Abstract
Objectives
In parallel with the increase in pediatric esophagastroduodenoscopy (EGD) procedures since the 1970s, the incidence of disorders that require EGD for diagnosis in children has increased. The aim of this study was to identify changes in subject characteristics and endoscopic procedures over a 20 year interval in children undergoing EGD at a single center.
Methods
All children undergoing first EGD with biopsy in 1985, 1995, or 2005 were identified. Details of the clinical presentation and EGD were abstracted from medical records in a random sample of subjects within each time point.
Results
The number of first time EGDs rose dramatically from 107 in 1985 to 1,294 in 2005. The proportion of subjects that were less than one year of age varied significantly from 13% in 1985, to 23% in 1995, to 8% in 2005 (p < 0.001). The proportion of subjects with gastrointestinal bleeding declined from 34% to 5% over the 20 year interval (p < 0.001) while the proportion with abdominal pain increased from 23% to 43% (p < 0.01). Over the same interval, the proportion of subjects with complete EGD (biopsies from the esophagus, stomach and duodenum) increased from 18% of EGDs in 1985 to 95% in 2005 (p < 0.001).
Conclusions
This study of children undergoing first time EGDs with biopsy over a 20 year interval demonstrated significant differences in subject characteristics and endoscopy practices. The inclusion of children with less severe clinical presentation and the collection of greater numbers of biopsies per procedure may contribute to the rising incidence rates of pediatric gastrointestinal disorders.
Keywords: endoscopy, indications, practice, pediatric, epidemiology
Introduction
The field of pediatric gastroenterology has experienced rapid growth since its inception in the 1960s. Pediatric gastroenterology is now an American Board of Pediatrics certified subspecialty that emerged from earlier training of pediatricians in adult gastroenterology units and an increased recognition of gastrointestinal disorders that are unique to children. Over the past 30 years, there has been a proliferation of pediatric gastroenterologists from a few select centers around the world, to an ever growing specialty that has approximately one pediatric gastroenterologist per 100,000 children in the United States.1 With the development of a subspecialty focused on the disorders of the pediatric gastrointestinal tract, new technologies were also developed to aid in diagnoses such as pediatric esophagogastroduodenoscopy (EGD).
Pediatric EGD began in the 1970s, and over a 30 year period has evolved from an infrequent procedure in the operating room with a single ocular for viewing the intestinal lining to a routine outpatient procedure using intravenous sedation and large viewing screens.2
In parallel with the growth of pediatric gastroenterology, disorders that require EGD for diagnosis have shown a rising incidence of diagnosis.3 This increasing incidence may be due in part to changing endoscopy practices over this time period. Therefore, we hypothesized that subjects in the earlier years would have a higher disease severity compared to subjects in more recent years. The aim of this study was to identify differences in subject characteristics and endoscopic practices among subjects undergoing endoscopy in 1985, 1995, and 2005.
Materials and Methods
The Children's Hospital of Philadelphia was the first pediatric hospital in the nation and represents the largest pediatric hospital in the 9-county greater Philadelphia area. The population catchment area serving The Children's Hospital of Philadelphia was geographically defined as Philadelphia, Bucks, Chester, Delaware, Montgomery, Burlington, Camden, Gloucester and Mercer Counties.
We conducted a retrospective analysis of children and adolescents that underwent EGD at a single center during the calendar years 1985, 1995, and 2005. All patients undergoing EGD with biopsy at The Children's Hospital of Philadelphia during the study time periods were initially with biopsy identified through a systematic review of a pathology database comprised of paper and electronic records. The samples were limited to subjects undergoing first-time EGD with biopsy. Subject demographics (age, gender, and race) were available for all subjects undergoing first time EGD in 1985, 1995, and 2005.
Additional data regarding clinical presentation and details of the EGD procedure were obtained through abstraction of the medical records in a random sample of 100 subjects undergoing first time EGD in 1985, 1995, and 2005 (sample command; STATA 9.0, Stata, College Station, TX). Inpatient and outpatient medical records were available in 78, 84, and 83 subjects at the 1985, 1995, and 2005 time points, respectively.
A severity index for indications for EGD was created as a dichotomous variable of high versus low severity. Components indicative of high severity included any of the following documented prior to EGD: a diagnosis of failure to thrive, gastrointestinal bleeding, severe systemic disease, abnormal upper gastrointestinal (UGI) radiographic findings, and abnormal screening laboratory results. Severe systemic disease included conditions such as malignancy, genetic syndromes, systemic inflammatory conditions or conditions requiring hospitalization in the intensive care unit. Abnormal UGI radiographic findings were defined as documentation of stricture, ulceration or mass. Abnormal screening laboratories included elevation of inflammatory markers, hemoglobin values of less than 10.5 mg/dL, abnormal celiac serologies, abnormal inflammatory bowel disease (IBD) serologies, and an albumin of less than 3.5 mg/dL. Additional data included medications at the time of the EGD. Data abstraction was confined to these defined endpoints because of the limited data available in 1985 subject charts.
The EGD procedure characteristics included the anatomic biopsy sites and the number of biopsies obtained at each site. A complete EGD was defined as a study that included biopsies from the esophagus, stomach, and duodenum. The presence of any abnormalities on visual inspection was noted as well as the histological results.
Statistical analyses were conducted using STATA® 9.0 (Stata, College Station, TX) and Pass® 2005 (NCSS, Kaysville, Utah). Descriptive statistics for subject characteristics and basic demographics were reported as means and standard deviations (SD) or medians with ranges depending on the variable distributions. Subject characteristics and EGD practices were compared across time points using ANOVA test for normally distributed variables, K-Wallis tests for non-normally distributed variables, and Chi-Square test for dichotomous outcome variables. All tests were considered significant at the p < 0.05 level. This study was approved by The Children's Hospital of Philadelphia Institutional Review Board.
Results
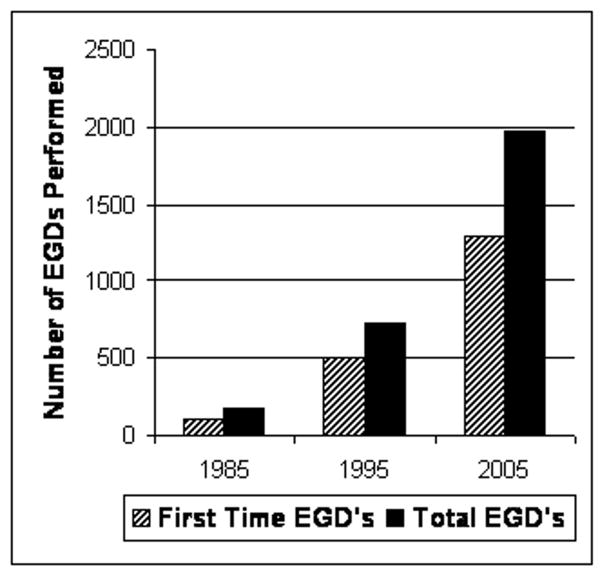
The number of first time and total EGDs in the time points of 1985, 1995 and 2005 are summarized in Figure 1 and Table 1. Given that the number of children and adolescents in the greater Philadelphia catchment area was stable over this interval, the first time EGD rate increased 12-fold (from 7.9 to 95.2 procedures per 100,000 children per year) over this 20 year interval (p < 0.001) (Table 1). Over the same interval, the number of pediatric gastroenterologists and outpatient gastroenterology clinic visits also increased significantly.
Figure 1. Total & First EGD in 1985, 1995 & 2005.
Subjects who underwent EGD with biopsy at The Children's Hospital of Philadelphia during the calendar years 1985, 1995, and 2005.
Table 1. Total & Incident EGDs Relative to Population Catchment Area, Number of Endoscopists and Gastroenterology Clinic Visits at the Children's Hospital of Philadelphia in 1985, 1995 & 2005.
Total and incident EGD with biopsy cohorts are represented in the context of their corresponding pediatric U.S. Census population estimates and the numbers of pediatric endoscopists and gastroenterology clinic visits at The Children's Hospital of Philadelphia. Proportions reflect a dramatic rise in the number of pediatric subjects undergoing endoscopy, but that the number of endoscopies relative to the number gastroenterologists and the number of outpatient visits has remained constant over the last decade.
| 1985 | 1995 | 2005 | |
|---|---|---|---|
| Total EGDs | 171 | 723 | 1,980 |
| First EGDs | 107 | 507 | 1,294 |
| Population Catchment Area (Census Data) | 1,359,046 | 1,257,689 | 1,359,599 |
| First EGDs per 100,000 children | 7.9 | 40.3 | 95.2 |
| Number of GI Attendings | 4 | 10 | 25 |
| First EGD per Attending | 26.8 | 50.7 | 51.8 |
| Total GI Outpatient Visits | ** | 7,033 | 18,586 |
| First EGD per total outpatient visits | ** | 7.2 | 6.96 |
| New GI Outpatient Visits | ** | 2,055 | 5,950 |
Data not available
Patient demographics in subjects undergoing first time EGD with biopsy in the time points of 1985, 1995 and 2005 are summarized in Table 2. Data are provided for the entire population of children undergoing EGD with biopsy in each of the three years, as well as the random subsamples of 78, 84, and 83 subjects in 1985, 1995 and 2005, respectively. The age, gender and race distributions in the total sample and the subsamples within each of the three eras are comparable, confirming that the random subsamples are representative of the entire populations in each era. The distributions of sex and race were not significantly different across the three eras. However, the proportion of subjects that were less than one year of age decreased significantly from 1995 to 2005 within the total population (p < 0.001) as well as the random subsample (p < 0.005).
Table 2. Demographic Characteristics in Incident EGD Subjects in 1985, 1995 & 2005.
Incident EGD with biopsy subject cohort demographics in 1985, 1995 and 2005. Subsamples represent subject data available from subject medical records from a random sample of 100 EGD subjects from the respective time points. A striking difference in the number of infants (less than 1 year of age) undergoing EGD in 1985, 1995 and 2005 was confirmed in two separate databases for the total cohorts as well as the subsample.
| 1985 | 1995 | 2005 | p-value | |
|---|---|---|---|---|
| Total Subsample Eligible | 107 | 507 | 1294 | |
| 78 of 100 | 84 of 100 | 83 of 100 | ||
|
Total Age Subsample Age (mean, year) |
7.7 ± 6.0 | 6.4±5.7 | 9.0± 6.0 | P=ns* |
| 7.6 ± 6.1 | 6.9±6.4 | 8.5 ± 5.3 | ||
|
Total Infants Subsample Infants (% <1 year) |
13.1% | 23.3% | 7.7% | P<0.005** |
| 16.7% | 28.6% | 6.0% | ||
|
Total Sex Subsample Sex (% male) |
52.3% | 53.9% | 54.6% | P=ns** |
| 53.2% | 51.2% | 55.4% | ||
|
Total Race Subsample Race (% Caucasian) |
78.9% | 82.8% | 79.2% | P=ns** |
| 81.0% | 81.0% | 84.3% | ||
Subjects in 1985, 1995, & 2005 compared using ANOVA test
Subjects in 1985, 1995, & 2005 compared using Chi-Square test
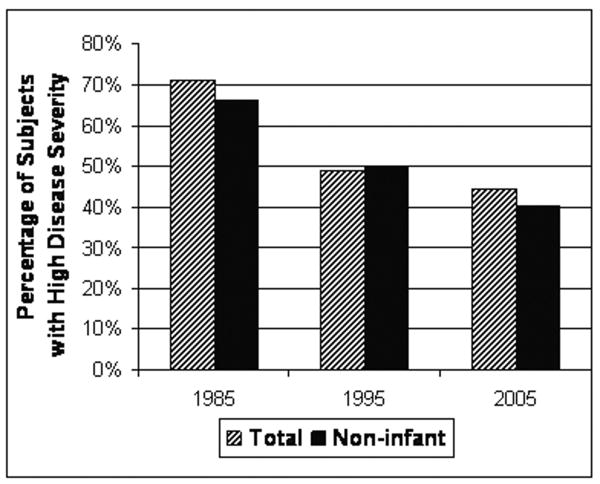
The clinical presentations, indications for EGD, and disease severity in the subsamples were compared across the three eras (Table 3). The proportion of subjects with gastrointestinal bleeding declined significantly from 34% to 5% over the 3 eras (p < 0.001). In contrast, the proportions with abdominal pain increased from 23% to 43% (p < 0.01). The proportion of subjects with a high severity index for indications for EGD decreased significantly over the three eras (p < 0.005), as summarized in Figure 2.
Table 3. EGD Subject Subsample Clinical Characteristics in 1985, 1995 & 2005.
Indications for EGD, patient characteristics and the use of acid suppression medications were abstracted from the medical records of a subsample of subjects in 1985, 1995 and 2005. Reciprocal trends of isolated abdominal pain and GI bleeding indications support the direction of the aggregate conclusions of the symptom severity index. As expected, the proportion of subjects undergoing EGD for failure to thrive remained constant. The use of acid suppression medication prior to endoscopy has risen dramatically.
| 1985 | 1995 | 2005 | p-value | |
|---|---|---|---|---|
| Eligible of Subsample | 78 of 100 | 84 of 100 | 83 of 100 | |
| Height Z-score | -0.82 ± 1.92 | -0.47 ± 2.02 | -0.40 ± 1.20 | P=ns |
| BMI Z-score | -0.78 ± 1.78 | -0.17 ± 1.64 | -0.51 ± 1.27 | P=ns |
| Isolated Abdominal Pain | 22.8% | 42.9% | 43.4% | P<0.01 |
| High Severity | 71.8% | 48.8% | 44.6% | P<0.005 |
| GI Bleeding | 34.2% | 15.5% | 4.8% | P<0.001 |
| Failure to Thrive | 26.6% | 20.2% | 21.7% | P=ns |
| H2RA or PPI prior to EGD | 14.1% | 35.7% | 55.4% | P<0.001 |
Subjects in 1985, 1995, & 2005 compared using Chi-Square test for dichotomous variables and ANOVA test for continuous variables
Figure 2. Severity Index Among Incident EGD Subjects in 1985, 1995 & 2005.
Among subjects undergoing incident EGD with biopsy, the indications for EGD were abstracted from medical records in a subsample and dichotomized into a severity index. Components indicative of high severity included: failure to thrive, gastrointestinal bleeding, severe systemic disease, abnormal UGI radiographic findings, and abnormal screening laboratory results.
Acid suppression medication prescribing practices prior to EGD were also examined. Histamine 2 Receptor Antagonists (H2RA) medications or proton pump inhibitors were prescribed prior to EGD in 14.1% of subjects in 1985, 35.7% of subjects in 1995 and 55.4% of subjects in 2005 (p < 0.001). In the respective years, proton pump inhibitors alone were prescribed in 1.3%, 4.8% and 41.0% of subjects undergoing first time EGD (p < 0.001).
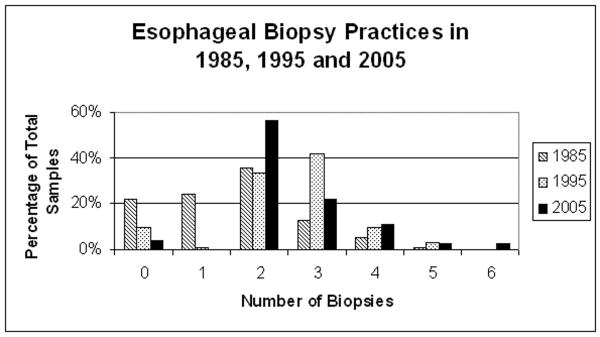
EGD practices and findings changed significantly from 1985 to 2005 as summarized in Table 4. A complete EGD (with biopsies obtained in the esophagus, stomach and duodenum) was performed in 17.7% of EGDs in 1985, 79.8% in 1995, and 95.2% in 2005 (p < 0.001). The proportion of biopsies with histologic abnormalities declined from 1985 to 2005 at each site. Finally, the number of esophageal biopsies obtained during each EGD procedure increased significantly over the three eras (p < 0.001) (Figure 3). In 1985, 53.2% had two or more esophageal biopsies compared to 97.3% in 1995 and 98.8% in 2005.
Table 4. EGD Biopsy Practices in 1985, 1995 & 2005.
Table 4. The EGD biopsy practices among the subsample cohorts are described. As the practice of obtaining routine biopsies from the duodenum, stomach and esophagus has changed from 1985, 1995, and 2005, the proportion of subjects with any histologic abnormality from the duodenum, stomach and esophagus has declined.
| 1985 | 1995 | 2005 | p-value | |
|---|---|---|---|---|
| Eligible of Subsample | 78 of 100 | 84 of 100 | 83 of 100 | |
| Complete EGD (esophageal, stomach & duodenal biopsies) |
17.7% (14/78) |
79.8% (67/84) |
95.2% (79/83) |
P < 0.001* |
| Esophageal Histologic Abnormality | 56.7% (34/60) |
49.3% (37/75) |
35.4% (28/79) |
P=0.037* |
| Stomach Histologic Abnormality | 34.5% (10/29) |
21.6% (16/74) |
14.8% (12/81) |
P=0.078* |
| Duodenal Histologic Abnormality | 45.8% (22/48) |
17.3% (13/75) |
17.1% (14/82) |
P=0.000* |
Subjects in 1985, 1995, & 2005 compared using Chi-Square test
Figure 3. Number of Esophageal Biopsies Among Incident EGD Subjects in 1985, 1995 & 2005.
The number of esophageal biopsies obtained during incident EGDs in 1985, 1995, and 2005. This graphic representation of fewer esophageal biopsies obtained in 1985 compared to 1995 and 2005 supports the trends in declining proportions of esophageal histologic abnormalities in 1985, 1995, and 2005 depicted in Table 4. Subjects in 1985 were less likely to have screening esophageal biopsies obtained.
Discussion
This study of children undergoing first EGDs with biopsy in the years 1985, 1995 and 2005 demonstrated significant differences in subject characteristics and endoscopy practices. Children who underwent EGD in 1985 were less likely to have isolated abdominal pain, were more likely to have upper gastrointestinal bleeding and had a higher disease severity index compared to subjects in 1995 and 2005. These findings support the hypothesis that the indications for pediatric EGD have changed over the past 20 years.
In the earlier years, pediatric EGD was a new technology and pediatric gastroenterology was a developing field. The differences in absolute numbers of first time EGDs, EGDs per population and EGDs per GI attending support the concepts of relative inexperience with pediatric endoscopy, lower number of referrals from general pediatricians and the use of other diagnostic modalities such as pH probes, manometry and Crosby capsules between 1985 and the later years. High disease severity and gastrointestinal bleeding rates in the earlier years may also have been influenced by acid suppression medication prescribing practices and improvements in the management of diseases that predispose to GI bleeding such as liver transplantation for portal hypertension associated variceal bleeding. In addition, the numbers of EGDs performed by pediatric gastroenterologists and by outpatient gastroenterology clinic visits were comparable in 1995 and 2005. This suggests that the overall increases in EGD rates were related to increases in the number of gastroenterologists, outpatient gastroenterology visits, referrals from pediatricians and education about disorders diagnosed with EGD in recent years.
In 1996, The North American Society of Pediatric Gastroenterology stated that “Diagnostic upper endoscopy is generally not indicated for uncomplicated gastroesophageal reflux [or] uncomplicated functional abdominal pain.” 6 In other words, children with uncomplicated GERD that were likely to be treated with acid suppression therapy were not undergoing routine EGD in 1985 and 1995. In 2001, the North American Society for Pediatric Gastroenterology Guidelines for the management of symptoms of gastroesophageal reflux recommended a trial of acid suppression therapy prior to endoscopy.7 These statements imply that the practice of EGD in children has shifted from a procedure seldom performed in the setting of low-severity indications, such as uncomplicated GERD, to one routinely performed after a trial of acid suppression therapy to exclude other etiologies. Our data support the clinical application of these practice parameters with more children with lower disease severity undergoing EGD with biopsy in the later years. Of particular interest in this data is the higher proportion of infants undergoing EGD in 1995 compared to 1985 and 2005. It is postulated that infants were less likely to undergo EGD in 1985 given technical limitations and physician inexperience with EGD in this age group. However, with increasing physician experience in 1995, the proportion of infants undergoing EGD increased. In 2005, although the absolute number of infants undergoing EGD remained approximately the same in compared to 1995, the two fold increase in total first time EGD procedures decreases the relative proportion of this age group. This suggests that while the increasing experience of pediatric endoscopists performing infant EGD is reflected in the rise of infant EGDs from 1985 to 1995, the use of acid suppression therapy in this age group likely contributed to a decreased proportion of infants undergoing EGD from 1995 to 2005.
In addition to improved technology and physician technical experience, there have been new discoveries and interest in pediatric gastrointestinal inflammatory disorders that may also influence the number of EGDs performed. In particular, in the past decade Eosinophilic Esophagitis (EoE) has received considerable attention as a “new” disease, with the first consensus report being published in 2007. EoE is a disorder that requires EGD with biopsy for diagnosis and is defined by lack of response to gastric acid suppression medications. In a cohort of 381 children with EoE, 82% (312) were subclassified into a gastroesophageal reflux category of which 61% (191) were described as having epigastric pain, heartburn or waterbrash. 8 Subjects presenting with isolated epigastric abdominal pain, heartburn, waterbrash, nausea, and vomiting would be classified as having a low symptom severity index in our study. Therefore, EoE subjects with a low symptom severity index may have been underdetected in the earlier years. Further, the recognition of EoE as a distinct clinical entity from GERD and functional abdominal pain has increased the use of pediatric EGD in the 2005 era relative to 1985 and 1995, as an important diagnostic modality for a disorder that currently cannot be diagnosed by any other means. Therefore, EGD practices may serve as an important confounding variable in the description of rising EoE incidence rates.
Changing indications for pediatric endoscopy over this 20 year period may have also influenced other disease detection rates such as that of IBD. In a prospective study in 2000-2001, Kugathasan and colleagues reported pediatric population-based IBD incidence rates with a higher proportion of children with Crohn's disease than had been reported previously.9,10, 11 Practices of routine EGD evaluation in conjunction with total colonoscopy with ileal intubation during the initial evaluation for IBD were not commonly practiced until the 1990s. IBD disease detection rates may be confounded by the changing practices of pediatric EGD.
Celiac disease is another important pediatric gastrointestinal disorder for which endoscopy is the gold standard to establish the diagnosis. In the United States, Olmsted County, MN described a 51 year experience of the diagnosis of celiac disease across all ages in their population after having an endoscopic biopsy to establish the diagnosis.3 They report respective annual incidence rates of celiac disease with a dramatic rise from 0.9 per 100,000 in 1950-1989, 3.3 per 100,000 in the 1990s and 9.1 per 100,000 in 2000-2001. 3, 12-16. In another study, serologic testing across a random sample of 4,126 subjects from the United States population from 1996-2001 estimated a prevalence of celiac disease in subjects without risk factors to be as high as 1:133 (0.8%).15 Fasano and colleagues have described this phenomenon as an iceberg effect.13 Subjects with severe symptomatic disease who are likely to go to their physician, be referred to a gastroenterologist and have their diagnosis of celiac disease confirmed with EGD represent only a small fraction of the true population with milder or even asymptomatic (latent) disease.
In addition to changing patient characteristics described in our study, changing EGD practices also serve as a confounding variable in the description of rising incidence rates. In our study, 46.8% of subjects in 1985 had 0 or 1 esophageal biopsies compared to 2.4% in 1995 and 1.2% in 2005. Only 19.0% of subjects in 1985 had 3 or more esophageal biopsies taken compared to 52.4% in 1995 and 38.5% in 2005 (p < 0.001). Gonsalves and colleagues have shown previously that the sensitivity of detecting EoE is dependent upon the number of esophageal biopsies taken at the time of diagnosis.17 If only one biopsy is taken the sensitivity is 55%, compared to 94% if four or more biopsies are taken. The fewer number of esophageal biopsies taken in the earlier years may have underestimated the true incidence and prevalence of this disorder.
In conclusion, this study of children undergoing first time EGDs in the years 1985, 1995, and 2005 support the hypothesis that the subject characteristics and EGD practices among children undergoing EGD have changed over the past 20 years. In the context of a 12-fold rise in the number if EGDs performed from 1985 to 2005, it is not surprising that subjects with the highest disease severity would undergo EGD in 1985 relative to 2005. As the patient characteristics and endoscopic practices among those subjects who undergo EGD change, disease detection rates will also change. Therefore caution must be exercised in reporting increasing incidence of disease rates when these disease rates may instead reflect increasing rates of diagnosis of disease rather than true increase in disease occurrence.
Acknowledgments
Grant Support: NIH T32-DK007740, “Clinical Epidemiology Training in Gastroenterology”
Footnotes
Financial Disclosures: none
Writing Assistance: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pediatric Gastroenterology Workforce Survey, 2003-2004. Journal of pediatric gastroenterology and nutrition. 2005;40(4):397–405. doi: 10.1097/01.mpg.0000158523.92175.50. [DOI] [PubMed] [Google Scholar]
- 2.Gilger MA. Gastroenterologic endoscopy in children: past, present, and future. Current opinion in pediatrics. 2001;13(5):429–34. doi: 10.1097/00008480-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Murray JA, Van Dyke C, Plevak MF, Dierkhising RA, Zinsmeister AR, Melton LJ., 3rd Trends in the identification and clinical features of celiac disease in a North American community, 1950-2001. Clin Gastroenterol Hepatol. 2003;1(1):19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 4.Thayu M, Shults J, Burnham JM, Zemel BS, Baldassano RN, Leonard MB. Gender differences in body composition deficits at diagnosis in children and adolescents with Crohn's disease. Inflammatory bowel diseases. 2007;13(9):1121–8. doi: 10.1002/ibd.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 6.Squires RH, Jr, Colletti RB. Indications for pediatric gastrointestinal endoscopy: a medical position statement of the North American Society for Pediatric Gastroenterology and Nutrition. Journal of pediatric gastroenterology and nutrition. 1996;23(2):107–10. doi: 10.1097/00005176-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Rudolph CD, Mazur LJ, Liptak GS, et al. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. Journal of pediatric gastroenterology and nutrition. 2001;32 2:S1–31. doi: 10.1097/00005176-200100002-00001. [DOI] [PubMed] [Google Scholar]
- 8.Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3(12):1198–206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 9.Kugathasan S, Judd RH, Hoffmann RG, et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. The Journal of pediatrics. 2003;143(4):525–31. doi: 10.1067/s0022-3476(03)00444-x. [DOI] [PubMed] [Google Scholar]
- 10.Abdullah BA, Gupta SK, Croffie JM, et al. The role of esophagogastroduodenoscopy in the initial evaluation of childhood inflammatory bowel disease: a 7-year study. Journal of pediatric gastroenterology and nutrition. 2002;35(5):636–40. doi: 10.1097/00005176-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Castellaneta SP, Afzal NA, Greenberg M, et al. Diagnostic role of upper gastrointestinal endoscopy in pediatric inflammatory bowel disease. Journal of pediatric gastroenterology and nutrition. 2004;39(3):257–61. doi: 10.1097/00005176-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Hill ID, Dirks MH, Liptak GS, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Journal of pediatric gastroenterology and nutrition. 2005;40(1):1–19. doi: 10.1097/00005176-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Fasano A. Where have all the American celiacs gone? Acta Paediatr Suppl. 1996;412:20–4. doi: 10.1111/j.1651-2227.1996.tb14242.x. [DOI] [PubMed] [Google Scholar]
- 14.Fasano A. European and North American populations should be screened for coeliac disease. Gut. 2003;52(2):168–9. doi: 10.1136/gut.52.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Archives of internal medicine. 2003;163(3):286–92. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 16.Fasano A, Catassi C. Coeliac disease in children. Best practice & research. 2005;19(3):467–78. doi: 10.1016/j.bpg.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Gonsalves N, Policarpio-Nicolas M, Zhang Q, Rao MS, Hirano I. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointestinal endoscopy. 2006;64(3):313–9. doi: 10.1016/j.gie.2006.04.037. [DOI] [PubMed] [Google Scholar]