Abstract
Objective
The aim of this study was to determine if there are racial disparities in regard to the age at which SLE patients experience CVD and CVD associated death.
Methods
Using the 2003–2006 National Inpatient Sample, we calculated the age difference between SLE patients and their race and gender-matched controls at the time of hospitalization for a cardiovascular (CVD) event and for CVD-associated death. In addition, we also calculated the age difference for the same outcomes between White SLE patients and gender-matched controls for each minority group.
Results
The mean age difference at the time of CVD event between women with and without SLE was 10.5 years. All age differences between women with SLE (n=3,625) and women without SLE admitted for CVD were significant (p<0.0001). Black women were the youngest female SLE racial group to be admitted with CVD (53.9 years) and have a CVD associated inhospital mortality (52.8 years; n=218). Black SLE women were 19.8 years younger than race and gender-matched controls at the time of CVD associated death. Admission trends for CVD were reversed for Black women such that the highest proportions of these patients were admitted before age 55 and then steadily decreased across age categories. There were 805 men with SLE admitted with a CVD event, with Black and Hispanic groups being the youngest.
Conclusions
There are significant racial disparities with regard to age at the time of hospital admission for CVD events and a CVD-related hospitalization resulting in death in patients with SLE.
Cardiovascular disease (CVD) is a significant cause of morbidity and mortality in patients with systemic lupus erythematosus (SLE). Recent data have demonstrated that ischemic heart disease is the leading cause of death in SLE patients, representing 36% of all deaths (1).
SLE is an independent risk factor for myocardial infarction, after controlling for other known CVD risks (2). Given that SLE can be particularly severe in certain ethnic groups, including African-Americans, and that inflammation is a key mechanism in the pathogenesis of CVD (3), it is logical to theorize that the epidemiology of CVD and CVD – related death may be altered in those racial groups. In addition, the mean age at onset of SLE is younger in African-Americans, as compared to Caucasians, thus the duration of inflammation and disease may result in CVD and/or CVD death at earlier ages than age-matched non-SLE patients and age-matched Caucasian SLE patients. The goal of our study was to examine the race-specific age differences in SLE patients experiencing CVD and CVD associated death in a large national population-based in-patient population. We also examined the pattern of admissions for different racial groups in age categories in SLE and non-SLE patients for CVD and CVD-related death.
Patients and Methods
Data Source
Data from the Health Care Utilization Project (HCUP) National Inpatient Sample (NIS) Database between 2003 and 2006 were analyzed (4). The NIS is the largest all-payer inpatient care database in the United States. It contains data from approximately 8 million hospital stays each year. The sampling frame for the 2006 NIS is a sample of hospitals that comprises approximately 20 percent of all hospital discharges in the United States. The NIS contains clinical and resource use information included in a typical discharge abstract, with safeguards to protect the privacy of individual patients, physicians, and hospitals. The 2006 NIS contains all discharge data from 1,045 hospitals located in 38 States, approximating a 20-percent stratified sample of U.S. community hospitals. The NIS’s large sample size enables analyses of rare conditions, such as SLE.
Study subjects: inclusion and exclusion criteria
All adult patients (≥ age 18 years) were included in the analysis. All SLE patients were identified via the primary or secondary diagnosis codes, using the International Classification of Diseases, 9th revision, Clinic Modification (ICD-9-CM) (5) code 710.0 (SLE). Diagnosis codes were coded as either the primary diagnosis or as one of 14 secondary diagnoses. Patients were excluded if they had ICD-9 code 714.0 (rheumatoid arthritis) or 710.1 (scleroderma), as both of these conditions may potentially overlap with SLE and/or be associated with CVD. We avoided these ICD-9 codes in order not to falsely include patients who may not truly have SLE. HCUP also supplies a primary procedure and up to 14 secondary procedure codes. Inclusion of acute CVD included “MI” (ICD-9 410.0 through 411.9) and “angina” (ICD-9 413.0 through 413.9). We also examined all hospitalizations for which principal procedures or secondary procedures included cardiac revascularization (including angioplasty and stent placement and coronary artery bypass (ICD-9 36.0 through 36.34). Any admission which did not include gender, race, age, or median income by zip code was excluded from the analyses.
Outcomes
Age at the time of a CVD event or CVD associated death was the primary outcome of interest. Our unadjusted and adjusted models used age as a continuous variable as the dependent variable. Cardiovascular disease was defined as any of the above noted ICD-9 codes for MI and/or angina and/or above noted procedural codes. “CVD associated death” was defined as any in-hospital mortality with the above noted cardiac ICD-9 and/or procedural codes. We also examined CVD-associated death as a secondary outcome of interest.
Covariates
Gender, age at the time of admission, and race were identified from the dataset. Racial groups were identified as Black, White, Hispanic, Asian/Pacific Islander, or Other (which included Native Americans). Race was available from 39 states for the 2003 and 2004 data and from 41 states from the 2004–2006 data. The median income of the patients’ zip codes was used as a surrogate of socioeconomic status, since individual income levels were not available. Zip code quartiles were defined as < $36,000, $36,000 – $44,999, $45,000–$58,999, and > $59,000. We identified patients with specific concomitant comorbidities using the Elixhauser Comorbidity Index, which is incorporated in HCUP software (6). Congestive heart failure, diabetes mellitus, neurological disease, obesity, and renal failure were included in the comorbidities included in regression analyses.
Statistical Analysis
To examine the age difference at the time of CVD admission and CVD death between women and men of the same ethnicity, unadjusted regression models were used in each ethnic/gender group using the diagnosis of SLE as the independent variable and age as the dependent variable. To examine the age differences between white SLE subjects versus minority SLE subjects with CVD or CVD death, unadjusted regression analyses were utilized with ethnicity as the independent variable and age as the dependent variable. An adjusted regression analysis of CVD death in SLE patients was also done controlling for age, gender, race, female-race interactions, and the five above-mentioned comorbidities. Due to colinearity between race and income by zip code, the income variable was not included in this regression analysis.
RESULTS
Sample characteristics
There were 31,927,484 hospitalizations for non-SLE patients and 124, 688 hospitalizations for SLE patients. Patients whose data did not include race, age, gender, and/or median income by zip code were eliminated from the analyses. This resulted in 18,939,916 hospitalizations for non-SLE patients and 90,444 hospitalizations for SLE patients (See Table 1). 89% (80,744) of the SLE population were women as compared to 61% of the non-SLE population. SLE affects women 9 times as frequently as men, matching the gender ratio of this population. The mean age of the female SLE patients was 50.2 years versus 54.7 years for the female non-SLE group, and the mean age for the men was 52.3 versus 60.4 years, respectively The racial composition for the SLE group was as follows; White 55%, Black 28%, Hispanic 12%, Asian 2%, Other 3%. There was a higher proportion of Blacks in the SLE group compared to non-SLE, 28% versus 14%. This was primarily due to the higher proportion of Black to White ratio in the SLE group, 2:1 versus 5:1 in the non-SLE group. Though statistically different due to large sample size, the distribution of the median income by zip codes for the SLE and non-SLE groups are somewhat similar.
Table 1.
Sociodemographic Characteristics of Hospitalized SLE Patients versus Hospitalized Non-SLE Population
| Non-SLE |
SLE |
p-value |
||
|---|---|---|---|---|
| N=18, 939,916 | N=90,444 | |||
| Gender | Women | 11,552,552 (61%) | 80,744 (89%) | <0.0001 |
| Men | 7,387,364 (39%) | 9,700 (11%) | ||
| Age (years) | Women | 54.7 ± 22.5 | 50.2 ± 16.8 | <0.0001 |
| Men | 60.4 ± 18.1 | 52.3 ± 17.7 | ||
| Race | White | 13,261,642 (70%) | 50,084 (55%) | <0.0001 |
| Black | 2,545,085 (14%) | 25,170 (28%) | ||
| Hispanic | 2,133,627 (11%) | 10,997 (12%) | ||
| Asian | 415,041 (2%) | 1,944 (2%) | ||
| Other | 584,521 (3%) | 2,249 (3%) | ||
| Median Income | <$35,999 | 5,472,302 (29%) | 28,841 (32%) | <0.0001 |
| $36,000–44,999 | 4,722,823 (25%) | 22,309 (25%) | ||
| $45,000–58,999 | 4,472,827 (24%) | 20,418 (22%) | ||
| >$59,000 | 4,271,964 (22%) | 18,876 (21%) |
Due to the large population, these small differences were still significant (p<0.0001)
Age at CVD
We were particularly interested in determining if there were racial disparities in regards to the age at hospitalization for CVD and age at death associated with CVD. Table 2 demonstrates the age at which hospitalized SLE and non-SLE patients were with CVD event. In addition, Table 2 also shows the age differences between racially matched controls and SLE patients with CVD. Since the mean age for all of the controls groups was higher than the SLE patients, the mean age of the SLE group was subtracted from the mean age of the control group to determine the “age difference” for each racial category. We also calculated the age difference between the White SLE group and each of the other racial groups. Since the mean age of the White SLE groups were older than each of the other racial groups, this was calculated as the mean age of the White SLE group minus the mean age of each of the racial groups with SLE.
Table 2.
Mean age at time of CVD admission (in Years)
| SLE |
Control |
Age difference (control- SLE) |
p-value |
Age difference (white SLE- minority SLE) |
p-value |
|
|---|---|---|---|---|---|---|
| WOMEN | N=3,627 | N= 608,543 | 10.5 | <0.0001 | ||
| 60.8 | 71.3 | |||||
| White | 63.5 | 72.5 | 9.0 | <0.0001 | ||
| N (%) | 2,352 (65%) | 466,056 (76%) | ||||
| Black | 53.9 | 65.8 | 11.9 | <0.0001 | 9.6 | <0.0001 |
| N (%) | 786 (21%) | 67,602 (11%) | ||||
| Hispanic | 57.5 | 68.6 | 11.1 | <0.0001 | 6.0 | <0.0001 |
| N (%) | 343 (9%) | 48,085 (8%) | ||||
| Asian | 60.6 | 71.5 | 10.8 | <0.0001 | 2.9 | 0.11 |
| N (%) | 55 (2%) | 10,994 (2%) | ||||
| Other | 57.0 | 68.6 | 11.6 | <0.0001 | 6.4 | <0.0001 |
| N (%) | 91 (3%) | 15,806 (3%) | ||||
| MEN | N=805 | N=828,608 | 5.5 | <0.0001 | ||
| 60.4 | 65.9 | |||||
| White | 63.0 | 66.7 | 3.7 | <0.0001 | ||
| N (%) | 603 (75%) | 665,999 (80%) | ||||
| Black | 52.3 | 61.4 | 9.1 | <0.0001 | 10.7 | <0.0001 |
| N (%) | 114 (14%) | 59,851 (7%) | ||||
| Hispanic | 51.4 | 63.5 | 12.1 | <0.0001 | 11.6 | <0.0001 |
| N (%) | 58 (7%) | 59,556 (7%) | ||||
| Asian | 59.2 | 65.9 | 6.7 | 0.16 | 3.8 | 0.41 |
| N (%) | 8 (1%) | 16,914 (2%) | ||||
| Other | 55.4 | 62.6 | 7.2 | 0.009 | 7.6 | 0.007 |
| N (%) | 22 (3%) | 26,288 (3%) |
Women with CVD
There were 3,627 admissions for SLE women with acute CVD. The mean age of SLE women hospitalized with CVD was 60.8 ± 13.7 years, whereas the mean age of non-SLE women admitted with CVD was 71.3 ± 13.4 years. The range of age differences between the female controls and female SLE patients was 9.0 – 11.9 years. Of the women with CVD, the Black SLE group was the youngest, at 53.9 years of age; this was in comparison to 65.8 years of age for Black women without SLE. This 11.9 year difference was the largest age difference between any SLE versus control racial group comparison. The mean age at CVD event for controls versus SLE were each examined via unadjusted regression models. All were significant (p<0.0001). Although White lupus patients were significantly younger than their non-SLE controls at the time of CVD event and CVD death, they were significantly older than most of the other SLE racial groups. On examination of age at CVD hospitalization between White and minority SLE subjects, significant age differences were demonstrated for the Black (p<0.0001), Hispanic (p<0.0001), and the Other groups (p<0.0001), but not for the Asian group. The largest age difference between White and minority groups was for the Black SLE group. There was a 9.6 year difference for the age of hospitalization for CVD with Blacks being 53.9 years versus 63.5 years for the White SLE group.
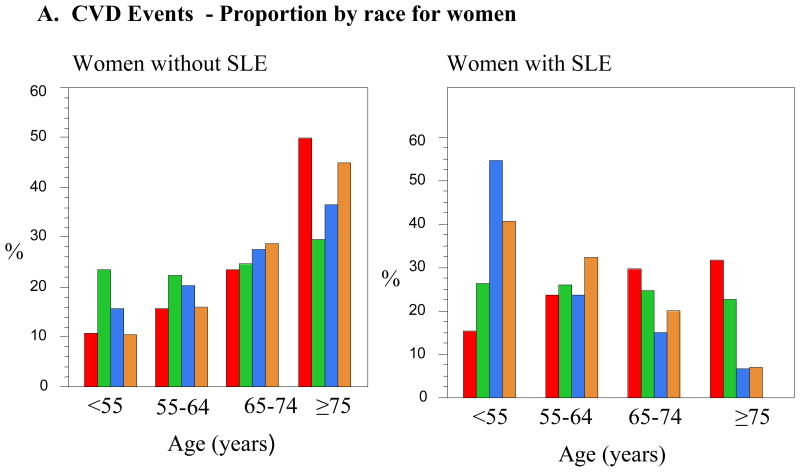
We were interested in demonstrating any trends that differ between racial groups in regards to not only the mean age at presentation for CVD events, but also the proportion of patients with CVD events in different decades of life. We chose age categories similar to those previously used in the general population of age <55, 55–64, 65–74, and ≥75 years of age (7). The specific proportions of patients with and without SLE in each race with either CVD events or CVD mortality are listed in Table 4. The proportion of SLE women admitted with CVD less than the age of 55 was 2.4 to 3.3 times higher than non-SLE women. Fifty-five percent of Black women with SLE were admitted with CVD in the youngest age group, 41% of the Hispanic women, 33% of the Asian women, and 26% of the White women with SLE. Figure 1 demonstrates these trends visually. In women without SLE, the general tendency in women was that the proportion of patients admitted with a CVD event increased with age. This trend was reversed in patients with SLE, with the effect being most dramatic in Black and Hispanic women. This apparent reversal is likely in part due to the young age at which these SLE patients experience their CVD events.
Table 4.
Proportions of CVD and CVD Death in Men and Women with and Without SLE by Race
| Proportion of Women by Age Categories WITHOUT SLE with CVD | ||||
|---|---|---|---|---|
| <55 | 55–64 | 65–74 | ≥75 | |
| White | 11% | 16% | 23% | 50% |
| Black | 23% | 22% | 25% | 30% |
| Hispanic | 16% | 20% | 28% | 36% |
| Asian | 10% | 16% | 29% | 45% |
|
Proportion of Women by Age Categories WIH SLE with CVD | ||||
| White | 26% | 26% | 25% | 23% |
| Black | 55% | 24% | 15% | 6% |
| Hispanic | 41% | 32% | 20% | 7% |
| Asian | 33% | 24% | 20% | 23% |
|
Proportion of Women by Age Categories WITHOUT SLE with CVD Death | ||||
| White | 3% | 7% | 16% | 73% |
| Black | 12% | 15% | 23% | 50% |
| Hispanic | 7% | 13% | 23% | 57% |
| Asian | 5% | 8% | 21% | 66% |
|
Proportion of Women by Age Categories WITH SLE with CVD Death | ||||
| White | 17% | 24% | 27% | 32% |
| Black | 51% | 23% | 17% | 9% |
| Hispanic | 23% | 36% | 29% | 12% |
| Asian | 22% | 34% | 22% | 22% |
|
Proportion of Men by Age Categories WITHOUT SLE with CVD | ||||
| White | 19% | 24% | 26% | 31% |
| Black | 33% | 26% | 23% | 18% |
| Hispanic | 26% | 25% | 26% | 23% |
| Asian | 21% | 24% | 27% | 28% |
|
Proportion of Men by Age Categories WITH SLE with CVD | ||||
| White | 26% | 26% | 28% | 20% |
| Black | 58% | 26% | 12% | 4% |
| Hispanic | 67% | 9% | 14% | 10% |
| Asian | 25% | 25% | 50% | - |
|
Proportion of Men by Age Categories WITHOUT SLE with CVD Death | ||||
| White | 6% | 11% | 22% | 61% |
| Black | 17% | 20% | 26% | 37% |
| Hispanic | 13% | 15% | 26% | 46% |
| Asian | 8% | 10% | 23% | 59% |
|
Proportion of Men by Age Categories WITH SLE with CVD Death | ||||
| White | 18% | 25% | 25% | 32% |
| Black | 67% | 33% | - | - |
| Hispanic | - | - | - | - |
| Asian | 100% | - | - | - |
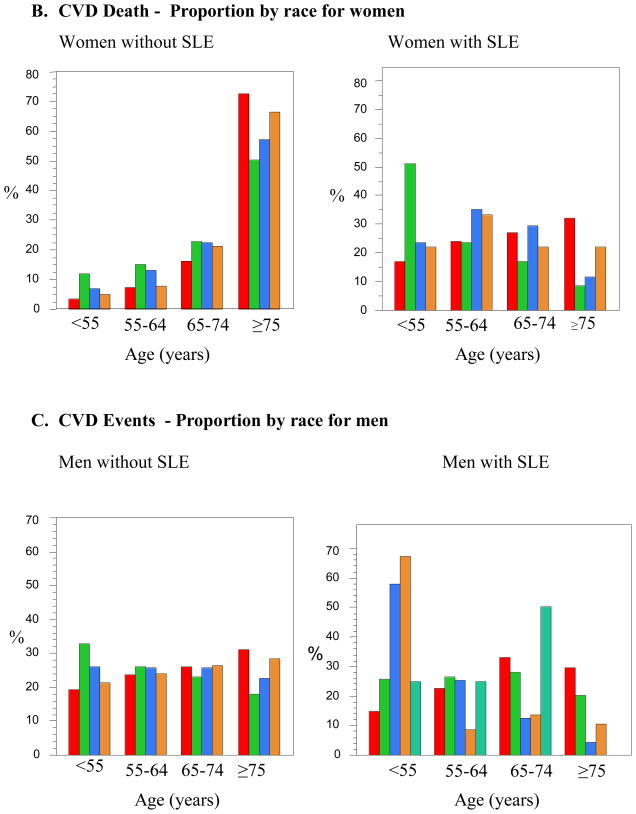
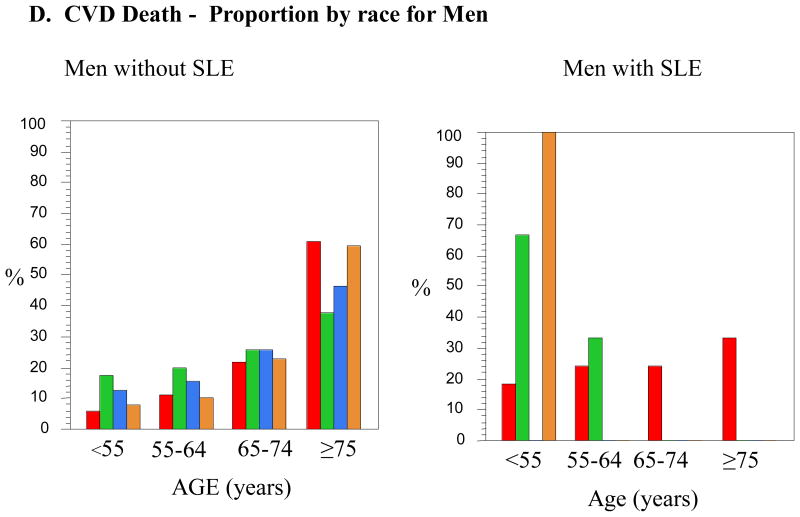
Figure 1.
A. CVD Events - Proportion by race for women
B. CVD Death - Proportion by race for women
C. CVD Events - Proportion by race for men
D. CVD Death - Proportion by race for Men
Men with CVD
There were 805 admissions for SLE men with CVD. The mean age for men with SLE admitted with CVD was 60.4 ± 13.9 years, versus 65.9 years for men without SLE. The range for age differences between racially-matched men without SLE and men with SLE was 3.7 to12.1 years. The greatest difference, 12.1 years, was for the Hispanic men with SLE who had a mean age of 51.4 years versus 63.5 years of age for Hispanic men without SLE. The Hispanic men also had the greatest age difference when compared with the White men with SLE, 11.6 years younger (p<0.0001). The next youngest group was the Black SLE men who were 9.1 years younger than their racially matched non-SLE group (p<0.0001) and 10.7 years younger than White men with SLE (p<0.0001). Mean ages at time of CVD between male controls and male SLE as well as between white men with SLE and minority men with SLE were all significantly different, except for the Asian group in each comparison (See Table 2). Table 4 demonstrates that Hispanic and Black men were admitted with CVD less than the age of 55 years 67 and 58 percent of cases, respectively. In men without SLE, the general trend for presentation of CVD slowly decreased over time and age categories. This effect was accentuated in men with SLE, especially Hispanics and Blacks.
Age at CVD-Associated Mortality
We performed similar analyses of age at the time of death associated with a CVD event. Table 3 demonstrates the mean ages of women and men with and without SLE at the time of their CVD-associated mortality.
Table 3.
Mean age at time of CVD Mortality (in years)
| SLE | Control | Age difference (control-SLE) | p-value | Age difference (white SLE- minority SLE) | p-value | |
|---|---|---|---|---|---|---|
| WOMEN | N=218 | N=35,003 | 14.8 | <0.0001 | ||
| 63.4 | 78.2 | |||||
| White | 67.1 | 79.3 | 12.2 | <0.0001 | ||
| N (%) | 141 (65%) | 27,142 (78%) | ||||
| Black | 52.8 | 72.6 | 19.8 | <0.0001 | 14.3 | <0.0001 |
| N (%) | 47 (21%) | 3,674 (10%) | ||||
| Hispanic | 62.0 | 74.9 | 12.9 | <0.0001 | 5.1 | 0.09 |
| N (%) | 17 (8% ) | 2,472 (7%) | ||||
| Asian | 63.8 | 77.5 | 13.7 | 0.0004 | 3.3 | 0.41 |
| N (%) | 9 (4%) | 825 (2%) | ||||
| Other | 63.5 | 75.4 | 11.9 | 0.07 | 3.6 | 0.54 |
| N (%) | 4 (2%) | 890 (3%) | ||||
| MEN | N=38 | N=35,501 | 11.0 | <0.0001 | ||
| 63.7 | 74.7 | |||||
| White | 66.2 | 75.7 | 9.5 | <0.0001 | ||
| N (%) | 33 (86%) | 28,178 (79%) | ||||
| Black | 52.4 | 68.4 | 16.0 | .05 | 13.9 | 0.06 |
| N (%) | 3 (8%) | 2,785 (8%) | ||||
| Hispanic | 71.0 | N/A | N/A | N/A | N/A | |
| N (%) | 0 | 2,588 (7%) | ||||
| Asian | 45.0 | 75.4 | 30.4 | 0.02 | 21.2 | 0.09 |
| N (%) | 1 (3%) | 965 (3%) | ||||
| Other | 36.0 | 71.7 | 35.7 | 0.005 | 30.2 | 0.02 |
| N (%) | 1(3%) | 985 (3%) |
Women with CVD-Associated Death
There were 218 admissions for women with SLE who suffered CVD-associated in-hospital mortality. The mean age of SLE women suffering a CVD-related death was 63.4 ± 15.5 years. The range of age differences between women without SLE compared to those with SLE was 11.9 to 19.8 years. Black women with SLE were the youngest group hospitalized with CVD-associated death. The mean age at the time of death was 52.8 years of age, 19.8 years younger (p<0.0001) than Black women without SLE. Black SLE women who died were 14.3 years younger than White women with SLE who died during their CVD-related hospitalization (p<0.0001). The other racial groups were again all much younger than their non-SLE matched group, in order of highest to lowest age differences, Asian (13.7 years (p=0.0004)), Hispanic (12.9 years (p<0.0001)), Whites, (12.2 years (p<0.0001)), and Other (11.9 years younger (p=0.07)). The Black women with SLE were the youngest of the minorities with SLE to have CVD-associated death, as compared to the white women with SLE. The mean ages for the other minorities, as compared to White women with SLE were not significant in unadjusted regression models. Table 4 demonstrates that over half of the Black women with SLE who died with CVD were less than age 55. Black women were the only group of SLE women who had a consistent decline in mortality sequentially through the age categories. As compared to women without SLE, women with SLE in the other three racial groups had attenuated rises in the percentages of patients dying with CVD with advancing age.
Men with CVD-Associated Death
There were 38 men with SLE who experienced CVD-associated death (see Table 3). The mean age for men with SLE having a CVD-related mortality in-hospital was 63.7 ± 14.8 years, almost exactly the same age as the women with SLE and CVD death. 33 of the 38 men were White, 3 were Black, one was Asian, and one was Other. No Hispanic men with SLE were identified. There was a 16.0 year age difference between Black men with and without SLE (p=0.05), but interpretation is limited by the few men included in the analysis. There was a 9.5 year age difference between White men with and without SLE who died during their CVD-related hospitalization (p<0.0001).
CVD-related mortality regression
An adjusted logistic regression model controlling for age, gender, race, gender-race interactions, CHF, diabetes, neurologic disease, obesity, and renal failure revealed that age, CHF, obesity, and renal failure were significant variables in determining CVD-related mortality in SLE patients. Increasing age was overall significantly related to CVD mortality. The presence of concomitant CHF and renal failure increased the odds of CVD mortality in SLE patients by 2.2 and 3 times respectively. Obesity was not a risk for dying with a CVD event; rather those patients who were not obese were at a slightly higher risk of dying with CVD while admitted to the hospital.
DISCUSSION
We have demonstrated, through a large national database of inpatient hospitalizations, that there are significant racial disparities with regard to age at the time of hospital admission for CVD and for CVD-related hospitalization resulting in death in patients with SLE. The mean ages at time of CVD admission for men and women in our study are similar to those that have been previously published for patients in the general population (8). Women with SLE were, on average, 10.5 years younger than women without SLE at the time of CVD admission and 14.8 years younger at the time of their CVD mortality. Manzi et al has previously shown that age-specific incidence rates for MI and angina are much higher in women with SLE as compared to participants in the Framingham Offspring Study. The mean age at the time of the first CVD event in their 33 patients with SLE was 48 years of age (age range 22–72 years) (9). A more recent study identified 53 British SLE patients with a mean age of 53 ± 10 years (age range 33 to 73 years) at the time of first CVD event (10). The mean age at the time of CVD admissions in our study, 60.8 years for women with SLE, is older than reported in these two studies. This difference may be due to the larger number of patients and/or the wider age range (18–98 years of age) of the identified SLE patients in our study. Alternatively, we were unable to discriminate whether the CVD admission was a first event, such that if an individual had had one or more previous non-life threatening CVD events, any subsequent events occurring at an older age were included in our analysis. Very little information about minority men with SLE is available for outcomes such as CVD, given that most cohorts are unable to report a meaningful number of men with this outcome. This is the first study we know of that has been able to compare the age at the time of CVD and CVD death in men with and without SLE. On average, men with SLE were 5.5 years younger than men without SLE at the time of CVD admission and 11.0 years younger at the time of CVD death.
Hospitalizations due to acute MI have been shown to be 2.3 times more common in SLE women aged 18–44 than in women without SLE in the same age group. Younger women with SLE had a particularly high risk of dying with a CVD related hospitalization (11). Race differences were not reported in these studies. Our data show that in specific racial groups the mean ages for CVD and CVD-related mortality in SLE versus non-SLE women were all statistically significant lower when compared to racially matched controls. In the National Registry of Myocardial Infarction-2, characteristics of over 500,000 Black versus White patients in the general population experiencing acute MI and MI-related death were compared. The proportion of Black females was higher than the proportion of white females, especially in younger age groups. There was also a higher proportion of Blacks younger than the age of 65 years at the time of MI as compared to Whites. In all age groups less than age 65 years, Blacks had higher mortality rates than Whites, whereas there was trend toward lower mortality rates compared to Whites in patients 80 years of age or older (7). Our study illustrates similar results with a larger proportion of Black females in the general population presenting with CVD and CVD-associated mortality in younger age groups as compared to Whites, with a similar trend also seen in Hispanic females. As age increased, the proportion of females without SLE, in each of the individual racial categories, rose as age increased. The opposite trend was seen in female lupus patients who were Black or Hispanic, and to a lesser extent in those who were Asian. The proportion of White women with SLE admitted with CVD events remained fairly constant across age groups.
Overall, in all racial and gender groups, SLE either reversed or attenuated the proportions of patients admitted with CVD and CVD-related death across the age categories. The proportion of women admitted for CVD-related death increased across the age categories in non-SLE patients, whereas the highest proportion of Black women with SLE was in the <55 year old age group (55%), which progressively decreased as age increased. Some of the most startling results from this study include the vast age difference between SLE patients and their age-matched controls at the time of CVD death. Black women with SLE were almost 20 years younger than Black women without SLE (52.8 versus 72.6 years of age). Reasons for such significant age differences are not clear but may be related to specific race and SLE-related causes. Overall, American Black race is independently associated with a worsened probability of survival. In the general population, after adjusting for CVD risk factors, associated comorbidities, insurance, medications, and MI characteristics, Black race is associated with a higher mortality rate as age decreases. This is true for men and women, although more pronounced in men (7). American Black females have been shown to have an early age of onset of SLE, aggressive disease at onset, and have a high frequency of renal involvement (12, 13). Our adjusted model demonstrated that concomitant comorbidities are associated with CVD-associated mortality. Patients with SLE were 2.2 times more likely to experience CVD-associated death with concomitant CHF and 3 times more likely with renal failure. It may be that the higher risk for CVD and CVD-related death in certain racial groups at younger ages is a result of higher SLE disease activity including renal and central nervous system complications. Data from the Third National Health and Nutrition Examination Survey (NHANES) demonstrated that there is increased CVD risk factor clustering among Americans with lower socioeconomic status, particularly among non-Hispanic Blacks (14). Shah et al. has shown that SLE patients hospitalized with a myocardial infarction have a higher mortality with associated CHF (15). In addition to SLE-related comorbidities, clustering of CVD risks may be contributing to earlier CVD events and death.
Given that many of the traditional CVD risk factors are modifiable, active risk factor management should be targeted to women with SLE. In particular, identification of SLE patients at particular risk, including young Black and Hispanic patients, should be a high priority. Risk factor management and recognition in SLE remains a pertinent issue. Urowitz et al. demonstrated that even though hypertension and hyperlipidemia had improved in a closely monitored cohort, there was still room for tighter control. Over a 12-year period of observation, only 22% of patients with hypercholesterolemia were adequately controlled (16). The definition of hypercholesterolemia varies greatly between studies, but its prevalence occurs anywhere between 4 and 75% in SLE cohorts (9, 16, 17). Studies are warranted in ethnically diverse lupus populations to examine the success in addressing risk factor management and what barriers may exist in optimizing risk factor management and whether there are specific racial disparities to prescription habits, adherence to prescribed medications, optimization of risk factor management, awareness of heart attack warning signs, and appropriate management of acute CVD events. In the general population all of these issues have been shown to be different between the races, with poorer management in ethnic minorities (18–23).
The strengths of this study include that it is based in the NIS, a large database that is well suited for diseases with a low incidence such as SLE. SLE is a disease that is predominantly female, 9:1 ratio of women to men, and the large dataset that includes men with lupus has provided us with unique insight about the incidence of CVD in men with lupus. Due to the rigorous nature of the sampling techniques, the NIS is free of sampling and referral bias. The NIS does not contain information about SLE severity, SLE disease duration, medications, or whether a CVD event was a first event or not. This information would be helpful to determine associations of SLE-related indices with CVD events, biases that could exist in regard to SLE and/or race, and whether the age noted at the time of admission may be different between first CVD events versus all CVD events. There may be an under-diagnosis of SLE, such that not all patients with SLE were captured in the database. We also cannot confirm that all patients diagnosed with SLE met clinical criteria for the disease, but all diagnoses were made by physicians. The colinearity of race and the measure of income by zip code prevented us from reliably including it in the regression analysis for CVD mortality.
We have shown significant differences in the time at which ethnic minorities, including African-Americans and Hispanics, are admitted with CVD and CVD-associated death. Large multicenter and multi-ethnic studies are needed to examine if there are SLE specific indices that may be contributing to these age disparities. Physician awareness and patient education should be considered in all SLE patients in the third and fourth decades of life, especially for Blacks and Hispanics. In this way, primary and secondary preventative measures can be undertaken at the most favorable time.
Table 5.
Multivariate logistic regression analysis of characteristics associated with CVD-related mortality in SLE patients
| Covariate | OR* (95% CI) | p-value |
|---|---|---|
| Age | 1.044(1.034, 1.055) | < 0.0001 |
| CHF | 2.220 (1.606, 3.070) | < 0.0001 |
| Renal Failure | 3.024 (2.240, 4.083) | < 0.0001 |
| Obesity | 0.382 (0.157, 0.932) | 0.03 |
OR=odds ratio; 95% CI = 95% confidence interval
References
- 1.Anderson E, Nietert PJ, Kamen DL, Gilkeson GS. Ethnic disparities among patients with systemic lupus erythematosus in South Carolina. J Rheumatol. 2008;35(5):819–25. [PMC free article] [PubMed] [Google Scholar]
- 2.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2331–7. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 4.HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: [Google Scholar]
- 5.Hart AC, MSS . ICD-9-CM Expert for Physicians. Salt Lake City, UT: Ingenix; 2006. [Google Scholar]
- 6.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Manhapra A, Canto JG, Vaccarino V, Parsons L, Kiefe CI, Barron HV, et al. Relation of age and race with hospital death after acute myocardial infarction. Am Heart J. 2004;148(1):92–8. doi: 10.1016/j.ahj.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Dittrich H, Gilpin E, Nicod P, Cali G, Henning H, Ross J., Jr Acute myocardial infarction in women: influence of gender on mortality and prognostic variables. Am J Cardiol. 1988;62(1):1–7. doi: 10.1016/0002-9149(88)91356-2. [DOI] [PubMed] [Google Scholar]
- 9.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145(5):408–15. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 10.Haque S, Gordon C, Isenberg D, Rahman A, Lanyon P, Bell A, et al. Risk Factors for Clinical Coronary Heart Disease in Systemic Lupus Erythematosus: The Lupus and Atherosclerosis Evaluation of Risk (LASER) Study. Journal of Rheumatology. 2010;37(1) doi: 10.3899/jrheum.090306. [DOI] [PubMed] [Google Scholar]
- 11.Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42(2):338–46. doi: 10.1002/1529-0131(199902)42:2<338::AID-ANR17>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 12.Reveille JD, Bartolucci A, Alarcon GS. Prognosis in systemic lupus erythematosus. Negative impact of increasing age at onset, black race, and thrombocytopenia, as well as causes of death. Arthritis Rheum. 1990;33(1):37–48. doi: 10.1002/art.1780330105. [DOI] [PubMed] [Google Scholar]
- 13.Hochberg MC, Boyd RE, Ahearn JM, Arnett FC, Bias WB, Provost TT, et al. Systemic lupus erythematosus: a review of clinico-laboratory features and immunogenetic markers in 150 patients with emphasis on demographic subsets. Medicine (Baltimore) 1985;64(5):285–95. [PubMed] [Google Scholar]
- 14.Sharma S, Malarcher AM, Giles WH, Myers G. Racial, ethnic and socioeconomic disparities in the clustering of cardiovascular disease risk factors. Ethn Dis. 2004;14(1):43–8. [PubMed] [Google Scholar]
- 15.Shah MA, Shah AM, Krishnan E. Poor outcomes after acute myocardial infarction in systemic lupus erythematosus. J Rheumatol. 2009;36(3):570–5. doi: 10.3899/jrheum.080373. [DOI] [PubMed] [Google Scholar]
- 16.Urowitz MB, Gladman DD, Ibanez D, Berliner Y. Modification of hypertension and hypercholesterolaemia in patients with systemic lupus erythematosus: a quality improvement study. Ann Rheum Dis. 2006;65(1):115–7. doi: 10.1136/ard.2005.038802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruce IN, Gladman DD, Urowitz MB. Detection and modification of risk factors for coronary artery disease in patients with systemic lupus erythematosus: a quality improvement study. Clin Exp Rheumatol. 1998;16(4):435–40. [PubMed] [Google Scholar]
- 18.Sonel AF, Good CB, Mulgund J, Roe MT, Gibler WB, Smith SC, Jr, et al. Racial variations in treatment and outcomes of black and white patients with high-risk non-ST-elevation acute coronary syndromes: insights from CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines?) Circulation. 2005;111(10):1225–32. doi: 10.1161/01.CIR.0000157732.03358.64. [DOI] [PubMed] [Google Scholar]
- 19.Condon JV, Miller KM, Le AH, Quasem M, Looney SW. Acute myocardial infarction and race, sex, and insurance types: unequal processes of care. Health Care Manag (Frederick) 2008;27(3):212–22. doi: 10.1097/01.HCM.0000285057.32235.5e. [DOI] [PubMed] [Google Scholar]
- 20.Zerwic JJ, Ryan CJ, DeVon HA, Drell MJ. Treatment seeking for acute myocardial infarction symptoms: differences in delay across sex and race. Nurs Res. 2003;52(3):159–67. doi: 10.1097/00006199-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Disparities in adult awareness of heart attack warning signs and symptoms--14 states, 2005. MMWR Morb Mortal Wkly Rep. 2008;57(7):175–9. [PubMed] [Google Scholar]
- 22.Jha AK, Staiger DO, Lucas FL, Chandra A. Do race-specific models explain disparities in treatments after acute myocardial infarction? Am Heart J. 2007;153(5):785–91. doi: 10.1016/j.ahj.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yood MU, McCarthy BD, Kempf J, Kucera GP, Wells K, Oliveria S, et al. Racial differences in reaching target low-density lipoprotein goal among individuals treated with prescription statin therapy. Am Heart J. 2006;152(4):777–84. doi: 10.1016/j.ahj.2006.02.036. [DOI] [PubMed] [Google Scholar]