Abstract
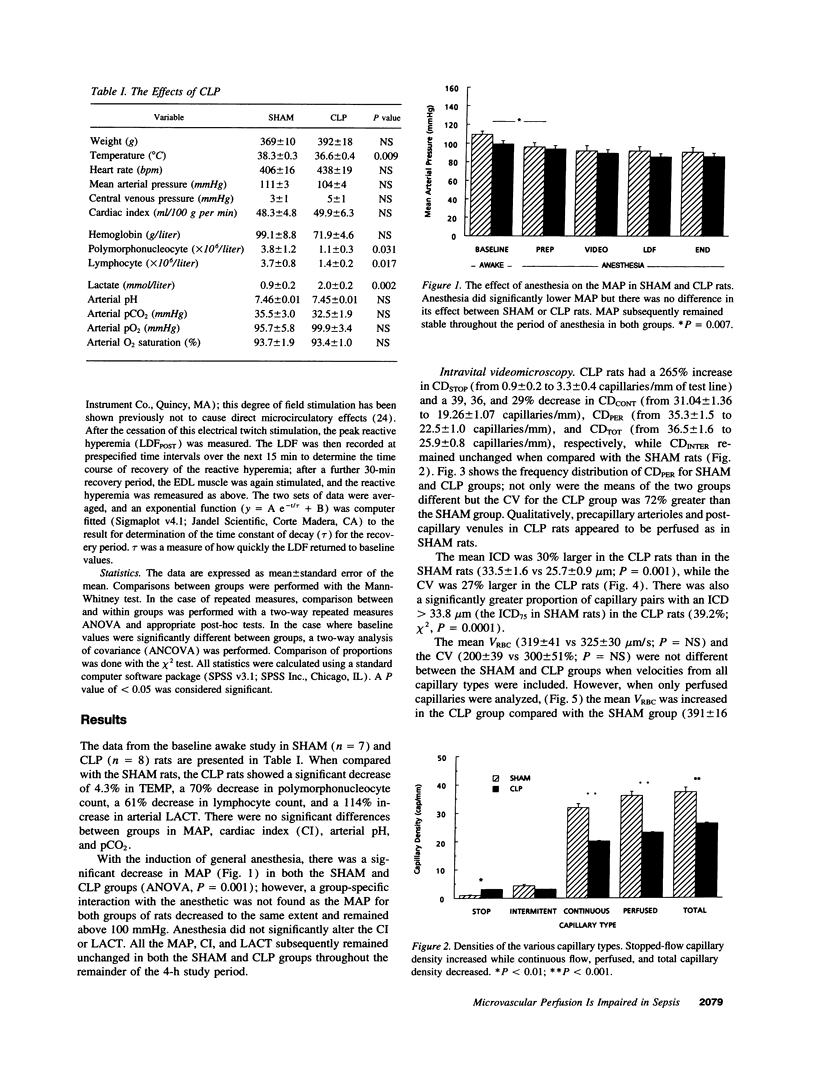
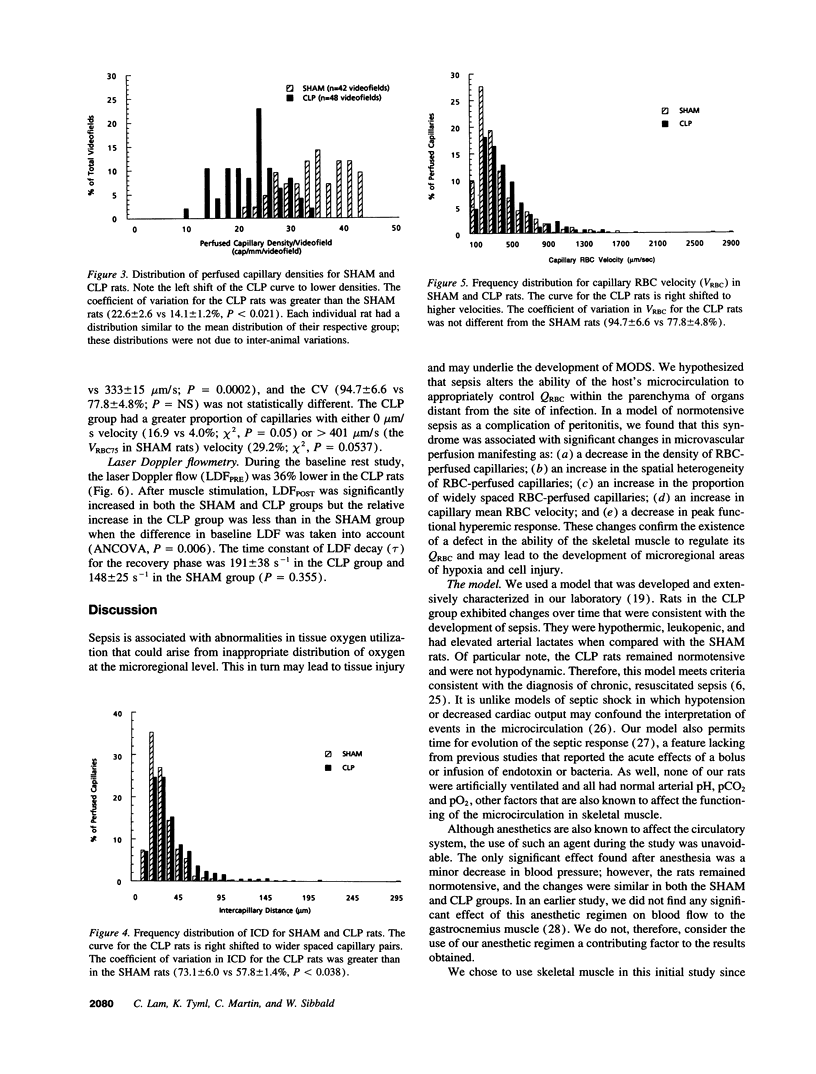
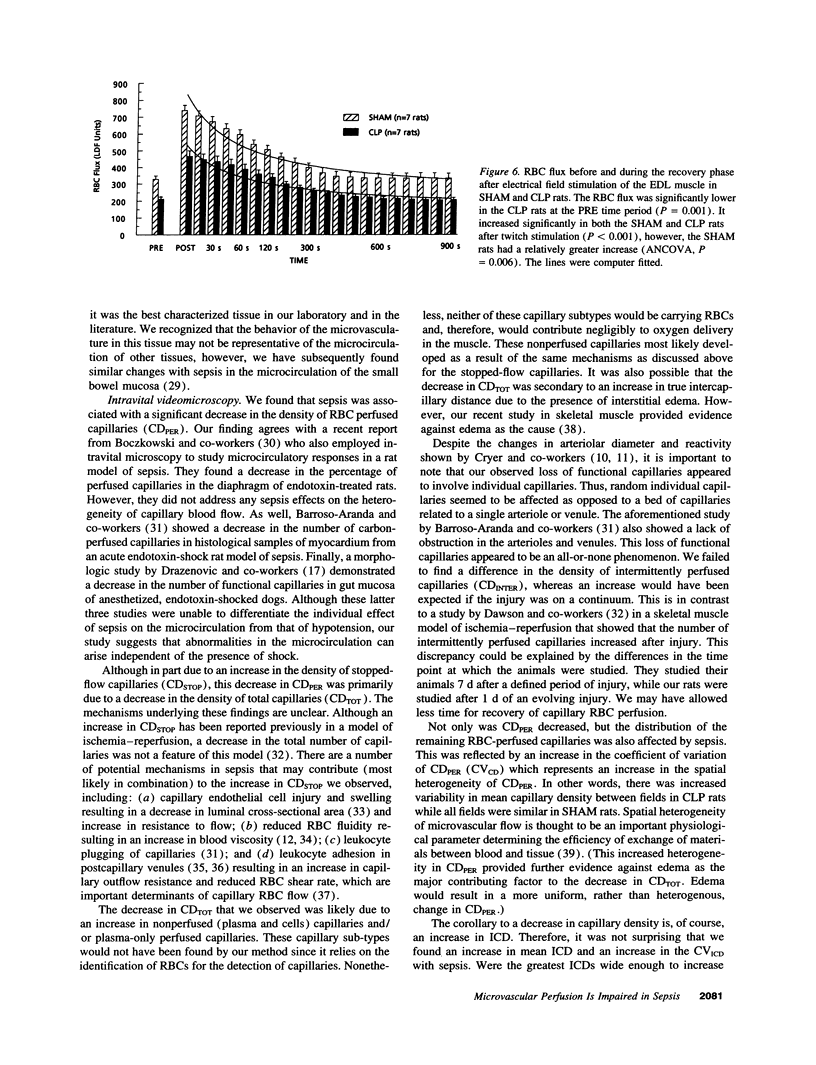
We hypothesized that normotensive sepsis affects the ability of the microcirculation to appropriately regulate microregional red blood cell (RBC) flux. An extensor digitorum longus muscle preparation for intravital study was used to compare the distribution of RBC flux and the functional hyperemic response in SHAM rats and rats made septic by cecal ligation and perforation (CLP). Using intravital microscopy, we found that sepsis was associated with a 36% reduction in perfused capillary density (from 35.3 +/- 1.5 to 22.5 +/- 1.0 capillaries/mm of test line) and a 265% increase in stopped-flow capillaries (from 0.9 +/- 0.2 to 3.3 +/- 0.4 capillaries/mm); the spatial distribution of perfused capillaries was also 72% more heterogeneous. Mean intercapillary distance (ICD) increased 30% (from 25.7 +/- 0.8 to 33.5 +/- 1.6 microns), and the proportion of capillary pairs with intercapillary distances > 33.8 microns (the 75th percentile of ICDSHAM) was greater with sepsis. Mean capillary RBC velocity increased 17% in CLP rats (391 vs 333 microns/s). Laser Doppler flowmetry was used to assess the functional hyperemic response of the extensor digitorum longus muscle before and after a period of maximal twitch contraction designed to increase oxygen demand. RBC flux was 36% lower in the CLP rats at rest. After contraction, RBC flux increased in both SHAM and CLP rats; however, the relative increase was less in the CLP group. We concluded that sepsis affects the ability of the skeletal muscle microcirculation to appropriately distribute RBC flux and to respond to increases in oxygen need.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astiz M. E., Tilly E., Rackow E. D., Weil M. H. Peripheral vascular tone in sepsis. Chest. 1991 May;99(5):1072–1075. doi: 10.1378/chest.99.5.1072. [DOI] [PubMed] [Google Scholar]
- Baker C. H., Davis D. L. Endotoxin effects on capillary transit times of RBC and plasma as measured by indicator dilution. Microvasc Res. 1980 Sep;20(2):242–252. doi: 10.1016/0026-2862(80)90011-4. [DOI] [PubMed] [Google Scholar]
- Baker C. H., Sutton E. T., Zhou Z., Reynolds D. G. Reduced microvascular adrenergic receptor activity due to opioids in endotoxin shock. Circ Shock. 1990 Oct;32(2):101–112. [PubMed] [Google Scholar]
- Baker C. H., Wilmoth F. R. Microvascular responses to E. coli endotoxin with altered adrenergic activity. Circ Shock. 1984;12(3):165–176. [PubMed] [Google Scholar]
- Baker C. H., Wilmoth F. R., Sutton E. T. Reduced RBC versus plasma microvascular flow due to endotoxin. Circ Shock. 1986;20(2):127–139. [PubMed] [Google Scholar]
- Barroso-Aranda J., Schmid-Schönbein G. W., Zweifach B. W., Mathison J. C. Polymorphonuclear neutrophil contribution to induced tolerance to bacterial lipopolysaccharide. Circ Res. 1991 Nov;69(5):1196–1206. doi: 10.1161/01.res.69.5.1196. [DOI] [PubMed] [Google Scholar]
- Bersten A. D., Gnidec A. A., Rutledge F. S., Sibbald W. J. Hyperdynamic sepsis modifies a PEEP-mediated redistribution in organ blood flows. Am Rev Respir Dis. 1990 May;141(5 Pt 1):1198–1208. doi: 10.1164/ajrccm/141.5_Pt_1.1198. [DOI] [PubMed] [Google Scholar]
- Boczkowski J., Vicaut E., Aubier M. In vivo effects of Escherichia coli endotoxemia on diaphragmatic microcirculation in rats. J Appl Physiol (1985) 1992 Jun;72(6):2219–2224. doi: 10.1152/jappl.1992.72.6.2219. [DOI] [PubMed] [Google Scholar]
- Bone R. C., Balk R. A., Cerra F. B., Dellinger R. P., Fein A. M., Knaus W. A., Schein R. M., Sibbald W. J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992 Jun;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Bone R. C. Gram-negative sepsis. Background, clinical features, and intervention. Chest. 1991 Sep;100(3):802–808. doi: 10.1378/chest.100.3.802. [DOI] [PubMed] [Google Scholar]
- Bredle D. L., Samsel R. W., Schumacker P. T., Cain S. M. Critical O2 delivery to skeletal muscle at high and low PO2 in endotoxemic dogs. J Appl Physiol (1985) 1989 Jun;66(6):2553–2558. doi: 10.1152/jappl.1989.66.6.2553. [DOI] [PubMed] [Google Scholar]
- Cryer H. M., Garrison R. N., Harris P. D. Role of muscle microvasculature during hyperdynamic and hypodynamic phases of endotoxin shock in decerebrate rats. J Trauma. 1988 Mar;28(3):312–318. doi: 10.1097/00005373-198803000-00006. [DOI] [PubMed] [Google Scholar]
- Cryer H. M., Garrison R. N., Kaebnick H. W., Harris P. D., Flint L. M. Skeletal microcirculatory responses to hyperdynamic Escherichia coli sepsis in unanesthetized rats. Arch Surg. 1987 Jan;122(1):86–92. doi: 10.1001/archsurg.1987.01400130092014. [DOI] [PubMed] [Google Scholar]
- D'Orio V., Mendes P., Carlier P., Fatemi M., Marcelle R. Lung fluid dynamics and supply dependency of oxygen uptake during experimental endotoxic shock and volume resuscitation. Crit Care Med. 1991 Jul;19(7):955–962. doi: 10.1097/00003246-199107000-00022. [DOI] [PubMed] [Google Scholar]
- Dahn M. S., Wilson R. F., Lange P., Stone A., Jacobs L. A. Hepatic parenchymal oxygen tension following injury and sepsis. Arch Surg. 1990 Apr;125(4):441–443. doi: 10.1001/archsurg.1990.01410160027004. [DOI] [PubMed] [Google Scholar]
- Dawson J. M., Okyayuz-Baklouti I., Hudlickà O. Skeletal muscle microcirculation: the effects of limited blood supply and treatment with torbafylline. Int J Microcirc Clin Exp. 1990 Nov;9(4):385–400. [PubMed] [Google Scholar]
- Drazenovic R., Samsel R. W., Wylam M. E., Doerschuk C. M., Schumacker P. T. Regulation of perfused capillary density in canine intestinal mucosa during endotoxemia. J Appl Physiol (1985) 1992 Jan;72(1):259–265. doi: 10.1152/jappl.1992.72.1.259. [DOI] [PubMed] [Google Scholar]
- Fink M. P., Heard S. O. Laboratory models of sepsis and septic shock. J Surg Res. 1990 Aug;49(2):186–196. doi: 10.1016/0022-4804(90)90260-9. [DOI] [PubMed] [Google Scholar]
- Garrison R. N., Cryer H. M., 3rd Role of the microcirculation to skeletal muscle during shock. Prog Clin Biol Res. 1989;299:43–52. [PubMed] [Google Scholar]
- Gutierrez G. The rate of oxygen release and its effect on capillary O2 tension: a mathematical analysis. Respir Physiol. 1986 Jan;63(1):79–96. doi: 10.1016/0034-5687(86)90032-0. [DOI] [PubMed] [Google Scholar]
- Hersch M., Gnidec A. A., Bersten A. D., Troster M., Rutledge F. S., Sibbald W. J. Histologic and ultrastructural changes in nonpulmonary organs during early hyperdynamic sepsis. Surgery. 1990 Apr;107(4):397–410. [PubMed] [Google Scholar]
- Lübbe A. S., Garrison R. N., Cryer H. M., Alsip N. L., Harris P. D. EDRF as a possible mediator of sepsis-induced arteriolar dilation in skeletal muscle. Am J Physiol. 1992 Mar;262(3 Pt 2):H880–H887. doi: 10.1152/ajpheart.1992.262.3.H880. [DOI] [PubMed] [Google Scholar]
- Martin C. M., Yaghi A., Sibbald W. J., McCormack D., Paterson N. A. Differential impairment of vascular reactivity of small pulmonary and systemic arteries in hyperdynamic sepsis. Am Rev Respir Dis. 1993 Jul;148(1):164–172. doi: 10.1164/ajrccm/148.1.164. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Rubinstein I., Robbins R. A., Koyama S., Joyner W. L., Rennard S. I. Role of neutrophils in endotoxin-mediated microvascular injury in hamsters. J Appl Physiol (1985) 1991 Jul;71(1):307–313. doi: 10.1152/jappl.1991.71.1.307. [DOI] [PubMed] [Google Scholar]
- Olive P. L., Vikse C., Trotter M. J. Measurement of oxygen diffusion distance in tumor cubes using a fluorescent hypoxia probe. Int J Radiat Oncol Biol Phys. 1992;22(3):397–402. doi: 10.1016/0360-3016(92)90840-e. [DOI] [PubMed] [Google Scholar]
- Popel A. S. Theory of oxygen transport to tissue. Crit Rev Biomed Eng. 1989;17(3):257–321. [PMC free article] [PubMed] [Google Scholar]
- Puranapanda V., Hinshaw L. B., O'Rear E. A., Chang A. C., Whitsett T. L. Erythrocyte deformability in canine septic shock and the efficacy of pentoxifylline and a leukotriene antagonist. Proc Soc Exp Biol Med. 1987 Jun;185(2):206–210. doi: 10.3181/00379727-185-42536. [DOI] [PubMed] [Google Scholar]
- Schumacker P. T., Samsel R. W. Oxygen delivery and uptake by peripheral tissues: physiology and pathophysiology. Crit Care Clin. 1989 Apr;5(2):255–269. [PubMed] [Google Scholar]
- Stahl T. J., Alden P. B., Ring W. S., Madoff R. C., Cerra F. B. Sepsis-induced diastolic dysfunction in chronic canine peritonitis. Am J Physiol. 1990 Mar;258(3 Pt 2):H625–H633. doi: 10.1152/ajpheart.1990.258.3.H625. [DOI] [PubMed] [Google Scholar]
- Svensjö E., Erlansson M., van den Bos G. C. Endotoxin-induced increase in leukocyte adherence and macromolecular permeability of postcapillary venules. Agents Actions. 1990 Jan;29(1-2):21–23. doi: 10.1007/BF01964708. [DOI] [PubMed] [Google Scholar]
- Tuchschmidt J., Fried J., Astiz M., Rackow E. Elevation of cardiac output and oxygen delivery improves outcome in septic shock. Chest. 1992 Jul;102(1):216–220. doi: 10.1378/chest.102.1.216. [DOI] [PubMed] [Google Scholar]
- Tuchschmidt J., Oblitas D., Fried J. C. Oxygen consumption in sepsis and septic shock. Crit Care Med. 1991 May;19(5):664–671. doi: 10.1097/00003246-199105000-00013. [DOI] [PubMed] [Google Scholar]
- Tyml K., Budreau C. H. A new preparation of rat extensor digitorum longus muscle for intravital investigation of the microcirculation. Int J Microcirc Clin Exp. 1991 Nov;10(4):335–343. [PubMed] [Google Scholar]
- Tyml K., Ellis C. G. Simultaneous assessment of red cell perfusion in skeletal muscle by laser Doppler flowmetry and video microscopy. Int J Microcirc Clin Exp. 1985;4(4):397–406. [PubMed] [Google Scholar]
- Tyml K. Heterogeneity of microvascular flow in rat skeletal muscle is reduced by contraction and by hemodilution. Int J Microcirc Clin Exp. 1991 Feb;10(1):75–86. [PubMed] [Google Scholar]
- Tyml K., Mathieu-Costello O., Budreau C. H. Distribution of red blood cell velocity in capillary network, and endothelial ultrastructure, in aged rat skeletal muscle. Microvasc Res. 1992 Jul;44(1):1–13. doi: 10.1016/0026-2862(92)90097-9. [DOI] [PubMed] [Google Scholar]
- Tyml K. Red cell perfusion in skeletal muscle at rest and after mild and severe contractions. Am J Physiol. 1987 Mar;252(3 Pt 2):H485–H493. doi: 10.1152/ajpheart.1987.252.3.H485. [DOI] [PubMed] [Google Scholar]



