Abstract
Risky decision-making is a complex process that involves weighing the probabilities of alternative options that can be desirable, undesirable, or neutral. Individuals vary greatly in how they make decisions either under ambiguity and/or under risk. Such individual differences may have genetic bases. Based on previous studies on the genetic basis of decision making, two decision making tasks [i.e., Iowa Gambling Task (IGT) and Loss Aversion Task (LAT)] were used to test the effect of 5-HTTLPR polymorphism on decision making under ambiguity and under risk in a large Han Chinese sample (572 college students, 312 females). Basic intelligence and memory tests were also included to control for the influence of basic cognitive abilities on decision making. We found that 5-HTTLPR polymorphism significantly influenced performance in both IGT and LAT. After controlling for intellectual and memory abilities, subjects homozygous for s allele had lower IGT scores than l carriers in the first 40 trials of the IGT task. They also exhibited higher loss aversion than l carriers in the LAT task. Moreover, the effects of 5-HTTLPR were stronger for males than for females. These results extend the literature on the important role of emotion in decision under ambiguity and risk, and provide additional lights on how decision-making is influenced by culture as well as sex differences. Combining our results with existing literature, we propose that these effects might be mediated by a neural circuitry that comprises the amygdala, ventromedial prefrontal cortex, and insular cortex. Understanding the genetic factors affecting decision in healthy subjects may allow us better identify at-risk individuals, and target better the development of new potential treatments for specific disorders such as schizophrenia, addiction, and depression.
Keywords: 5-HTTLPR, Iowa Gambling Task, Loss Aversion, Decision Making, Emotion, Amygdala, Ventromedial Prefrontal Cortex, Insula
Introduction
Making economic decisions under uncertainty, such as whether or not to invest in stocks or buy insurance, is common and essential in everyday life. It is a complex process that involves weighing alternative outcomes’ desirability and their probabilities (Fox and Poldrack, 2009; Xue et al., 2010; Xue et al., 2009). There are two main types of economic decisions, decisions under ambiguity and decisions under risk, distinguished by the degree of uncertainty about the outcome probability. For decisions under ambiguity, the decision maker lacks the knowledge of the precise probability distribution of the possible outcomes. On the other hand, decisions under risk are those with known outcome probabilities (Fox and Poldrack, 2009; Stoltenberg and Vandever, 2010).
Individuals vary greatly in how they make decisions under uncertainty. Such individual differences may have genetic bases. Twin studies have estimated that genetic effect accounts for about 20% of the variance in risk taking behavior (Cesarini et al., 2009). It is important to explore the genetic factors affecting decision making in both healthy subjects (van den Bos et al., 2009) and patients with certain psychiatric disorders (such as addiction, schizophrenia, or depression) who are known to have deficit in decision making (e.g., Jollant et al., 2007; Lenze et al., 2005; Roiser et al., 2006; Tremeau et al., 2008; Tremeau et al., 2007). In this study, we investigated the influence of serotonin transporter gene-linked polymorphic region (5-HTTLPR) on decision making under uncertainty, using a large healthy sample.
Serotonin modulates the synaptic activities of neurons in brain regions that have been shown to be functionally important when making economic decisions (e.g., the prefrontal cortex, see Bechara and Damasio, 2005). The serotonin neuronal cell bodies, which reside in the brainstem, project their axons into many brain regions related to decision making, including the prefrontal cortex (orbital, ventromedial and dorsolateral), amygdala, striatum and insular cortex (Baumgarten and Grozdanovic, 1999; Wai et al., 2008). The most often studied genetic polymorphism in the serotonin system is the serotonin transporter gene-linked polymorphic region (5-HTTLPR). The 5-HTTLPR consists of a 44-bp insertion/deletion, resulting in either a long (l) or short (s) allele. The s allele is reported to be associated with reduced serotonin transporter expression as compared to the l allele (Heils et al., 1996; Lesch et al., 1996). Polymorphism of the 5-HTTLPR is associated with activation in the amygdala (e.g., Hariri et al., 2005; Hariri and Holmes, 2006; Hariri et al., 2002a; Hariri et al., 2002b; Labus et al., 2008; see Munafo et al., 2008 for a review), ventromedial prefrontal cortex (e.g., Heinz et al., 2005; Labus et al., 2008), and insular cortex (e.g., Labus et al., 2008).
Recent studies using behavioral and neuroimaging techniques have examined the effect of 5-HTTLPR on economic decision making in both healthy subjects (Crisan et al., 2009; Homberg et al., 2008; Kuhnen and Chiao, 2009; Roiser et al., 2009; Stoltenberg and Vandever, 2010; van den Bos et al., 2009; Zhong et al., 2009) and patients (Jollant et al., 2007; Must et al., 2007). The results are mixed (see Table 1 for a summary of these studies). Five studies (including 2 patient studies) focused on the Iowa Gambling Task (IGT), which is a widely used task with mixed gambles (Bechara et al., 1994; Bechara et al., 2000). Three of them showed that s allele homozygotes performed worse than those with l allele in the overall IGT score (Homberg et al., 2008; Must et al., 2007; van den Bos et al., 2009). Such difference also presented when dividing the IGT into the first 40 (decisions under ambiguity) and the last 60 trials (decisions under risk) (Homberg et al., 2008; van den Bos et al., 2009). Although the other two studies failed to show a significant main effect of genotype, one did suggested that l carriers (but not s/s) showed significant performance improvement from the early to the late stage of the IGT (Jollant et al., 2007), and the other one suggested a significant gender by genotype interaction in first 20 trials (Stoltenberg and Vandever, 2010). Using a different task (Balloon Analogue Risk Task, BART) aimed to test decision making under ambiguity, it had been showed that the s allele was associated with fewer pumps (i.e., taking less risk under ambiguity) in BART (Crisan et al., 2009). In addition, researchers have also examined decision making under risk, using the investment task (Kuhnen and Chiao, 2009), framing design (Crisan et al., 2009; Roiser et al., 2009), and multiple price list design (Zhong et al., 2009). These studies revealed that the s allele was associated with more risk aversion, such as less investment in risky asset (Kuhnen and Chiao, 2009) and a larger framing effect (Crisan et al., 2009; Roiser et al., 2009). However, Zhong et al. (2009) only showed a trend (non-significant) that s allele was associated with more risk aversion.
Table 1.
Summary of previous studies investigating the 5-HTTLPR effect on economic decision making
| Study | N | Race | Genotypes | Task | Results | ||
|---|---|---|---|---|---|---|---|
| s/s | s/l | l/l | |||||
| Crisan et al., 2009 | 32 (23F) | NA* | 9 | 9 | 14 | BART and Framing Design | BART numbers of pumps: s/s < l carriers Gamble in loss frame: s carriers > l/l |
| Homberg et al., 2008 | 88 (88F) | NA | 22 | 49 | 17 | IGT | IGT score: s/s < l carriers (larger in trials 41–100) |
| Jollant et al., 2007** | 162 (66.1% F) | Caucasian | 32 | 69 | 61 | IGT | No genotype main effect, but significant performance improvement in l carriers but not s/s |
| Kuhnen et al., 2009 | 65 (26F) | NA* | 21 | 10 | 34 | Investment Task | Amount invest in risky asset: s/s < l carriers |
| Must et al., 2007*** | 124 (83F) | NA | 35 | 58 | 31 | IGT | IGT score: s/s < l/l with l/s in the middle |
| Roiser et al., 2009 | 30 (13F) | Caucasian | 15 | 0 | 15 | Framing Design | Framing effect: s/s > l/l; frontal-amygdala coupling: s/s < l/l |
| Stoltenberg et al., 2010 | 188 (117F) | Caucasian | 39 | 86 | 62 | IGT | No genotype effect, but a significant gender by genotype interaction in first 20 trials. IGT score for males: s carriers > l/l; IGT score for females: s carriers < l/l |
| van den Bos et al., 2009 | 70 (70F) | NA | 18 | 39 | 13 | IGT | IGT score: s/s < l carriers (larger in trials 41–100) |
| Zhong et al., 2009 | 325 (53.7% F) | Chinese | 168 | 118 | 39 | Multiple Price List Design | No genotype effect, but a trend of higher risk aversion in subjects with s allele |
Subjects were recruited from university campus.
Subjects were all suicide attempters.
Subjects were all diagnosed as unipolar major depressive disorder according to DSM-IV.
F: females. NA: not available. IGT: Iowa Gambling Task. BART: Balloon Analogue Risk Task.
A number of factors may have contributed to the inconsistency in the literature on the influence of 5-HTTLPR on decision making. First and most important, previous studies used different tasks. Different tasks not only involve different cognitive and emotional processing, but also target different brain areas that are involving different neurotransmitter systems with different genetic architectures (Stoltenberg and Vandever, 2010). It is thus important to choose tasks whose cognitive and neural mechanisms have been well understood. Second, the sample size of most previous studies are relatively small (the largest sample is the study by Zhong et al., N = 325). Small sample size has low statistical power and is difficult to yield stable results (Munafo et al., 2008). Third, some of these studies only included female subjects (Homberg et al., 2008; van den Bos et al., 2009), which prevented them from examining the gender differences in decision making (e.g., Bolla et al., 2004; Overman et al., 2006; Reavis and Overman, 2001), as well as the interaction between gender and 5-HTTLPR polymorphism (Stoltenberg and Vandever, 2010). Finally, most previous studies did not control other individual differences such as intelligence and memory that play important roles in decision making (e.g., Gupta et al., 2009; Morsanyi and Handley, 2008; Yechiam and Busemeyer, 2005; Yechiam et al., 2008).
The present study tested the effect of 5-HTTLPR polymorphism on decision making under ambiguity and under risk, using two widely used tasks: the Iowa Gambling Task (Bechara et al., 1994) and the Loss Aversion Task (Tom et al., 2007). The initial stage of the IGT (typically the first 40 trials) is usually recognized as decision making under ambiguity because subjects do not yet know the outcome probabilities. And as the task progresses, subjects may acquire some subjective sense of what the outcome probabilities associated with different decisions might be, hence rendering at least the more subsequent trials of the IGT as a test of decision making under risk (usually the last 60 trials, see Bechara et al., 1997). The Loss Aversion Task (LAT) tests a specific type of decision making under risk (loss aversion in the prospect theory). A large Han Chinese sample (572 college students, 312 females) were included in the present study. Basic intelligence and memory tests were also included to control for the influence of cognitive abilities on decision making.
Materials and Methods
Subjects
Five hundred and seventy-two (312 females) undergraduate students (Han Chinese, aged from 17 to 27 years old, with a mean of 20.47 years, SD = 1.01) were recruited from Beijing Normal University (Beijing, China) to participate in a large-scale gene-brain-behavior project. All subjects had normal or corrected-to-normal vision. According to a modified version of Edinburgh Handedness Inventory (Xue et al., 2004), only a few subjects are left-handers (N = 35, 6.12%)1. Two questionnaires were used to measure drinking (the Alcohol Use Disorders Identification Test, Saunders et al., 1993) and smoking (the Fagerström Test for Nicotine Dependence, Heatherton et al., 1991) behavior separately. Only 4 subjects2 were identified to have high level of alcohol problems (scored 16 or higher on the AUDIT, Saunders et al., 1993); and no subject was found to show high (6–7 on the FTND) or very high (8 or higher on the FTND) dependence on nicotine (Heatherton et al., 1991). Based on self-report, they had not had neurological or psychiatric problems. Informed written consent was obtained from all subjects. This study was approved by the Beijing Normal University Institutional Review Board.
Using polymerase chain reaction (PCR), 569 subjects (310 females, 54.48%) were genotyped (3 subjects failed to be typed because of a technical error). Between 517 and 567 subjects completed and had valid data on tests described below: 549 (304 females) for IGT; 517 (288 females) for LAT; 564 (311 females) for RAPM; 560 (308 females) for WAIS-RC; 567 (311 females) for WMS-Recognition; 558 (307 females) for WMS-Recall; 535 (295 females) for WMT. Three factors contributed to the missing data: first, some subjects did not show up in some testing sessions; second, the program or computer crashed in some testing sessions, causing incomplete data recording; third, some subjects’ performance was classified as outliers because their data exceeded ±3 SD of the group means.
Decision making Tasks
The Iowa Gambling Task (IGT)
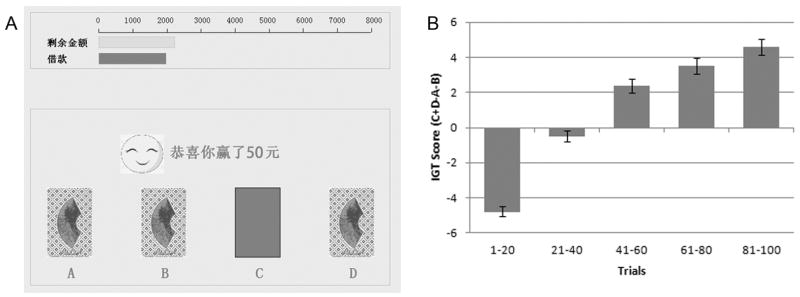
A computerized version of the IGT (Bechara et al., 2000) was used in the present study (Figure 1A). It was designed to assess decision making under ambiguity and risk (Bechara et al., 1994; Bechara et al., 1997, 2005; Bechara et al., 2000). To motivate subjects, they were informed that the amount of their winning would be converted into real money. Subjects were asked to select one card at a time (100 trials in total) from one of the four decks (labeled A, B, C, and D). As described in previous studies and the IGT manual (PAR, Inc.), two of the decks were disadvantageous because they yielded high immediate gain but even a greater loss in the long run (i.e., net loss of 250 yuan on average over 10 cards), and two decks were advantageous because they yielded lower immediate gain but even a smaller loss in the long run (i.e., net gain of 250 yuan on average over 10 cards).
Figure 1.
A) Illustration of the Iowa Gambling Task. Subjects were instructed to choose one card at a time from 4 decks of cards (labeled A, B, C and D). Green bar (the lighter one) on the top represents money in his/her wallet which changed in length as he/she “wins” or “loses”. Red bar (the darker one) represents his/her loan (2000 yuan). B) IGT score (number of choices from “good” decks minus choices from “bad” decks) showed a linear improvement over 5 blocks (20 cards each). Error bars indicate standard errors. Please note that to protect the validity of the IGT test, the payoffs on the cards are just for illustration purpose, and were not the actual ones subjects received in the task.
The Loss Aversion Task (LAT)
In the computerized loss aversion task (Tom et al., 2007), subjects were presented with 256 trials, each of which proposed a gamble of 50/50 chance to win or to lose real money (Figure 2A). Possible gains ranged from 10–40 yuan (in increments of 2 yuan) and possible losses ranged from 5–20 yuan (in increments of 1 yuan). The 256 trials were the total number of all possible combinations of wins and losses (16 × 16). Subjects were asked to evaluate whether or not they would like to play each of the gamble by pressing one of the four buttons (strongly accept, weakly accept, weakly reject, and strongly reject). Subjects had 3 sec to respond to each trial. They were informed before the game that, in three randomly selected trials, if they had accepted the gamble, the outcome would be decided with a coin toss; if they had rejected, the gamble would not be played. Responses of each subject were re-coded as to gamble or not. The responses were counterbalanced across subjects (i.e., half of the subjects responded to “accept” using their left hand and the other half using their right hand).
Figure 2.
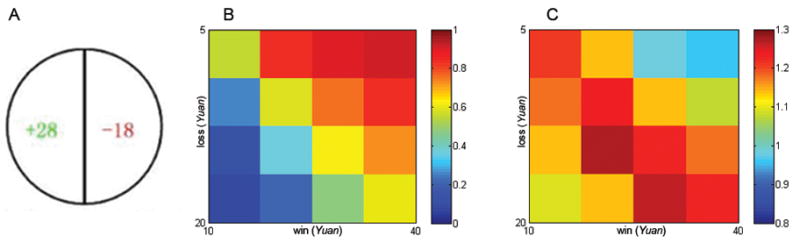
A) A sample trial of the Loss Aversion Task. On each trial, subjects were presented a display showing the amount of potential gain (in green) and loss (in red). They had to decide to what extent they would like to play this gamble. B) Color-coded heatmap of probability of gamble acceptance at each level of gain/loss. C) Color-coded heatmap of probability of response times (sec) at each level of gain/loss.
Intelligence and Memory Tests
Two intelligence tests [Raven’s Advanced Progressive Matrices (RAPM) and Wechsler Adult Intelligence Scale-Revised Chinese Version (WAIS-RC)] and three memory tests [Wechsler Memory Scale-Recognition (WMS-Recognition), Wechsler Memory Scale-Recall (WMS-Recall) and Working Memory Test (WMT)] were used to measure subjects’ basic cognitive and memory abilities (see Zhu et al., Inpress for detailed description of these tests). Several measures were generated to represent the basic intelligence and memory abilities, including the number of correct response to the test items of RAPM; the three IQ scores (verbal IQ, performance IQ, and total IQ) of WAIS-RC; the total scores of WMS-Recognition and WMS-Recall; and the accuracy of each WMT component (morphology, phonology and semantic). These tests were selected because they are not only among the most widely used intelligence and memory tests in China and elsewhere, but they also have been shown to have good reliability and validity for Chinese subjects.
Genotype analysis
DNA fragments were amplified by polymerase chain reaction using forward primer 5′-GGC GTT GCC GCT CTG AAT TGC-3′ and reverse primer 5′-GAG GGA CTG AGC TGG ACA ACC AC-3′. The PCR reaction mixture contained a total volume of 25 μl with composition as described in a previous protocol (Collier et al., 1996). After an initial 4 min denaturation step at 95 °C, 35 cycles were performed consisting of 45 sec at 96 °C, 90 sec at 61 °C, and 90 sec at 72 °C. The reaction was ended by a step of elongation at 72 °C for 10 min. PCR products were separated by the long (528 bp) and short (484 bp) variants on 2% agarose gels stained with ethidium bromide for 3 hr and then analyzed with a Gel Doc 2000 imaging system (Bio-Rad Laboratories Ltd, UK).
Procedure
A 4 ml venous blood sample was collected from each subject. Genomic DNA was extracted using the standard method within 2 weeks. 5-HTTLPR polymorphisms were typed using PCR as described above. Subjects were tested over four sessions spread across a period of 11 months. The RAPM test was administered in the first session. The WMS-Recognition and IGT were administered in the second session (5 months after first session). The WAIS-RC and WMS-Recall were administered in the third session (6 months after first session). The WMT and LAT were administered in the last session. Except for the individually administered paper version of the RAPM, WAIS-RC and WMS-Recall, all other tests were administered on computer.
Data Analysis
For IGT, the 100 trials were divided into 5 blocks of 20 trials. A net score was calculated by subtracting the total number of selections of the disadvantageous decks (A and B) from the total number of selections of the advantageous decks (C and D), separately for each block. In addition, the net score for the first 40 and last 60 trials were also calculated to represent performance in decision under ambiguity and decision under risk, respectively. Higher scores indicated superior performance.
For LAT, following Tom et al. (2007), we collapsed 256 trials into a 4×4 win-loss matrix, and calculated the gamble acceptance rate and mean reaction time for each cell. Logistic regression was performed for each subject, using acceptance/rejection as dependent variable (Y), amount of gain (Win) and loss (Loss) as independent variables. The equation was , where βWin and βLoss are the unstandardized regression coefficients for gain and loss, respectively. E represented estimated error. The percent of correct classification was used to represent the goodness of model fit (G2). Behavioral loss aversion parameter λ was computed as . Higher λ value indicates higher loss aversion.
After removing the outliers (exceeded ±3 SD of the mean), the data were inputted into SPSS software (SPSS Inc., Chicago, IL) for statistical analysis, using analysis of variance (ANOVA), analysis of covariance (ANCOVA), t-tests, chi-square tests and correlation analysis. ANOVAs and ANCOVAs were followed by post-hoc comparisons.
Results
Out of 569 subjects, 52 were l allele homozygotes (l/l), 219 were heterozygotes (l/s), and 298 were homozygous for the s allele (s/s). The distribution is consistent with Hardy-Weinberg Equilibrium (χ2 (1) = 1.615, p = .204). There were 24 males with two copies of the l allele, another 99 heterozygotes, and 143 males with two copies of the s allele, consistent with Hardy-Weinberg Equilibrium (χ2 (1) = 2.563, p = .109). And of all the females, 28 were l homozygotes, 127 were heterozygotes, and 155 were s homozygotes, consistent with Hardy-Weinberg Equilibrium (χ2 (1) = .074, p = .786). More specifically, the frequency of genotype (9.14 % of l/l, 38.49 % of l/s and 52.37 % of s/s) in the present study was comparable with other studies with Asian subjects (Huang et al., 2004; Joo et al., 2007; Katsuragi et al., 1999; Tsai et al., 2002; Zhong et al., 2009).
Intelligence and Memory Tasks
Intelligence and memory test scores for each genetic subgroup are summarized in Table 2. Overall, statistics showed no genotype group difference (all p’s > .05), i.e., subjects in different genetic subgroups got comparable scores in all the intelligence and memory tests.
Table 2.
Intelligence and memory scores for each genotype group
| l/l | l/s | s/s | Statistics | |
|---|---|---|---|---|
| RAPM | 26.00 (3.73) | 25.70 (3.84) | 25.46 (4.09) | F (2, 558) = .530, p = .589 |
| WAIS-RC | ||||
| Verbal | 125.37 (8.83) | 123.26 (9.00) | 124.24 (8.66) | F (2, 554) = 1.482, p = .228 |
| Performance | 123.71 (8.53) | 123.53 (9.69) | 122.94 (10.23) | F (2, 554) = .287, p = .751 |
| Total | 126.82 (7.56) | 125.33 (8.16) | 125.70 (8.12) | F (2, 554) = .707, p = .494 |
| WMS | ||||
| Recognition | 14.94 (1.31) | 14.60 (1.51) | 14.74 (1.34) | F (2, 561) = 1.455, p = .234 |
| Recall | 17.47 (1.68) | 17.58 (1.91) | 17.28 (2.07) | F (2, 552) = 1.471, p = .231 |
| WMT | ||||
| Semantic | .856 (.135) | .864 (.108) | .856 (.108) | F (2, 537) = .360, p = .698 |
| Phonology | .830 (.092) | .826 (.098) | .811 (.112) | F (2, 536) = 1.399, p = .248 |
| Morphology | .887 (.104) | .876 (.076) | .881 (.075) | F (2, 537) = .454, p = .626 |
Numbers represent the mean (SD).
RAPM: Raven’s Advanced Progressive Matrices; WAIS-RC: Wechsler Adult Intelligence Scale-Revised Chinese Version; WMS: Wechsler Memory Scale; WMT: Working Memory Test.
The Iowa Gambling Task
As can be seen in Figure 1B, subjects on average chose more advantageous cards as the task went on. One-way repeated-measure ANOVA showed significant trial block effect (F(4, 2192) = 149.567, p < .001). Indeed, multiple comparisons suggested that subjects increasingly chose advantageous decks from block to block (linear: F(1, 548) = 295.023, p < .001; quadratic: F(1, 548) = 48.910, p < .001). The first 40 trials were grouped together to obtain performance scores in decision under ambiguity and the last 60 trials were also grouped together to obtain performance scores in decision under risk. Although we did not ask subjects to report what they knew about the task when they were performing it, a previous study that adopted this method had shown that on average, decisions in the first 40 trials were made under ambiguous conditions, but after that, subjects began to develop some subjective sense of the probabilities and thereby they began to make decisions under risk (Bechara et al., 1997). This distinction has also been widely adopted by several other studies (e.g., Homberg et al., 2008; van den Bos et al., 2009).
The Loss Aversion Task
On average, the rate of gamble acceptance for all subjects was 55.06% (SD = 27%), with an average reaction time of 1.16 sec (SD = .31). Subjects showed indifference to gambles in which potential gains were twice as likely as losses (Figure 2B). Also, they needed more time to decide whether or not to accept those gambles (Figure 2C). The mean λ value was 1.92 (SD = .75), ranging from .34 to 6.65. Average G2 was 85.34% (SD = 7.4%, ranging from 52% to 100%), suggesting good fit of the model to the data. This result was consistent with Tom et al. (2007).
Correlations between tasks
Decision under ambiguity (i.e., performance in the first 40 trials) were highly correlated with decision under risk (i.e., performance in the last 60 trials) tested by the IGT (r = .377, p < .01), but neither of them was correlated with loss aversion λ (decision under ambiguity: r = .011; decision under risk: r = −.011; both ps > .05), suggesting they were testing different decision making processes.
Correlation analysis also showed a few significant correlations between decision making performance and basic intelligence and memory abilities (Table 3). Three correlations were significant: The net score of the first 40 IGT trials and phonological working memory (r = .096, p < .05), the net score of the last 60 IGT trials and the RAPM score (r = .089, p < .05), the net score of the last 60 IGT trials and the WAIS-RC performance IQ (r = .097, p < .05). The loss aversion λ did not correlate with basic intelligence and memory abilities (all ps > .05). These intelligence and memory test scores thus were used as covariables in ANCOVAs to determine the unique effect of 5-HTTLPR polymorphism on economic decision making, and also to increase the statistical power.
Table 3.
Correlations between decision making tasks and basic intelligence and memory tests
| RAPM | WAIS-RC | WMT | WMS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Verbal | Performance | Total | Semantic | Phonology | Morphology | Recognition | Recall | ||
| IGT-first 40 | −0.013 | −0.034 | −0.053 | −0.056 | −0.003 | .096* | −0.032 | −0.074 | −0.008 |
| IGT-last 60 | .089* | 0.017 | .097* | 0.066 | 0.075 | 0.05 | −0.004 | −0.037 | 0.01 |
| LAT λ | 0.013 | −0.017 | 0.061 | 0.02 | 0.046 | −0.059 | 0.038 | 0.000 | −0.008 |
p < .05
IGT: Iowa Gambling Task; LAT: Loss Aversion Task; RAPM: Raven’s Advanced Progressive Matrices; WAIS-RC: Wechsler Adult Intelligence Scale-Revised Chinese Version; WMS: Wechsler Memory Scale; WMT: Working Memory Test.
Gender, 5-HTTLPR and decision making under ambiguity
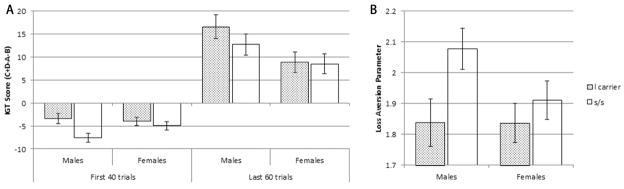
For decision making under ambiguity, two-way ANCOVA using 5-HTTLPR polymorphism and gender as independent variables showed that 5-HTTLPR polymorphism (s/s, s/l and l/l) had a significant effect on the IGT score in the first 40 trials (F(2, 489) = 3.703, p < .05). But there was no effect of gender (F(1, 489) = .026, p > .05) or gender by 5-HTTLPR interaction (F(2, 489) = 1.518, p > .05). Further analysis suggested that subjects with the l/s genotype (M = −3.85, SD = 10.569) had significantly higher scores (i.e., choosing more advantageous cards) than s/s (M = −6.43, SD = 10.459) in the first 40 IGT trials (t(496) = 2.709, p < .01, Cohen’s d = .25), but there was no difference between the l/l (M = −4.58, SD = 10.363) and l/s individuals (t(256) = −.437, p > .05, Cohen’s d = −.07) or l/l and s/s (t(334) = 1.134, p > .05, Cohen’s d = .18). We then grouped subjects with the l/l and l/s genotypes together (l carriers) to attain more statistical power. The pattern of results was similar but stronger: a significant effect of 5-HTTLPR polymorphism (F(1, 491) = 7.517, p < .01), no effect of gender (F(1, 491) = 1.129, p > .05), but with a trend of interaction of gender by 5-HTTLPR polymorphism (F(1, 491) = 2.902, p = .089). Subjects with the l allele (l carriers) had significantly higher IGT scores than s allele homozygotes (Figure 3A). This effect was significant for male subjects (F(1, 210) = 6.960, p < .01) but not significant for female subjects (F(1, 272) = .427, p > .05). To be comparable with Stoltenberg & Vandever (2010), we also examined the gender by genotype interaction using first 20 trials as decision making under ambiguity. The result was similar to that with the first 40 trials but stronger (F(1, 491) = 3.909, p < .05). Males with l allele performed more advantageously than s allele homozygotes.
Figure 3.
A) 5-HTTLPR polymorphism had a significant effect on decision under ambiguity (the first 40 trials) but not on decision under risk (the last 60 trials) tested by IGT. Subjects with l allele (l carriers) had a significantly higher mean IGT score than s allele homozygotes on the first 40 trials of IGT. Moreover, this effect was modulated by gender, with only males showing the effect. But for the last 60 trials of IGT, although there was no effect of 5-HTTLPR polymorphism, the IGT score was modulated by gender. Males chose more advantageous cards than females. B) 5-HTTLPR also influenced the loss aversion parameter λ tested by LAT task. Subjects homozygous for sallele showed higher λ value than l carriers. Error bars indicate standard errors.
Gender, 5-HTTLPR and decision making under risk
For decision making under risk, two-way ANCOVA suggested that gender had a significant effect on the IGT score in the last 60 trials (F(1, 489) = 4.026, p < .05). But there was no effect of 5-HTTLPR polymorphism (F(2, 489) = .414, p > .05) or gender by 5-HTTLPR interaction (F(2, 489) = .313, p > .05). After grouping the l/l and l/s genotypes together (l carriers), the analysis showed the same pattern but stronger results: a significant effect of gender (F(1, 491) = 6.516, p < .01), no effect of 5-HTTLPR polymorphism (F(1, 491) = .817, p > .05) or gender by 5-HTTLPR interaction (F(1, 491) = .578, p > .05). Male subjects (M = 12.84, SD = 27.535) showed a marginally significant higher IGT score than females (M = 8.55, SD = 24.456) (Figure 3A, t(547) = 1.933, p = .054, Cohen’s d = .17).
Gender, 5-HTTLPR and loss aversion
For LAT, two-way ANCOVA showed a marginally significant main effect of 5-HTTLPR on λ (F (2, 483) = 2.876, p = .057), but no effect of gender (F (1, 483) = .381, p > .05) or gender by 5-HTTLPR interaction (F (2, 483) = .722, p > .05). Multiple comparisons showed that subjects with the s/s (M = 1.99, SD = .836) genotype showed significantly higher loss aversion (i.e., higher λ value) than those with l/s (M = 1.84, SD = .639) genotype (t(465) = 2.149, p < .05, Cohen’s d = .20), and marginally significant higher loss aversion than those with l/l (M = 1.81, SD = .577) genotype (t(321) = 1.922, p = .058, Cohen’s d = .23), but there was no significant difference between the l/s and l/l groups (t(242) = .312, p > .05, Cohen’s d = .05). The results were similar but stronger after we grouped subjects with the l/l and l/s genotypes together. Two-way ANCOVA results showed a significant 5-HTTLPR polymorphism effect on λ (F(1, 485) = 5.384, p < .05) with the s allele homozygotes showing higher λ value than the l carriers (Figure 3B), but no effect of gender (F(1, 485) = 1.534, p > .05) or gender by 5-HTTLPR interaction (F(1, 485) = 1.488, p > .05).
Discussion
In the present study, two decision making tasks (i.e., the IGT and LAT) were used to test the effect of 5-HTTLPR polymorphism on decision making under ambiguity and risk. Basic intelligence and memory tests were also used to control for the potential confounding effects of basic cognitive abilities. The large Han Chinese sample used in this study enabled us to obtain stable and comprehensive results. Results indicated that 5-HTTLPR influence both decision under ambiguity and loss aversion, and this effect is modulated by Gender.
Consistent with previous studies (Bechara et al., 1994; Bechara et al., 2000), the healthy young college students in our study learned to choose cards from advantageous decks across five blocks of 20 trials. Although the overall performance showed no difference, males performed more advantageously than females in decision under risk but not under ambiguity, consistent with previous studies (Bolla et al., 2004; Overman et al., 2006; Reavis and Overman, 2001). The loss aversion parameter λ (close to 2) in LAT task was also comparable to previous studies (e.g., Pennings and Smidts, 2003; Schmidt and Traub, 2002; Tom et al., 2007; Tversky and Kahneman, 1992). A few studies showed that women were more risk-averse than men (e.g., Bajtelsmit and Bernasek, 1998; Charness and Gneezy, 2007; Schmidt and Traub, 2002), but there was no significant gender difference in the present study. One possible reason for this incongruity might be that we used a task that involved a relatively small amount of money (wins from 10–40 Yuan and loss from 5–20 Yuan) rather than large amounts of investment as in previous studies.
Results showed no correlation between λ and either of the two IGT scores, suggesting the LAT tested aspects of decision making that are different from those involved in the IGT. Loss aversion is a specific phenomenon that reflects people’s tendency of being more sensitive to the possibility of losing objects or money than to the possibility of gaining the same objects or amounts of money (Tom et al., 2007). Indeed, neuroimaging studies have found that these two tasks have involved distinct neural substrates, with the amygdala playing an important role in IGT(Li et al., 2009), but not in LAT (Tom et al., 2007). Correlations between decision making tasks and general intelligence and memory tests suggested that decision making process is partly correlated with general intelligence and memory abilities, which is consistent with previous reports of the importance of basic cognitive functions in decision making (e.g., Gupta et al., 2009; Morsanyi and Handley, 2008; Yechiam and Busemeyer, 2005; Yechiam et al., 2008). This result thus suggests that general intelligence and memory abilities should be controlled in evaluating effects of genetic factors.
Central to the present study, 5-HTTLPR polymorphism was found to significantly influence performance in both the IGT and LAT. Subjects homozygous for the s allele had lower IGT score than the l carriers in the first 40 trials of the IGT task, and the former also showed higher loss aversion than the latter in the LAT task. These results were consistent with previous studies with healthy European or American subjects (Crisan et al., 2009; Homberg et al., 2008; Kuhnen and Chiao, 2009; Roiser et al., 2009; van den Bos et al., 2009), but not with one recent study with Chinese population which only showed a trend (Zhong et al., 2009). Since this effect was independent of subjects’ intellectual or memory abilities, our results add to the literature that emphasizes the important role emotion plays in both decision under ambiguity and loss aversion (one specific form of decision under risk).
Cumulative evidence has suggested that decision making not only involves cognitive processes, but is also modulated by emotional (i.e., homeostatic) signals (e.g., Loewenstein et al., 2001). Decision making task usually engenders strong subjective excitement or arousal, which is associated with strong physiological changes in heart rate, blood pressure, electromyogram, cortisol level, skin temperature, and skin conductance response (see Goudriaan et al., 2004 for a review). There has been mounting evidence that 5-HTTLPR gene has a significant impact on emotional processing (for reviews, see Canli and Lesch, 2007; Hariri and Holmes, 2006), not only in healthy subjects (e.g., Caspi et al., 2003; Kendler et al., 2005), but also in depression patients (e.g., Smits et al., 2006; Zalsman et al., 2006). Serotonin transporter gene s allele carriers are more prone to succumbing to the depression effects of stressful life events than those homozygous for the l allele. We found that subjects homozygous for 5-HTTLPR s allele chose more disadvantageous cards than those with l allele, suggesting that the former subjects tended to be less efficient when performing the Iowa Gambling Task. They also showed an impaired emotional process that disrupted rational choice, which led to a higher level of loss aversion than the l carriers. This view is compatible with Sokol-Hessner’s (2009) recent report that using effective emotion regulation strategies (i.e., “thinking like a trader”, which results in less emotional involvement) significantly reduced both behavioral and physiological loss aversion. Low serotonin transporter activity (s allele) might also reflect a functional “hypofrontality” that is reminiscent of that seen in frontal lobe patients, in that they are insensitive to the distant consequences of their decisions. This view was supported by two neuroimaging studies showing that s carriers had lower functional coupling between amygdala and rostral anterior cingulate cortex (Pezawas et al., 2005) and amygdala and prefrontal cortex/anterior cingulate cortex (Roiser et al., 2009), although the s carriers have an increased functional coupling between the amygdala and ventromedial prefrontal cortex (Heinz et al., 2005). Also, in the study of Bechara et al. (2000), the frontal patients were risk averse (they avoided the high penalty decks) but also performed disadvantageously in the IGT. More recent versions of the somatic marker model (e.g., Bechara and Damasio, 2005) have speculated that somatic signals manifested in the body and carried through the vagus nerve, or manifested solely within the brain, ultimately reach the neurotransmitter cell bodies within the brainstem, including serotonin, which in turn exert influence on motor and cognitive systems in the brain concerned with the implementation of decisions. Thus it has been proposed that one way by which “somatic markers” bias decisions is actually through the release of neurotransmitters (Bechara and Damasio, 2005). The current study provides empirical support for this view.
Several brain regions are important for emotional decision making, including the amygdala, the ventromedial prefrontal cortex and the insula cortex. The amygdala has been emphasized by existing literature, because evidences had shown that 5-HTTLPR genotype influences amygdala activation which was a vital brain area associated with basic emotional process (see Munafo et al., 2008 for a review and meta-analysis). Lesion studies showed that amygdala (e.g., Bechara et al., 2003; Bechara et al., 1999), VMPFC (e.g., Bechara et al., 1994; Bechara et al., 1999) and insular cortex (e.g., Bar-On et al., 2003; Clark et al., 2008) are all important in decision making process, which had also been shown in neuroimaging studies (e.g., De Martino et al., 2006; Li et al., 2009; Tom et al., 2007; Xue et al., 2010; Xue et al., 2009). In addition, there are strong anatomical connections among these areas, which might also be modulated by 5-HTTLPR polymorphism. For example, a recent study showed that 5-HTTLPR modulated the amygdala-frontal coupling (Roiser et al., 2009). Future studies need to combine functional MRI and decision making tasks to examine how the 5-HTTLPR polymorphism can affect decision making under ambiguity and risk through modulating insular activation.
In decision under ambiguity (as measured by the early trials of the IGT), the effect of 5-HTTLPR tended to be modulated by gender: male subjects with the l allele had significantly higher IGT score than males who were s allele homozygotes, but only a trend in the same direction was found for females. Gender by 5-HTTLPR interaction was also found in the LAT task, with stronger genetic effect (i.e., higher loss aversion for s/s than l carriers) for males than for females (see Figure 3B). One study also showed gender by 5-HTTLPR interaction on the performance of IGT (Stoltenberg and Vandever, 2010), that is, the IGT score in the first 20 trials was lower for l/l than for s carriers in males, but higher for l/l than for s carriers in females. Although the results for the female subjects are generally consistent (Homberg et al., 2008; van den Bos et al., 2009), the lack of significant 5-HTTLPR effect in our female subjects might be due to higher variance in this group. For example, females have higher variance in mood status than males (e.g., Kessler et al., 2007; Kessler et al., 2005). In addition, the mRNA and binding site densities for 5-HTT were shown to be regulated by estrogen (e.g., McQueen et al., 1997; McQueen et al., 1999), which varied at different stage of menstrual cycle. It should be noted that the menstrual cycle per se might not affect the overall IGT performance of females as one group (Reavis and Overman, 2001; Van den Bos et al., 2007), and future studies need to examine the interaction between menstrual cycle and 5-HTTLPR polymorphism in affecting decision making under risk and ambiguity. Our results for the male subjects, however, are in opposite direction to that of Stoltenberg and Vandever (2010). It remains unclear as to why this difference emerged. Potential reasons include the fact that there is a difference in allele frequency among Eastern and Western population (e.g., relatively more l/l in Western population and more s/s in Eastern population, see Table 1), Furthermore, subjects were grouped in different ways in the two studies (s carries vs. l/l in their study and s/s vs. l carries in the present study). Another potential reason is that the gene-behavior relationship is modulated by ethnicity and/or culture (e.g., Mizuno et al., 2006). Culture differences in decision-making remains one of the most understudied topics, and future studies are certainly needed to examine these intriguing differences.
The present study focused on the effect of 5-HTTLPR polymorphism on decision making under ambiguity and risk. Many previous studies have found that decision making is affected by other genes, including COMT (e.g., Boettiger et al., 2007; Camara et al., In Press; Dreher et al., 2009; Kang et al., 2010; Roussos et al., 2008; van den Bos et al., 2009; Yacubian et al., 2007), DRD4 (e.g., Camara et al., In Press; Ha et al., 2009; Kuhnen and Chiao, 2009), DAT (e.g., Dreher et al., 2009; Yacubian et al., 2007; Zhong et al., 2009), MAOA (e.g., Jollant et al., 2007), or BDNF (e.g., Kang et al., 2010), as well as their interactions (e.g., Camara et al., In Press; Dreher et al., 2009; Kang et al., 2010; van den Bos et al., 2009; Yacubian et al., 2007). Future studies should examine the effects of multiple genes and their interactions on decision making to significantly advance our understanding on the neural mechanisms of decision making.
In conclusion, this study found significant effects of 5-HTTLPR polymorphism on both decision making under ambiguity and risk in a large sample. These effects were independent of subjects’ intelligence and memory abilities, but tended to be modulated by gender. Our results add to the cumulative evidence emphasizing the important role of emotion in decision making under both ambiguity and risk. Future studies need to combine genetic, behavioral, and functional imaging techniques to examine the neural mechanisms underlying the genetic effects on decision making, which could potentially contribute to the diagnosis and treatment of specific psychiatric disorders associated with decision deficits, such as addiction, schizophrenia, or depression.
Acknowledgments
This research was supported by The 111 Project from the Ministry of Education of China, and grants from the National Institute on Drug Abuse (NIDA) R01 DA023051, and the National Institute of Neurological Disorders and Strokes (NINDS) P01 NS019632. We thank Dr. Russell Poldrack for letting us use the Loss Aversion Task and for his helpful comments on an earlier version of this manuscript. We would also like to thank all the members in the laboratory who helped with the data collection.
Footnotes
Conflict of interests: None
There was no correlation between handedness score and IGT performance (i.e., either IGT score in the first 40 or the last 60 trials, both rs < .03, p > .05). A small correlation was found between handedness score and loss aversion parameter λ (r = −.099, p = .025). One-way ANOVA also suggested that handedness score was not biased by 5-HTTLPR genotype (F(2, 566) = .019, p > .05). Furthermore, there was virtually no change in results after we excluded the left-handed subjects.
There’s virtually no change in results after we excluded those subjects with high alcohol problems.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bajtelsmit VL, Bernasek A. Why Do Women Invest Differently than Men? Financial Counseling and Planning. 1998 doi: 10.2139/ssrn.2238. Available at SSRN: http://ssrn.com/abstract=2238 or. [DOI]
- Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126:1790–1800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Baumgarten HG, Grozdanovic Z. Antomy of central serotoergic projection systems. In: Baumgarten HG, Gothert M, editors. Serotonergic neurons and 5-HT receptors in the CNS. Springer-Verlag; Berlin: 1999. [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behavior. 2005;52:336–372. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Ann N Y Acad Sci. 2003;985:356–369. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Sci. 2005;9:159–162. doi: 10.1016/j.tics.2005.02.002. discussion 162–154. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123 ( Pt 11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D’Esposito M, Fields HL. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27:14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cerebral Cortex. 2004;14:1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Camara E, Kramer UM, Cunillera T, Marco-Pallares J, Cucurell D, Nager W, Mestres-Misse A, Bauer P, Schule R, Schols L, Tempelmann C, Rodriguez-Fornells A, Munte TF. The Effects of COMT (Val108/158Met) and DRD4 (SNP -521) Dopamine Genotypes on Brain Activations Related to Valence and Magnitude of Rewards. Cereb Cortex. doi: 10.1093/cercor/bhp263. In Press. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cesarini D, Dawes CT, Johannesson M, Lichtenstein P, Wallace B. Genetic Variation in Preferences for Giving and Risk Taking. Quarterly Journal of Economics. 2009;124:809–842. [Google Scholar]
- Charness G, Gneezy U. Strong Evidence for Gender Differences in Investment. SSRN eLibrary; 2007. [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier DA, Stober G, Li T, Heils A, Catalano M, DiBella D, Arranz MJ, Murray RM, Vallada HP, Bengel D, Muller CR, Roberts GW, Smeraldi E, Kirov G, Sham P, Lesch KP. A novel functional polymorphism within the promoter of the serotonin transporter gene: Possible role in susceptibility to affective disorders. Molecular Psychiatry. 1996;1:453–460. [PubMed] [Google Scholar]
- Crisan LG, Pana S, Vulturar R, Heilman RM, Szekely R, Druga B, Dragos N, Miu AC. Genetic contributions of the serotonin transporter to social learning of fear and economic decision making. Soc Cogn Affect Neurosci. 2009;4:399–408. doi: 10.1093/scan/nsp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci U S A. 2009;106:617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CR, Poldrack RA. Prospect Theory and the Brain. In: Glimcher PW, Camerer CF, Fehr E, Poldrack RA, editors. Neuroeconomics: Decision Making and the Brain. Academic Press; New York: 2009. pp. 145–173. [Google Scholar]
- Goudriaan AE, Oosterlaan J, de Beurs E, Van den Brink W. Pathological gambling: a comprehensive review of biobehavioral findings. Neurosci Biobehav Rev. 2004;28:123–141. doi: 10.1016/j.neubiorev.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Gupta R, Duff MC, Denburg NL, Cohen NJ, Bechara A, Tranel D. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia. 2009;47:1686–1693. doi: 10.1016/j.neuropsychologia.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha RY, Namkoong K, Kang JI, Kim YT, Kim SJ. Interaction between serotonin transporter promoter and dopamine receptor D4 polymorphisms on decision making. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33:1217–1222. doi: 10.1016/j.pnpbp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002a;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002b;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Homberg JR, van den Bos R, den Heijer E, Suer R, Cuppen E. Serotonin transporter dosage modulates long-term decision-making in rat and human. Neuropharmacology. 2008;55:80–84. doi: 10.1016/j.neuropharm.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Huang L-j, Xu L-p, Xiao B, Jing-zhong L. The association between serotonin transporter gene polymorphism and anxiety-related personality traits in Chinese college students. Chinese Journal of Neurology (in Chinese) 2004;37:114–117. [Google Scholar]
- Jollant F, Buresi C, Guillaume S, Jaussent I, Bellivier F, Leboyer M, Castelnau D, Malafosse A, Courtet P. The influence of four serotonin-related genes on decision-making in suicide attempters. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:615–624. doi: 10.1002/ajmg.b.30467. [DOI] [PubMed] [Google Scholar]
- Joo YH, Oh HB, Kim B, Jung SH, Chung JK, Hong JP, Kim CY. No Association between 5-HTTLPR and Harm Avoidance in Korean College Students. J Korean Med Sci. 2007;22:138–141. doi: 10.3346/jkms.2007.22.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JI, Namkoong K, Ha RY, Jhung K, Kim YT, Kim SJ. Influence of BDNF and COMT polymorphisms on emotional decision making. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Katsuragi S, Kunugi H, Sano A, Tsutsumi T, Isogawa K, Nanko S, Akiyoshi J. Association between serotonin transporter gene polymorphism and anxiety-related traits. Biological Psychiatry. 1999;45:368–370. doi: 10.1016/s0006-3223(98)00090-0. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Angermeyer M, Anthony JC, RDEG, Demyttenaere K, Gasquet I, GDEG, Gluzman S, Gureje O, Haro JM, Kawakami N, Karam A, Levinson D, Medina Mora ME, Oakley Browne MA, Posada-Villa J, Stein DJ, Adley Tsang CH, Aguilar-Gaxiola S, Alonso J, Lee S, Heeringa S, Pennell BE, Berglund P, Gruber MJ, Petukhova M, Chatterji S, Ustun TB. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168–176. [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Chiao JY. Genetic determinants of financial risk taking. PLoS One. 2009;4:e4362. doi: 10.1371/journal.pone.0004362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus JS, Mayer EA, Hamaguchi T, Mizuno T, Kano M, Fukudo S. 5-Httlpr Gene Polymorphism Modulates Activity and Connectivity Within An Emotional Arousal Network of Healthy Control Subjects During Visceral Pain. Gastroenterology. 2008;134:A-121. doi: 10.1371/journal.pone.0123183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze EJ, Munin MC, Ferrell RE, Pollock BG, Skidmore E, Lotrich F, Rogers JC, Quear T, Houck P, Reynolds CF., 3rd Association of the serotonin transporter gene-linked polymorphic region (5-HTTLPR) genotype with depression in elderly persons after hip fracture. Am J Geriatr Psychiatry. 2005;13:428–432. doi: 10.1176/appi.ajgp.13.5.428. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li X, Lu ZL, D’Argembeau A, Ng M, Bechara A. The Iowa Gambling Task in fMRI images. Human Brain Mapping. 2009;31:410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychol Bull. 2001;127:267–286. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Fink G. Estradiol-17 beta increases serotonin transporter (SERT) mRNA levels and the density of SERT-binding sites in female rat brain. Brain Res Mol Brain Res. 1997;45:13–23. doi: 10.1016/s0169-328x(96)00233-1. [DOI] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Sumner BE, Fink G. Serotonin transporter (SERT) mRNA and binding site densities in male rat brain affected by sex steroids. Brain Res Mol Brain Res. 1999;63:241–247. doi: 10.1016/s0169-328x(98)00281-2. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Aoki M, Shimada Y, Inoue M, Nakaya K, Takahashi T, Itoyama Y, Kanazawa M, Utsumi A, Endo Y, Nomura T, Hiratsuka M, Mizugaki M, Goto J, Hongo M, Fukudo S. Gender difference in association between polymorphism of serotonin transporter gene regulatory region and anxiety. J Psychosom Res. 2006;60:91–97. doi: 10.1016/j.jpsychores.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Morsanyi K, Handley SJ. How smart do you need to be to get it wrong? The role of cognitive capacity in the development of heuristic-based judgment. Journal of Experimental Child Psychology. 2008;99:18–36. doi: 10.1016/j.jecp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin Transporter (5-HTTLPR) Genotype and Amygdala Activation: A Meta-Analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must A, Juhasz A, Rimanoczy A, Szabo Z, Keri S, Janka Z. Major depressive disorder, serotonin transporter, and personality traits: why patients use suboptimal decision-making strategies? J Affect Disord. 2007;103:273–276. doi: 10.1016/j.jad.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Overman W, Graham L, Redmond A, Eubank R, Boettcher L, Samplawski O, Walsh K. Contemplation of moral dilemmas eliminates sex differences on the Iowa Gambling Task. Behavioral Neuroscience. 2006;120:817–825. doi: 10.1037/0735-7044.120.4.817. [DOI] [PubMed] [Google Scholar]
- Pennings JME, Smidts A. The Shape of Utility Functions and Organizational Behavior. MANAGEMENT SCIENCE. 2003;49:1251–1263. [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Reavis R, Overman WH. Adult sex differences on a decision-making task previously shown to depend on the orbital prefrontal cortex. Behavioral Neuroscience. 2001;115:196–206. doi: 10.1037/0735-7044.115.1.196. [DOI] [PubMed] [Google Scholar]
- Roiser JP, de Martino B, Tan GC, Kumaran D, Seymour B, Wood NW, Dolan RJ. A genetically mediated bias in decision making driven by failure of amygdala control. J Neurosci. 2009;29:5985–5991. doi: 10.1523/JNEUROSCI.0407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Rogers RD, Cook LJ, Sahakian BJ. The effect of polymorphism at the serotonin transporter gene on decision-making, memory and executive function in ecstasy users and controls. Psychopharmacology (Berl) 2006;188:213–227. doi: 10.1007/s00213-006-0495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Pavlakis S, Bitsios P. Planning, decision-making and the COMT rs4818 polymorphism in healthy males. Neuropsychologia. 2008;46:757–763. doi: 10.1016/j.neuropsychologia.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, Delafuente JR, Grant M. Development of the Alcohol-Use Disorders Identification Test (Audit) - Who Collaborative Project on Early Detection of Persons with Harmful Alcohol-Consumption .2. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schmidt U, Traub S. An Experimental Test of Loss Aversion. Journal of Risk and Uncertainty. 2002;25:233–249. [Google Scholar]
- Smits BM, Mudde JB, van de Belt J, Verheul M, Olivier J, Homberg J, Guryev V, Cools AR, Ellenbroek BA, Plasterk RH, Cuppen E. Generation of gene knockouts and mutant models in the laboratory rat by ENU-driven target-selected mutagenesis. Pharmacogenet Genomics. 2006;16:159–169. doi: 10.1097/01.fpc.0000184960.82903.8f. [DOI] [PubMed] [Google Scholar]
- Sokol-Hessner P, Hsu M, Curley NG, Delgado MR, Camerer CF, Phelps EA. Thinking like a trader selectively reduces individuals’ loss aversion. Proc Natl Acad Sci U S A. 2009;106:5035–5040. doi: 10.1073/pnas.0806761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Vandever JM. Gender moderates the association between 5-HTTLPR and decision-making under ambiguity but not under risk. Neuropharmacology. 2010;58:423–428. doi: 10.1016/j.neuropharm.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Tremeau F, Brady M, Saccente E, Moreno A, Epstein H, Citrome L, Malaspina D, Javitt D. Loss aversion in schizophrenia. Schizophr Res. 2008;103:121–128. doi: 10.1016/j.schres.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Tremeau F, Cacioppo JT, Antonius D, Ziwich R, Saccente E, Butler PD, Javitt DC. Hedonic capacities in schizophrenia. Schizophrenia Bulletin. 2007;33:1. [Google Scholar]
- Tsai SJ, Hong CJ, Cheng CY. Serotonin transporter genetic polymorphisms and harm avoidance in the Chinese. Psychiatr Genet. 2002;12:165–168. doi: 10.1097/00041444-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Advances in prospect theory: Cumulative representation of uncertainty. Journal of Risk and Uncertainty. 1992;5:297–323. [Google Scholar]
- Van den Bos R, Den Heijer E, Vlaar S, Houx B. Psychology of Decision Making in Education. In: Elsworth JE, editor. Behavior & High Risk Situations. Nova Science Publishers Inc; 2007. pp. 207–226. [Google Scholar]
- van den Bos R, Homberg J, Gijsbers E, den Heijer E, Cuppen E. The effect of COMT Val158 Met genotype on decision-making and preliminary findings on its interaction with the 5-HTTLPR in healthy females. Neuropharmacology. 2009;56:493–498. doi: 10.1016/j.neuropharm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Wai MS, Shi C, Kwong WH, Zhang L, Lam WP, Yew DT. Development of the human insular cortex: differentiation, proliferation, cell death, and appearance of 5HT-2A receptors. Histochem Cell Biol. 2008;130:1199–1204. doi: 10.1007/s00418-008-0497-5. [DOI] [PubMed] [Google Scholar]
- Xue G, Dong Q, Jin Z, Chen C. Mapping of verbal working memory in nonfluent Chinese-English bilinguals with functional MRI. Neuroimage. 2004;22:1–10. doi: 10.1016/j.neuroimage.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Bechara A. The impact of prior risk experiences on subsequent risky decision-making: The role of the insula. Neuroimage. 2010;50:709–716. doi: 10.1016/j.neuroimage.2009.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A. Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cereb Cortex. 2009;19:1019–1027. doi: 10.1093/cercor/bhn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, Glascher J, Kalisch R, Leuenberger B, Braus DF, Buchel C. Gene-gene interaction associated with neural reward sensitivity. Proc Natl Acad Sci U S A. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechiam E, Busemeyer J. On the examination of basic assumptions embedded in learning models. Journal of Mathematical Psychology. 2005;49:108–108. [Google Scholar]
- Yechiam E, Hayden EP, Bodkins M, O’Donnell BF, Hetrick WP. Decision making in bipolar disorder: A cognitive modeling approach. Psychiatry Research. 2008;161:142–152. doi: 10.1016/j.psychres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA, Ellis SP, Goldman D, Mann JJ. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- Zhong S, Israel S, Xue H, Sham PC, Ebstein RP, Chew SH. A neurochemical approach to valuation sensitivity over gains and losses. Proc Biol Sci. 2009;276:4181–4188. doi: 10.1098/rspb.2009.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Chen C, Loftus EF, Lin C, He Q, Chen C, Li H, Xue G, Lu Z, Dong Q. Individual Differences in False Memory from Misinformation: Cognitive Factors. Memory. doi: 10.1080/09658211.2010.487051. Inpress. [DOI] [PubMed] [Google Scholar]