–8. doi: 10.1097/01.mao.0000244355.34577.85. [DOI] [PubMed] [Google Scholar]
- 11.Gstoettner W, Kiefer J, Baumgartner W. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Otolaryngol. 2004;124:348–352. doi: 10.1080/00016480410016432. [DOI] [PubMed] [Google Scholar]
- 12.Gantz BJ, Turner C, Gfeller KE. Preservation of hearing in cochlear implant surgery: Advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- 13.Meshik X, Holden TA, Chole RA, et al. Optimal cochlear implant insertion vectors. Otol Neurotol. 2010;31(1):58–63. doi: 10.1097/MAO.0b013e3181b76bb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skarzynski H, Lorens A, Piotrowska A, et al. Results of partial deafness cochlear implantation using various electrode designs. Audiol Neurotol. 2009;14(Suppl 1):39–45. doi: 10.1159/000206494. [DOI] [PubMed] [Google Scholar]
- 15.Gantz BJ, Hansen MR, Turner CW. Hybrid 10 Clinical Trial. Audiol Neurotol. 2009;14(Suppl 1):32–8. doi: 10.1159/000206493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorens A, Polak M, Piotrowksa A. Outcomes of treatment of partial deafness with cochlear implantation: A DUET study. Laryngoscope. 2008;118:288–94. doi: 10.1097/MLG.0b013e3181598887. [DOI] [PubMed] [Google Scholar]
- 17.Uchanski RM, Davidson LS, Quadrizius S, et al. Two ears and two (or more?) devices: A pediatric case study of bilateral profound hearing loss. Trends Amplif. 2009;13(2):107–23. doi: 10.1177/1084713809336423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James C, Albegger K, Battmer R. Preservation of residual hearing with cochlear implantation: How and why. Acta Otolaryngol. 2005;125:481–91. doi: 10.1080/00016480510026197. [DOI] [PubMed] [Google Scholar]
- 19.Dye LM, House WF, O’Conor C. Measurable residual hearing following cochlear implantation. In: Meyers EN, Bluestone CD, Brackmann DE, Krause CJ, editors. Advances in Otolaryngology Head and Neck Surgery. Vol. 4. St Louis: Mosby Year Book; 1990. pp. 61–79. [Google Scholar]
- 20.Hodges AV, Schloffman J, Balkany T. Conservation of residual hearing with cochlear implantation. Am J Otol. 1997;18:179–183. [PubMed] [Google Scholar]
- 21.Skarzynski H, Lorens A, Piotrowska, et al. Preservation of low frequency hearing in partial deafness cochlear implantation (PDCI) using the round window surgical approach. Acta Otolaryngol. 2007;127:41–8. doi: 10.1080/00016480500488917. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald MB, Sagi E, Jackson M, et al. Reimplantation of hybrid cochlear implant users with a full-length electrode after loss of residual hearing. Otol Neurotol. 2008;29:168–73. doi: 10.1097/mao.0b013e31815c4875. [DOI] [PubMed] [Google Scholar]


