Abstract
Chronic HBV infections cause hepatocellular carcinoma (HCC). Activities of the HBV HBx protein regulate HBV replication and may contribute to the development of HCC. We previously reported that HBx causes primary rat hepatocytes to exit G0 but stall in G1 phase of the cell cycle; entry into G1 stimulated HBV replication. We now report that the activity of the mitochondria permeability transition pore is required for HBx regulation of cell cycle proteins and HBV replication in primary rat hepatocytes, that progression from G0 to G1 stimulates HBV polymerase activity, and that HBV replication is facilitated by the HBx-induced G1 arrest. HBx stimulation of HBV replication was linked to elevation of the R2 subunit of ribonucleotide reductase. Our studies suggest that HBx uses mitochondrial-dependent calcium signaling to cause hepatocytes to exit G0 but stall in G1 and that this HBx activity alters the cellular environment and stimulates HBV replication.
Keywords: Hepatitis B Virus, HBx, viral replication, calcium, cell cycle, primary hepatocytes
INTRODUCTION
Worldwide, there are over 350 million individuals chronically infected with the human hepatitis B virus (HBV); approximately 25% of chronically HBV-infected individuals will develop hepatocellular carcinoma (HCC) (Beasley et al. 1981; Seeger et al. 2007). The HBV genome is a partially double-stranded DNA genome containing four overlapping open-reading frames (ORFs) that encode the viral polymerase/reverse transcriptase, capsid, envelope, and X (HBx) proteins (Seeger et al. 2007). Although the exact mechanisms that underlie the development of HBV-associated HCC have not been entirely elucidated, the results of many studies suggest that HBV-associated HCC develops in the context of a continual inflammatory response to HBV-infected hepatocytes and is also influenced by expression of HBV proteins such as HBx (Chisari 2000; Seeger et al. 2007).
HBx is a multifunctional protein that can modulate cellular transcription, protein-degradation, calcium signaling, cell cycle, and apoptotic pathways (reviewed in (Bouchard and Schneider 2004)). HBx activities have frequently been studied in transformed or immortalized cell lines, sometimes not of liver origin, and the precise effect of HBx on cellular signal transduction pathways and on HBV replication has varied in different experimental systems (reviewed in (Bouchard and Schneider 2004)). Consequently, the role of HBx during HBV replication in normal hepatocytes, and which HBx activities might contribute to the development of HCC, remains incompletely understood. The results of studies in HBx-transgenic mice suggest that HBx can impact the development of HCC. However, whether HBx is directly oncogenic or a co-factor in HCC progression can vary in different experimental models. For example, in some transgenic mice HBx has been shown to directly cause the formation of liver tumors while in other transgenic mouse models HBx did not directly cause HCC but sensitized mice to hepatocarcinogen treatment (Buendia 1998; Kim et al. 1991; Madden et al. 2001; Slagle et al. 1996; Terradillos et al. 1997; Yu et al. 1999). The reasons for the variation in oncogenic potential in these transgenic mouse models is unclear, but may reflect differences in the method of HBx expression or in the genetic background of the mice used in the study. The results of multiple studies also suggest that HBx strongly influences HBV replication in various tissue culture and in vivo models (Blum et al. 1992; Bouchard et al. 2001a; Keasler et al. 2007; Leupin et al. 2005; Melegari et al. 1998; Tang et al. 2005), and several HBx functions, including regulation of cytosolic calcium signaling, Src kinases, and proteasome activity, as well as interactions with components of the ultraviolet-damaged DNA binding (UVDDB) complex, can affect HBV replication (Bouchard et al. 2001a; Bouchard et al. 2003; Gearhart and Bouchard 2010; Klein et al. 1999; Leupin et al. 2005; Sitterlin et al. 2000; Zhang et al. 2004). HBx might also influence HBV replication by regulating cell proliferation pathways; however, the precise impact of HBx on cell proliferation pathways in normal hepatocytes, and how cell cycle progression influences HBV replication, remains incompletely understood.
Several studies have examined the effect of cell cycle progression on HBV replication. In HepG2.2.15 cells, human hepatoblastoma cells that contain an integrated copy of the HBV genome and can produce infectious HBV, HBV replication was highest in G0/G1, G1, and G2 phases as compared to S phase (Huang et al. 2004; Ozer et al. 1996). One study in HBV-transgenic mice suggested that HBV replicates equally well in resting or proliferating hepatocytes; however, because HBV replication is derived from a copy of the HBV genome that is integrated into mouse chromosomal DNA, whether replication of HBV in the livers of these mice recapitulates all aspects of HBV replication in an authentic HBV infection is unclear (Guidotti et al. 1997). We recently demonstrated that cell cycle progression affects HBV replication in cultured primary rat hepatocytes, a biologically relevant model of normal hepatocytes (Gearhart and Bouchard 2010). In these studies, rat hepatocytes were infected with recombinant adenoviruses that contain a greater-than-unit length copy of the HBV genome to allow HBV replication and then transfected with a p16 over-expression plasmid (Gearhart and Bouchard 2010). p16 prevents progression from G0 to G1 phase of the cell cycle, and its level must be decreased in order for a cell to enter G1 phase (Harper and Brooks 2005; Sherr and Roberts 1999). Over-expressed p16 prevented hepatocytes from exiting G0 and entering G1 and inhibited HBV replication (Gearhart and Bouchard 2010). These results demonstrate that HBV replication requires that quiescent hepatocytes exit G0 and enter G1 phase of the cell cycle. Whether HBV replication is affected by progression beyond G1 phase was not addressed (Gearhart and Bouchard 2010).
HBx can regulate the levels of p16, p21, p27, cyclin D1, cyclin A and cyclin B1, activate the p21 promoter, and increase the activity of CDK2 in various settings; although these responses to HBx expression, which have often been analyzed in immortalized or transformed cell lines, have varied in different experimental systems (Ahn et al. 2001; Chin et al. 2007; Han et al. 2002; Leach et al. 2003; Lee et al. 2002; Mukherji et al. 2007; Park et al. 2006; Park et al. 2000; Qiao et al. 2001). We previously showed that HBx modulates the levels and activities of cell cycle regulatory proteins in cultured, primary rat hepatocytes (Gearhart and Bouchard 2010). We demonstrated that HBx decreases the levels of proteins that inhibit progression into G1 phase, increases the levels of active G1 phase proteins such as p21, p27, and cyclin D1, and stimulates normally quiescent hepatocytes to exit G0 and stall in G1 phase of the cell cycle (Gearhart and Bouchard 2010). The results of these studies were similar to observations in primary mouse hepatocytes where HBx increased the levels of p21 and p27 and decreased cell proliferation (Qiao et al. 2001). We also demonstrated that cytosolic calcium signaling is required for HBx modulation of cell cycle regulatory proteins in primary rat hepatocytes (Gearhart and Bouchard 2010). These results showed that HBx modulates the levels and activities of cell cycle regulatory proteins to cause quiescent hepatocytes to exit G0 and arrest in G1 phase via a calcium-dependent mechanism.
In addition to being important for HBx modulation of cell proliferation, cytosolic calcium signaling also stimulates HBV replication in HepG2 cells, a human hepatoblastoma cell line (Bouchard et al. 2001a). HBx can increase the basal level of cytosolic calcium in HepG2 and Chang liver cells (Chami et al. 2003; McClain et al. 2007; Oh et al. 2003), and calcium mobilizing agents such as valinomycin, thapsigargin, and glibenclamide rescue the replication of a HBx-deficient mutant HBV (Bouchard et al. 2001a; Bouchard et al. 2003). Furthermore, treatment of HepG2 cells with calcium-chelating agents such as BAPTA-AM inhibited HBV replication (Bouchard et al. 2001a; Bouchard et al. 2003). Although the mechanism underlying HBx modulation of intracellular calcium remains undefined, the results of several studies have suggested that the mitochondria permeability transition pore (MPTP) may play a role. The MPTP is a pore present in mitochondria that controls the influx and efflux of ions, including calcium, from the mitochondrial matrix (Orrenius et al. 2003). The ability of HBx to increase the level of basal cytosolic calcium in HepG2 cells was blocked by cyclosporin A (CsA), an inhibitor of the MPTP (McClain et al. 2007). CsA treatment also inhibited HBV replication in HepG2 cells, linking MPTP activity and associated regulation of cellular calcium signaling to HBx modulation of HBV replication (Bouchard et al. 2001a). Whether HBV replication or HBx regulation of cell cycle proteins in normal hepatocytes is dependent on MPTP functions has not been addressed.
In studies reported here, we have examined the effect of cellular calcium signaling and cellular proliferation on HBV replication in cultured, primary rat hepatocytes, a model system for studying normal hepatocyte physiology and human liver diseases (Bingham et al. 1998; Chitturi and Farrell 2001; Diot et al. 1992; James et al. 2003; Rodriguez-Garay 2003). These studies were conducted with HBx that was expressed in the absence of other HBV proteins and in the context of HBV replication. Our results demonstrate that both cytosolic calcium signaling and MPTP activity are required for HBx stimulation of HBV replication and modulation of cell cycle regulatory proteins in primary hepatocytes. We demonstrate that HBx-induced elevation of p21 and p27, which are active in G1 phase, but prevent progression into S phase of the cell cycle, is required for the G1 phase arrest (Cheng et al. 1999; Sherr and Roberts 1999). We also show that an HBx-mediated G1 phase arrest is required for HBV replication in cultured, primary rat hepatocytes. While the purpose of this G1 phase arrest remains undefined, it may involve increasing the pool of available deoxynucleotide triphosphates (dNTPs) required for HBV replication by activating enzymes that synthesize dNTPs. One enzyme that synthesizes dNTPs is ribonucleotide reductase (Reichard 1987). We show that HBx increases the level of the R2 catalytic subunit of ribonucleotide reductase and that this increase in the level of R2 stimulates HBV replication. Interestingly, we observed that the activity of the HBV polymerase requires that hepatocytes progress from G0 to G1 but remained active when hepatocytes were allowed to progress into S phase even though HBV replication was significantly diminished. Our results suggest that HBV replication in primary hepatocytes requires HBx regulation of cytosolic calcium signaling pathways and MPTP activity to induce quiescent hepatocytes to exit G0 and enter and stall in G1 phase. HBx regulation of cell cycle progression, while beneficial for HBV replication, may provide a mechanism that alters hepatocyte physiology and contributes to the development of HBV-associated HCC.
RESULTS
Confirmation of authentic, differentiated hepatocytes
Studies were conducted in cultured, primary rat hepatocytes. Hepatocyte morphology and expression of differentiation-specific markers were assayed throughout the time course of our studies as previously described (Gearhart and Bouchard 2010). Briefly, RT-PCR analysis was performed on freshly isolated hepatocytes, and hepatocytes cultured for 72 hours, the time frame for our experiments. Albumin (ALB), transferrin (TFN), hepatocyte nuclear factor 4 (HNF4), and connexin 26 (CNX26) were used as accepted markers of differentiated hepatocytes (Block et al. 1996; Gearhart and Bouchard 2010; Piechocki et al. 1999; Runge et al. 2000). Additionally, we examined connexin 43 (CNX43), which is expressed in dedifferentiated hepatocytes, Kupffer cells, stellate cells, and liver sinusoidal endothelial cells. CNX43 was used to assess the purity of hepatocyte cultures (Gearhart and Bouchard 2010; Piechocki et al. 1999). Expression of ALB, TFN, HNF4, and CNX26 was observed in freshly isolated hepatocytes and in hepatocytes cultured for 72 hours ((Gearhart and Bouchard 2010) and Supplemental Fig. 1A–B). CNX43 expression was not detected in freshly isolated hepatocytes or hepatocytes that were maintained in culture for 72 hours ((Gearhart and Bouchard 2010; Rezvan et al. 2004) and Supplemental Fig. 1C). These results demonstrate that the highly purified, primary rat hepatocytes remain differentiated throughout the time course of our experiments.
Cytosolic calcium is required for HBV replication in primary rat hepatocytes
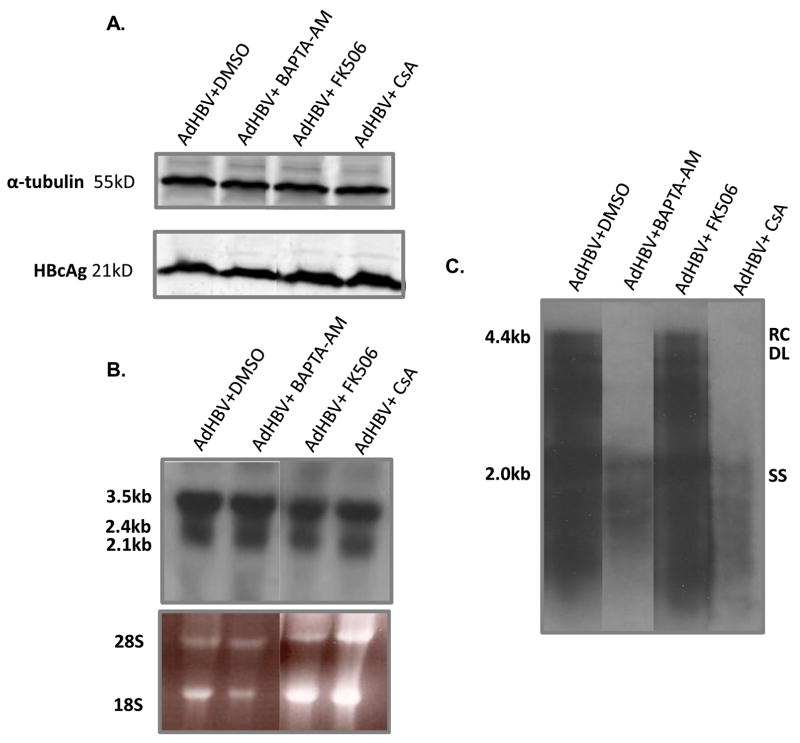
HBV replication in HepG2 cells is stimulated by cytosolic calcium signaling; however, the role of cytosolic calcium in HBV replication in primary hepatocytes has not been examined (Bouchard et al. 2001b). We analyzed the effect of the cytosolic calcium chelator, BAPTA-AM, on HBV replication in primary rat hepatocytes. Cultured, primary rat hepatocytes were infected with AdHBV and treated for 48 hours with BAPTA-AM. BAPTA-AM dramatically reduced the level of HBV replication but did not affect the levels of the HBV core protein (HBcAg) or viral RNAs (Fig. 1). These results show that HBV replication in primary hepatocytes requires modulation of intracellular calcium, similar to observations in HepG2 cells (Bouchard et al. 2001b).
FIG 1.
Cytosolic calcium and MPTP activity are required for HBV replication in primary rat hepatocytes: Hepatocytes were infected with AdHBV 24 hours after plating and treated with 50μM BAPTA-AM, 4μM CsA, 4μM FK506 or vehicle control. (A) Lysates were collected 72 hours post-infection, resolved via SDS-PAGE, and subjected to Western analysis for α-tubulin and HBcAg. (B) RNA was isolated from hepatocytes 72 hours post-infection, and viral RNAs were monitored via Northern blot analysis. (C) 72 hours after infection, HBV replication was analyzed with a standard HBV replication assay (see Materials and Methods); RC, relaxed circular; DL, double-stranded linear; SS, single stranded). Results shown are representative samples from at least 3 experiments performed in duplicate.
MPTP activity is required for HBV replication in primary rat hepatocytes
The results of previous studies demonstrated that HBx modulation of basal calcium levels requires modulation of the mitochondria permeability transition pore (MPTP), and MPTP activity is involved in HBV replication in HepG2 cells (Bouchard et al. 2001b; McClain et al. 2007). We next examined whether MPTP activity is required for HBV replication in primary rat hepatocytes. Primary rat hepatocytes were infected with AdHBV and treated for 48 hours with the MPTP inhibitor, cyclosporin A (CsA) (Clapham 1995). CsA treatment did not affect the level of HBcAg or HBV RNAs in AdHBV-infected primary rat hepatocytes (Fig. 1A and B) but did decrease the level of HBV replication (Fig. 1C). Because CsA inhibits both the MPTP and calcineurin, FK506, a calcineurin inhibitor that does not affect the MPTP, was also used to show that CsA inhibition of the MPTP was directly responsible for inhibiting HBV replication in primary rat hepatocytes. Similar to CsA treatment, FK506 had no effect on the level of viral RNAs or the level of HBcAg (Fig. 1A and B), but, unlike CsA treatment, FK506 did not alter the level of HBV replication (Fig. 1C). These results suggest that MPTP activity is required for HBV replication in cultured, primary rat hepatocytes.
HBx modulation of cell cycle proteins requires MPTP activity
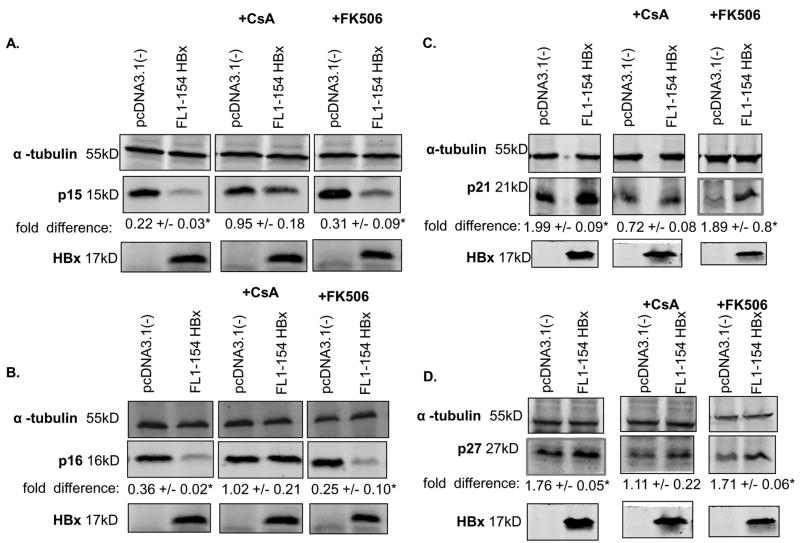
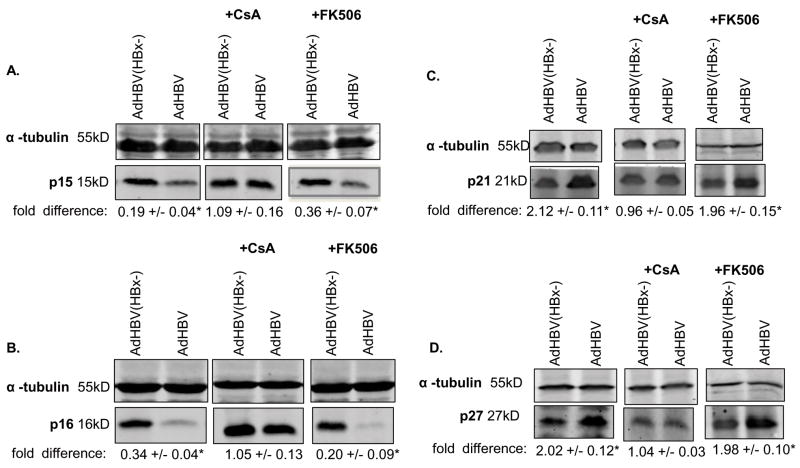
We recently demonstrated that HBx- and HBV-modulation of cell cycle regulatory proteins is calcium-dependent (Gearhart and Bouchard 2010). Because the results of previous studies demonstrated that HBx modulation of basal calcium levels was dependent on the activities of the MPTP, we asked whether HBx uses an MPTP-dependent pathway to regulate the levels of cell cycle proteins in cultured, primary rat hepatocytes. Cells were transfected with HBx (FL1-154 HBx) or vector control (pcDNA3.1(-)), or infected with AdHBV or AdHBV(HBx-), and treated for 18 hours with CsA. Hepatocytes were collected 24 hours post-transfection/infection and the levels of the cell cycle proteins p15, p16, p21 and p27 were examined via Western blot analysis. In the presence of CsA, HBx or AdHBV did not decrease the levels of p15 and p16 and did not increase the levels of p21 and p27, suggesting that HBx-modulation of p15, p16, p21, and p27 levels is through a MPTP-dependent mechanism (Fig. 2A–D and 3 A–D). Unlike CsA treatment, FK506 treatment of hepatocytes transfected with HBx (FL1-154 HBx) or infected with AdHBV did not inhibit the ability of HBx or AdHBV to decrease the levels of p15 and p16 or increase the levels of p21 and p27 (Fig. 2A–D and 3A–D). These results suggest that HBx modulates the levels of cell cycle regulatory proteins through an MPTP-dependent mechanism.
FIG 2.
HBx requires MPTP activity to modulate the levels of cell cycle regulatory proteins: Hepatocytes were transfected with FL1-154X or pcDNA3.1(-). Six hours after transfection, cells were treated with 4μM CsA, 4μM FK506 or vehicle control. (A–D) Lysates were collected 24 hours post-transfection, resolved via SDS-PAGE and subjected to Western blot analysis for α-tubulin, p15, p16, p21, p27, or HBx. Results shown are representative samples from at least 3 experiments performed in duplicate. * fold difference plus/minus standard error between FL1-154X and pcDNA3.1(-) was statistically significant as determined using a Student’s t-test (p ≤ 0.05).
FIG 3.
HBx, when expressed in the context of HBV replication, requires MPTP activity to modulate the levels of cell cycle regulatory proteins: Hepatocytes were infected with AdHBV or AdHBV(HBx-). Six hours after infection, cells were treated with 4μM CsA, 4μM FK506 or vehicle control. (A–D) Lysates were collected 24 hours post-infection, resolved via SDS-PAGE and subjected to Western blot analysis for α-tubulin, p15, p16, p21, or p27. Results shown are representative samples from at least 3 experiments performed in duplicate. * fold difference plus/minus standard error between AdHBV(HBx-) and AdHBV was statistically significant as determined using a Student’s t-test (p ≤ 0.05).
HBV replication requires that primary rat hepatocytes stall in G1 phase
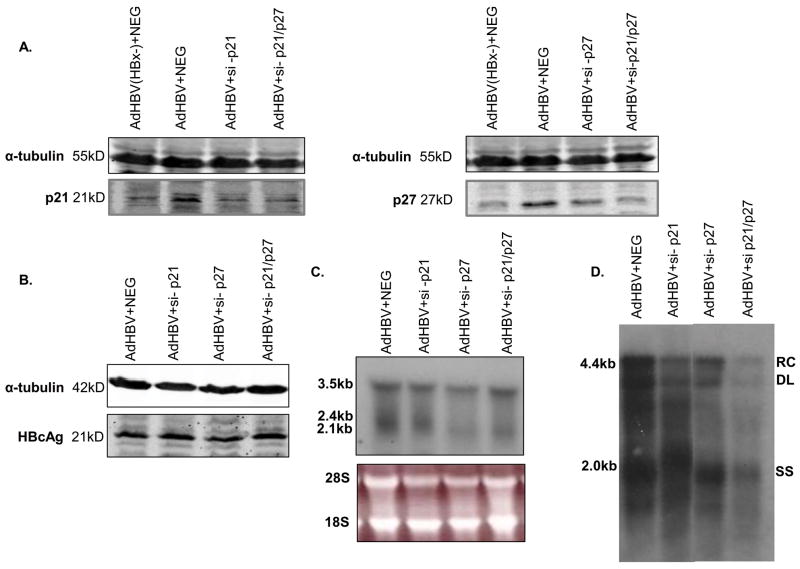
We previously demonstrated that HBx, expressed in the absence of other HBV proteins and in the context of replicating HBV, induces normally quiescent hepatocytes to exit G0 and enter G1 phase and that this HBx activity is necessary for efficient HBV replication in cultured primary rat hepatocytes. We also previously showed that HBx expression elevates the levels of p21 and p27, causing hepatocytes to stall before entering S phase (Gearhart and Bouchard 2010). We next sought to determine whether stalling hepatocytes in G1 phase and preventing progression into S phase is required for HBV replication in cultured primary rat hepatocytes. To prevent HBV-expressing primary hepatocytes from stalling in G1 phase, we knocked down both p21 and p27 proteins with small interfering (si) RNAs. siRNA transfection efficiency was approximately 40%, as measured by parallel transfection of a fluorescently labeled siRNA control (data not shown), and the efficiency of each siRNA knockdown was confirmed by Western blot analysis (Fig. 4A). Primary rat hepatocytes were infected with AdHBV and transfected with siRNAs for p21 (si-p21), p27 (si-p27), both p21 and p27 (si-p21/p27), or a negative control, scrambled siRNA sequence (NEG), and stained for BrdU incorporation (see Materials and Methods). The results in Table 2 illustrate the percent of cells found in S phase, as measured by the amount of BrdU incorporation. Hepatocytes infected with AdHBV and transfected with the scrambled siRNA sequence, si-p21 alone, or si-p27 alone showed little BrdU incorporation (Table 2); a low level of BrdU incorporation indicates that the cells have not progressed into S phase, and remain in G1 phase. However, hepatocytes that were infected with AdHBV and transfected with both si-p21 and si-p27 (si-p21/p27) showed a significant increase in BrdU-positive cells (Table 2). These results indicate that HBV induces primary rat hepatocytes to move into the cell cycle, but requires elevation of both p21 and p27 to stall the cells in G1 phase; without elevation of p21 and p27, HBx-expressing hepatocytes progress into S phase (Table 2). It is important to note that to confirm these results, we repeated these experiments with a second set of siRNAs, si-p21b and si-p27b, and similar results were observed both in efficiency of protein knockdown and BrdU incorporation (Table 2 and Supplemental Fig. 2A).
FIG 4.
HBV replication requires that cells stall in G1 phase: Hepatocytes were infected with AdHBV 24 hours after plating and transfected with si-p21, si-27, both si-p21 and si-p27 (si-p21/p27), or a negative control siRNA (NEG), or AdHBV(HBx-) + NEG (A only). (A–B) Lysates were collected 72 hours post-infection/transfection, resolved via SDS-PAGE, and subjected to Western analysis for α-tubulin, p21, p27 and HBcAg. (C) RNA was isolated from hepatocytes 72 hours post-infection, and viral RNAs were monitored via Northern blot analysis. (D) 72 hours after infection, HBV replication was analyzed with a standard HBV replication assay (see Materials and Methods); RC, relaxed circular; DL, double-stranded linear; SS, single stranded). Results shown are representative samples of at least 3 experiments performed in duplicate.
Table 2.
| % BrdU Negative | % BrdU Positive | |
|---|---|---|
| Untreated | 83.7 +/− 0.9% | 16.3 +/− 0.7% |
| AdHBV + Ø | 87.3 +/− 0.4% | 12.6 +/− 0.4% |
| AdHBV + NEG | 87.5 +/− 4.2% | 12.5 +/− 4.2% |
| AdHBV + si-p21 | 85.9 +/− 0.2% | 14.1 +/− 0.2% |
| AdHBV + si-p27 | 82.5 +/− 2.1% | 17.5 +/− 1.5% |
| AdHBV + si-p21b | 80.9 +/− 1.8% | 18.9 +/− 2.1% |
| AdHBV + si-p27b | 87.7 +/− 2.7% | 11.7 +/− 3.0% |
| AdHBV + si-p21/p27 | 67.3 +/− 0.8% | 32.7 +/− 0.8% |
| AdHBV + si-p21/p27b | 63.6 +/− 1.6% | 35.2 +/− 1.9% |
We next examined the effect of inhibiting the ability of hepatocytes to stall in G1 phase on HBV replication. The results of Western blot analysis demonstrate that knocking down p21 and p27, either alone or in combination, does not affect the levels of the HBcAg (Fig. 4B). We additionally examined the levels of viral RNAs in primary rat hepatocytes. In cells that were infected with AdHBV and transfected with the scrambled siRNA sequence (NEG), si-p21, si-p27, or si-p21/p27, we observed little to no effect on the levels of HBV RNAs as measured in Northern blot analysis (Fig. 4C). However, the level of HBV replication in AdHBV-infected hepatocytes that were transfected with si-p21/p27 was markedly reduced as compared to hepatocytes transfected with the scrambled siRNA sequence, si-p21 alone, or si-p27 alone (Fig. 4D). These results were confirmed using a second set of siRNAs (si-p21b, si-p27b, and si-p21/p27b; Supplemental Fig. 2B-D). These results show that HBV replication is reduced in primary rat hepatocytes when the cells are in S phase of the cell cycle. Additionally, these results are similar to results in HepG2.2.15 cells where HBV replication was low in S phase and high in G1 phase (Huang et al. 2004; Ozer et al. 1996); however, this is the first demonstration that efficient HBV replication requires that primary hepatocytes remain in G1.
HBV replication requires HBx modulation of ribonucleotide reductase
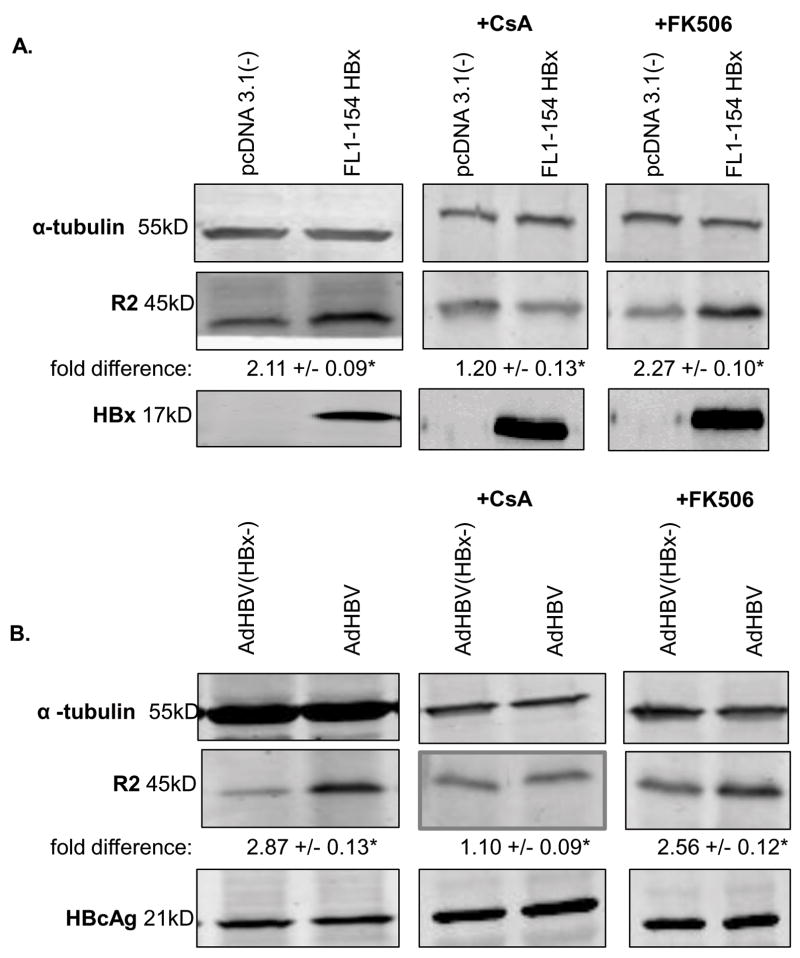
The results described above support the notion that HBV replication requires that hepatocytes do not progress into S phase of the cell cycle. Although there are likely to be many reasons that HBV replication requires that quiescent hepatocytes exit G0 and enter G1 phase, one reason that HBx causes hepatocytes to enter G1 phase may be to increase the pool of available deoxynucleotide triphosphates (dNTPs). HBV requires host cell dNTPs to replicate its genome. dNTPs are present at low levels in quiescent cells, but as the cell approaches S phase, the levels of dNTPs are increased (Yamashita and Emerman 2006). Ribonucleotide reductase, an enzyme responsible for synthesizing dNTPs, consists of two subunits, a constitutively expressed R1 subunit and the catalytic R2 subunit; the R2 subunit is only synthesized as cells approach S phase and prepare for DNA replication (Chabes and Thelander 2000; Reichard 1987). HBx-induced progression of hepatocytes into G1 phase, but inhibition of progression into S phase, could induce the production of dNTPs while preventing competition with the host cell, which would also require dNTPs for DNA replication in S phase. To determine if HBx affects the levels of the R2 subunit of ribonucleotide reductase, primary rat hepatocytes were transfected with HBx (FL1-154 HBx) or vector control (pcDNA(3.1-)), or infected with AdHBV or AdHBV(HBx-), and collected 24 hours post-transfection/infection. The level of R2 expression was examined with Western blot analysis. HBx, both alone and in the context of HBV replication, increased the level of R2 in primary rat hepatocytes as compared to controls (Fig. 5A–B). These results are similar to a recent report showing that HBV increases R2 mRNA levels and the concentration of intracellular dNTPs in HepG2.2.15 cells (Cohen et al. 2010).
FIG 5.
HBx, alone and in the context of HBV replication, increases the level of R2 in primary rat hepatocytes via a MPTP dependent mechanism: (A) Primary rat hepatocytes were transfected with pcDNA3.1(-) or FL1-154X 24 hours post-isolation, and treated with 4μM CsA, 4μM FK506, or vehicle control for 18 hours. 24 hours after transfection, hepatocytes were collected and resolved via SDS-PAGE, and subjected to Western analysis for α-tubulin, R2, and HBx. (B) Primary rat hepatocytes were infected with AdHBV(HBx-) or AdHBV recombinant adenoviruses 24 hours after plating and treated with 4uM CsA, 4uM FK506 or DMSO for 18 hours. 24 hours post-infection, hepatocytes were collected and resolved via SDS-PAGE, and subjected to Western analysis for α-tubulin, R2, and HBcAg. Results shown are representative samples from at least 3 experiments performed in duplicate. * fold difference plus/minus standard error between FL1-154X and pcDNA3.1(-), or AdHBV(HBx-) and AdHBV was statistically significant as determined using a Student’s t-test (p ≤ 0.05).
We next examined the mechanism underlying the HBx-induced increased level of the R2 subunit of ribonucleotide reductase. R2 is an E2F target gene, and E2F transcriptional activity is modulated by activity of cyclin D1/CDK4 (reviewed in (Harper and Brooks 2005)). The results of the studies described above demonstrated that HBx modulates cell cycle regulatory proteins using an MPTP-dependent mechanism (Fig. 3–4). To determine whether MPTP activity was involved in HBx modulation of R2, hepatocytes transfected with HBx (FL1-154 HBx) or vector control (pcDNA(3.1-)), or infected with AdHBV or AdHBV(HBx-), were treated with CsA for 18 hours; hepatocytes were collected 24 hours post-transfection/infection. CsA inhibited HBx-induction of R2 when HBx was expressed alone and in the context of HBV replication (Fig. 5A–B). FK506 had no effect on HBx induction of R2 when HBx was expressed alone and in the context of HBV replication (Fig. 5A–B). These results suggest that HBx modulates the level of R2, both alone and in the context of HBV replication, using an MPTP-dependent mechanism.
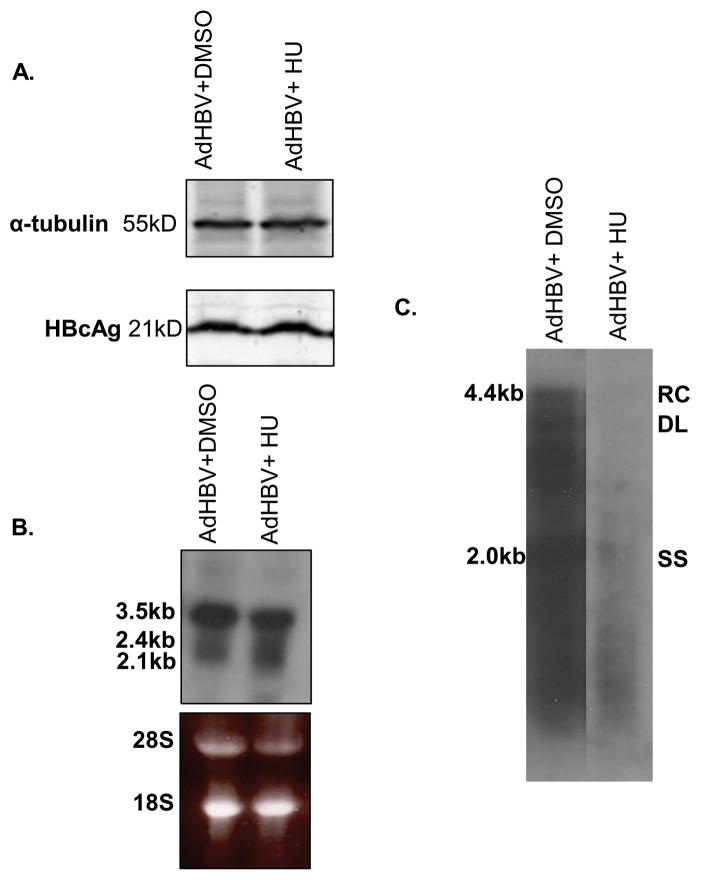
We next used two approaches to determine whether HBx modulation of ribonucleotide reductase activity was required for HBV replication. First, hepatocytes were infected with AdHBV and treated with hydroxyurea (HU) for 48 hours. HU arrests cells at the G1/S phase border by inhibiting ribonucleotide reductase (Thelander and Reichard 1979). When HBV replication was examined via Southern blot analysis, we observed that HU treatment reduced the level of HBV replication as compared to control-treated hepatocytes (Fig. 6C). Interestingly, HU did not affect the level of viral proteins or RNAs (Fig. 6A–B). These results suggest that HBV requires the activity of ribonucleotide reductase for HBV replication.
FIG 6.
HBV replication is inhibited by hydroxyurea treatment in primary rat hepatocytes: Hepatocytes were infected with AdHBV 24 hours after plating and treated with 100mM hydroxyurea (HU) or vehicle control. (A) Lysates were collected 72 hours post-infection, resolved via SDS-PAGE, and subjected to Western analysis for α-tubulin and HBcAg. (B) RNA was isolated from hepatocytes 72 hours post-infection, and viral RNAs were monitored via Northern blot analysis. (C) 72 hours after infection, HBV replication was analyzed with a standard HBV replication assay (see Materials and Methods); RC, relaxed circular; DL, double-stranded linear; SS, single stranded). Results shown are representative samples from at least 3 experiments performed in duplicate.
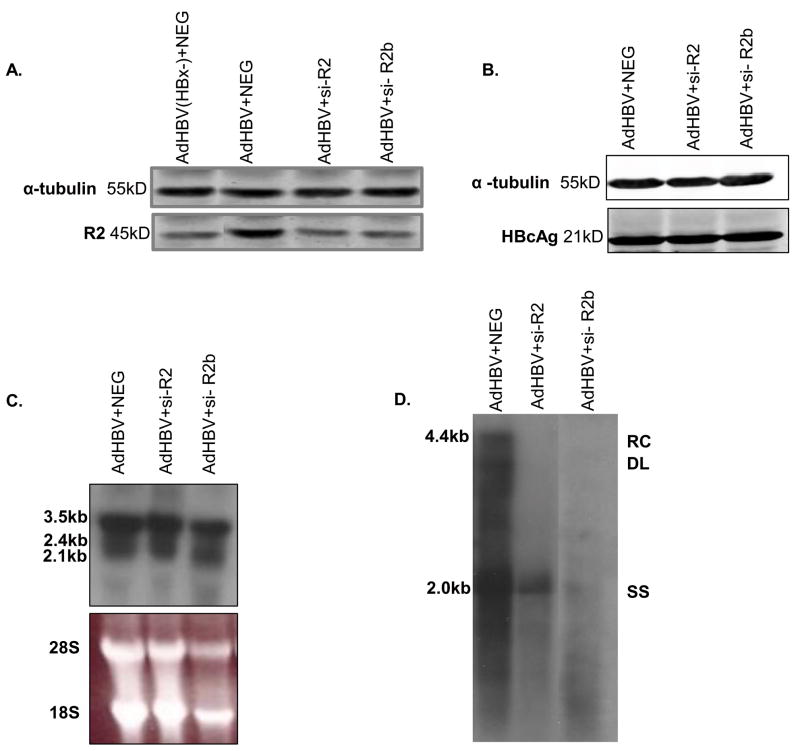
Because the mechanism of action for HU has not been precisely defined, our second approach was to specifically knockdown the level of the R2 catalytic subunit with siRNAs. In these studies, AdHBV-infected hepatocytes were transfected with a R2-specific siRNA (si-R2) or a scrambled, negative control siRNA sequence (NEG) and collected 72 hours post-infection (Fig. 7A). si-R2 mediated knockdown of R2 expression did not affect the level of HBV core protein or RNAs in primary rat hepatocytes (Figs. 7B–C); however, R2 knockdown dramatically reduced the level of HBV replication (Fig. 7D). Similar results were obtained using a second siRNA, si-R2b, to knock down R2 (Fig. 7D). These observations support the notion that HBV replication requires elevation of the R2 catalytic subunit of ribonucleotide reductase. Overall, these results suggest that HBV replication stimulates cells to enter G1 phase of the cell cycle, though stall before entry into S phase, to stimulate ribonucleotide reductase activity while preventing competition with the cell’s replication machinery. This is the first evidence that HBV requires a G1 phase block and elevation of the R2 catalytic subunit of ribonucleotide reductase for HBV replication in primary hepatocytes.
FIG 7.
HBV replication requires the dNTP-synthesizing enzyme ribonucleotide reductase: (A) Hepatocytes were infected with AdHBV 24 hours after plating and transfected with si-R2, si-R2b, or a negative control siRNA (NEG), or AdHBV(HBx-) + NEG (A only). Lysates were collected 72 hours post-infection/transfection, resolved via SDS-PAGE, and subjected to Western analysis for α-tubulin, R2, and HBcAg. (C) RNA was isolated from hepatocytes 72 hours post-infection, and viral RNAs were monitored via Northern blot analysis. (D) 72 hours after infection, HBV replication was analyzed with a standard HBV replication assay (see Materials and Methods); RC, relaxed circular; DL, double-stranded linear; SS, single stranded). Results shown are representative samples from at least 3 experiments performed in duplicate.
Cytosolic calcium, MPTP activity, and cell cycle entry are required for HBV polymerase activation in primary rat hepatocytes
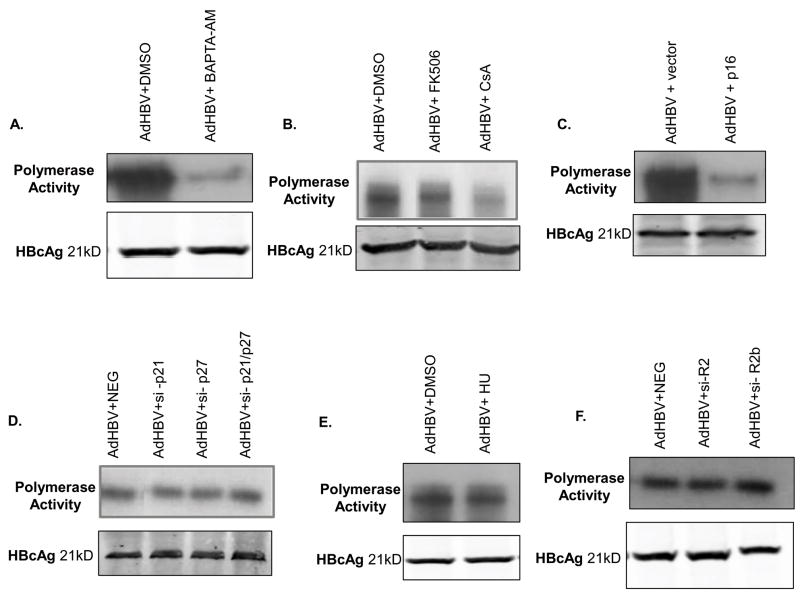
The results described above suggest that modulation of cytosolic calcium, cell proliferation, and MPTP activity are required for HBV replication. However, how these pathways stimulate HBV replication is unknown. To begin to address this, we examined whether cytosolic calcium signaling, MPTP activity, G0 to G1 phase progression, and stalling in G1 phase are required for activation of the HBV polymerase; the HBV polymerase must be active in order for HBV DNA replication to occur (reviewed in (Seeger et al. 2007)). To examine the effect of cytosolic calcium, MPTP activity, and cell proliferation on the HBV polymerase, we performed an endogenous polymerase assay as previously described (Bouchard et al. 2003; Bouchard et al. 2006; Bouchard et al. 2001b). We initially analyzed the role of cytosolic calcium and MPTP activity on activation of the HBV polymerase. Treating primary rat hepatocytes with the intracellular calcium chelating agent, BAPTA-AM, or the MPTP inhibitor, CsA, significantly reduced the activity of the HBV polymerase, while FK506 had no effect on HBV polymerase activity (Fig. 8A–B). These results demonstrate that both cytosolic calcium and activities of the MPTP are required for activation of the HBV polymerase in cultured, primary rat hepatocytes.
Fig. 8.
Activation of the HBV polymerase requires cytosolic calcium, MPTP activity and cell cycle entry in cultured, primary rat hepatocytes. Hepatocytes were infected with AdHBV 24 hours after plating and treated with vehicle control or (A) 50μM BAPTA-AM, (B) 4μM CsA or 4μM FK506, or (E) 100mM HU. (C) Hepatocytes were infected with AdHBV 24 hours after plating and co-transfected with a p16 overexpression plasmid. (D) Hepatocytes were infected with AdHBV 24 hours after plating and co-transfected with si-p21, si-p27, si-p21/27 or a negative control siRNA (NEG). (F) Hepatocytes were infected with AdHBV 24 hours after plating and co-transfected with si-R2, si-R2b, or a negative control siRNA (NEG). (A–F) Hepatocytes were collected 72 hours post-infection, and the activity of the HBV polymerase was determined via an endogenous polymerase assay (see Materials and Methods). Western blot analysis for HBcAg was performed on isolated core particles and used as a control for equal isolation of HBV core particles.
We next determined whether exit of quiescent hepatocytes from G0, entry into G1 phase, and stalling in G1 phase also impacted the activity of the HBV polymerase. Hepatocytes were infected with AdHBV and transfected with a scrambled siRNA sequence (NEG), si-p21, si-p27, si-p21/p27, or si-R2, or treated with HU. The results of the polymerase assay demonstrate that hepatocytes that could no longer arrest in G1 phase, or that could not activate ribonucleotide reductase or had reduced levels of the R2 catalytic subunit of ribonucleotide reductase had no deficiencies in HBV polymerase activity even though HBV replication was severely diminished, suggesting that stalling in G1 phase and ribonucleotide reductase are not required for activation of the HBV polymerase (Fig. 8D–F). These observations were also observed with a second set of siRNAs (Supplemental Fig. 2D). We next analyzed whether entry into G1 phase of the cell cycle, which we previously showed is required for HBV replication in primary rat hepatocytes (Gearhart and Bouchard 2010), is also required for activation of the HBV polymerase. In order for cells to leave quiescence and enter G1 phase of the cell cycle, p16 must be degraded (reviewed in (Harper and Brooks 2005; Sherr and Roberts 1999)). Primary rat hepatocytes were infected with AdHBV and transfected with a p16 over-expression plasmid, which we previously showed prevents cultured primary rat hepatocytes from exiting G0 and entering G1 phase (Gearhart and Bouchard 2010), and hepatocytes were collected 72 hours post-infection. The results of the endogenous polymerase assay demonstrate that when p16 is overexpressed there is a significant reduction in HBV polymerase activity as compared to control transfected, AdHBV-infected hepatocytes (Fig. 8C). Taken together, these results show that cytosolic calcium, MPTP activity, and entry into G1 phase of the cell cycle are required for activation of the HBV polymerase in cultured primary rat hepatocytes and that once hepatocytes have entered G1 phase, inhibition of HBV replication by progression of cells into S phase or reduction of ribonucleotide reductase activity occurs even though the HBV polymerase is activated.
DISCUSSION
Chronic infections with the hepatitis B virus (HBV) are associated with life-threatening liver diseases such as hepatocellular carcinoma (HCC) (Seeger et al. 2007). The development of HBV-associated HCC is believed to involve a combination of the continual inflammatory response to HBV-infected hepatocytes in individuals with a chronic HBV infection and activities of HBV proteins such as the HBV X protein (HBx). HBx is a multifunctional protein that can regulate cellular transcription, protein degradation, calcium signaling, proliferation, and apoptotic pathways (reviewed in (Benhenda et al. 2009; Bouchard and Schneider 2004)); the results of many studies also suggest that HBx is important for HBV replication (Bouchard et al. 2001b; Clippinger et al. 2009; Keasler et al. 2007; Melegari et al. 1998; Tsuge et al. 2010). In the studies described here, we have identified HBx activities that are required for HBV replication in cultured, primary rat hepatocytes, a biologically relevant hepatocyte system. These studies assessed the activities of HBx, when it was expressed alone and in the context of HBV replication. Although in some studies HBx was expressed at higher levels from a plasmid then would be expected when HBx is under the control of its endogenous promoter, our observations are unlikely to be an artifact of HBx over-expression because we observed similar effects when HBx was expressed in the context of HBV replication. Importantly, our studies link calcium signaling and MPTP activity to HBx modulation of cell cycle progression and HBV replication in primary hepatocytes. Significantly, these studies also demonstrate that HBx modulation of cell cycle progression is required to activate the HBV polymerase while establishing a favorable cellular environment for HBV replication.
One fundamental HBx activity is modulation of cellular calcium signaling pathways. HBx expression increases the basal level of cytosolic calcium in HepG2 cells, and this HBx activity is blocked by treatment with cyclosporin A (CsA), a mitochondria permeability transition pore (MPTP) inhibitor (McClain et al. 2007). Involvement of the MPTP in HBx activities is consistent with the observed co-localization of HBx and mitochondria and the reported interaction of HBx and the voltage dependent anion channel (VDAC) 3, a component of the MPTP (Clippinger and Bouchard 2008; Huh and Siddiqui 2002; McClain et al. 2007; Rahmani et al. 2000). Cytosolic calcium signaling and activities of the MPTP also stimulate HBV replication in HepG2 cells (Bouchard et al. 2001b), and we recently reported that HBx modulation of cell proliferation in cultured, primary rat hepatocytes requires cytosolic calcium signaling (Gearhart and Bouchard 2010). In the studies described here, we assessed the role of cytosolic calcium and MPTP activity in HBx regulation of cell cycle progression and HBV replication in cultured, primary rat hepatocytes. Treatment with the intracellular calcium chelator BAPTA-AM and the MPTP inhibitor CsA decreased the activity of the HBV polymerase and reduced HBV replication while not affecting the levels of HBV core protein or HBV RNAs (Figs. 1 and 8), suggesting that HBx activation of the HBV polymerase and stimulation of HBV replication in primary hepatocytes requires cytosolic calcium signaling and MPTP activity. We previously showed that calcium signaling is also required for HBx modulation of cell cycle regulatory proteins in rat hepatocytes and demonstrated here that CsA blocked the ability of HBx, alone and in the context of HBV replication, to decrease the levels of p15 and p16, cell cycle proteins that inhibit progression into G1 phase, and to increase the levels of p21 and p27, active G1 phase proteins that can block progression into S phase (Figs. 2 and 3). These results suggest that HBx regulation of cellular calcium signaling and HBV replication in hepatocytes requires activities of the MPTP. The precise impact of HBx on the MPTP remains undefined, and HBx may open, close, or cause the MPTP to flicker. Identification of the molecular mechanisms that underlie HBx regulation of the MPTP is the subject of our ongoing studies.
Many DNA viruses that replicate in differentiated cells modulate cell proliferation pathways to enhance viral replication (see Table 1 in (Mukherji and Kumar 2008)). We previously demonstrated that HBx induces normally quiescent hepatocytes to enter, but stall in, G1 phase of the cell cycle and that HBV replication requires that hepatocytes enter G1 phase of the cell cycle; HBx inhibits S phase entry by increasing the levels of p21 and p27 (Gearhart and Bouchard 2010). When siRNAs were used to simultaneously knockdown p21 and p27, HBV-expressing hepatocytes no longer stalled in G1 phase and progressed into S phase; progression beyond G1 phase and into S phase inhibited HBV replication in primary rat hepatocytes (Table 2, Fig. 4 and Supplemental Fig. 2). Inhibition of HBV replication in S phase was also previously observed in HepG2.2.15 cells (Huang et al. 2004; Ozer et al. 1996). Taken together our results show that HBx stimulates HBV replication by inducing quiescent hepatocytes to exit G0 and enter and stall in G1 phase of the cell cycle.
We next characterized mechanisms that could link HBx-induced G0 to G1 phase progression and subsequent stalling of hepatocytes in G1 phase to HBV replication. One possible consequence of progression into G1 phase could be to activate cellular factors that stimulate or are required for HBV replication. HBV replication requires deoxynucleotide triphosphates (dNTPs), which are present at low levels in quiescent cells, and we reasoned that HBx might induce hepatocytes to enter G1 phase to activate enzymes that increase the pool of available dNTPs (Yamashita and Emerman 2006). Ribonucleotide reductase is one cellular enzyme that is responsible for synthesizing dNTPs; this enzyme consists of a constitutively expressed R1 subunit and an R2 catalytic subunit that is expressed only when cells approach S phase (Reichard 1987). Ribonucleotide reductase is only active when both subunits combine to form the functional enzyme complex (Chabes and Thelander 2000). We observed that HBx, when expressed alone and in the context of HBV replication, increases the level of the R2 catalytic subunit of ribonucleotide reductase in primary rat hepatocytes (Fig. 5). Our observation that HBx increases the level of R2 is similar to a recent report of studies in primary mouse hepatocytes where HBx was shown to increase the level of the R2 mRNA (Cohen et al. 2010). We mechanistically linked the HBx-induced increase in the level of R2 to cytosolic calcium signaling and showed that HBx-modulation of R2 requires MPTP activity (Fig. 5). Overall, our observations suggest that HBx stimulates hepatocytes to progress far enough into G1 phase to activate ribonucleotide reductase.
To confirm that ribonucleotide reductase activity is required for efficient HBV replication, we treated primary rat hepatocytes with hydroxyurea (HU), a reagent that arrests cells at the G1/S border by inhibiting ribonucleotide reductase (Thelander and Reichard 1979); HU treatment inhibited HBV replication (Fig. 6). To directly assess the importance of the R2 subunit of ribonucleotide reductase and provide a second test of the importance of ribonucleotide reductase activity in HBV replication, we used siRNAs to specifically knockdown the levels of the R2 subunit. Our results demonstrate that specifically knocking down R2 dramatically reduces the level of HBV replication (Fig. 7). These results suggest that ribonucleotide reductase is required for HBV replication and are consistent with the results of recent studies in HepG2.215 cells that demonstrated that HBV induces an increase in intracellular dNTPs (Cohen et al. 2010). Although not directly tested, HBx may block progression into S phase while increasing the levels of the catalytic subunit of ribonucleotide reductase to provide a pool of dNTPs while also preventing competition with the DNA replication machinery of the cell which would require dNTPs in S phase. Alternatively, HBx might inhibit progression into S phase to prevent activation of apoptotic pathways that sense unscheduled cellular DNA synthesis that could be activated by the HBx-induced exit of cells from G0. In this scenario, HBx stalls hepatocytes in G1 phase to prevent activation of apoptotic pathways that could kill hepatocytes and potentially impede HBV replication. While we did not observe cell death during the time frame of our studies, even when siRNAs were used to simultaneously decrease the levels of p21 and p27, it is possible that cell death would become apparent after longer periods of time (Gearhart and Bouchard, unpublished observations).
Finally, we examined how cell cycle progression impacted the HBV replication machinery, and focused on the effect of cell cycle phase on activation of the HBV polymerase. We demonstrated that activation of the HBV polymerase requires that hepatocytes enter into G1 phase of the cell cycle. Interestingly, progression beyond G1 phase did not affect HBV polymerase activity even though HBV replication was diminished (Fig. 8). Precisely how progression into G1 stimulates the HBV polymerase is unknown, but one possibility is that activation of cellular factors could directly impact HBV proteins that regulate HBV replication. Phosphorylation of the HBV core protein is required for activation of the HBV polymerase (Melegari et al. 2005), but the kinase(s) responsible for phosphorylating the HBV core protein is unknown. Several candidate kinases have been identified, including protein kinase C (Kann and Gerlich 1994), glyceraldehydes-3-phosphate dehydrogenase (Duclos-Vallee et al. 1998), the stress response protein-specific kinases 1 and 2 (Daub et al. 2002), and the cell cycle-regulated kinase cdc2 (Yeh et al. 1993), and it is possible that the kinase responsible for phosphorylation of HBV core, and stimulation of HBV replication, is activated as hepatocytes exit G0 and enter G1 phase of the cell cycle. The mechanism(s) that underlies activation of the HBV polymerase as hepatocytes progress from G0 to G1 phase is the subject of our ongoing studies.
In summary, we have demonstrated that a fundamental HBx activity, modulation of cytosolic calcium signaling and MPTP activity, regulates hepatocyte proliferation pathways and stimulates HBV replication in a primary hepatocyte model system. We have also shown that HBx induces quiescent hepatocytes to exit G0 and enter and stall in G1 phase, and that both entry and stalling in G1 phase are essential for efficient HBV replication. Our studies suggest that exit from G0 and G1 phase entry activates the HBV polymerase and increases the levels of the R2 subunit of ribonucleotide reductase to stimulate the HBV replication machinery while creating a favorable cellular environment for HBV replication. HBx-induced G1 phase arrest caused by elevation of p21 and p27 also facilitates HBV replication, potentially by preventing competition with the cellular DNA replication machinery that might occur if cells progress into S phase. Finally, while we have shown that HBx modulation of cellular calcium signaling pathways and cell cycle progression is important for HBV replication in a primary hepatocyte model system, our studies also present potential mechanisms that could link HBx activities to the development of HCC. On the one hand, HBx regulation of calcium signaling and cell cycle progression regulates HBV replication, which is an essential contributor to the development of HBV-associated HCC. In addition, HBx alteration of normal hepatocyte cell cycle progression pathways likely disrupts normal hepatocyte physiology, providing another mechanism that could link chronic HBV infections to the development of HCC.
MATERIALS AND METHODS
Isolation and Maintenance of Primary Rat Hepatocytes
Primary rat hepatocytes were isolated using a 2-step perfusion method as previously described (Seglen 1993). The hepatocytes were plated on collagen-coated tissue culture plates at approximately 2.0x106 cells per 60 mm plate (~80% confluent) in Williams E medium supplemented with 2.0mM L-glutamine, 1.0mM sodium pyruvate, 4.0μg/ml Insulin/Transferrin/Selenium (ITS), 5.0μg/ml hydrocortisone, 5.0ng/ml epidermal growth factor (EGF), 10μg/ml gentamycin, and 2% Dimethyl Sulfoxide (DMSO) and maintained at 37°C in 5% CO2. Hepatocyte morphology and expression of hepatocyte-specific mRNAs were monitored throughout the time course of the experiments as previously described (Gearhart and Bouchard 2010). Animal surgery and hepatocyte isolation complied with all relevant federal and institutional policies.
Transfections and Reagents
Primary rat hepatocytes were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s directions. All transfections were performed 24 hours after plating. Hydroxyurea (HU), FK506 (Tacrolimus), and cyclosporin (CsA) were purchased from Sigma; 1,2-bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid-tetraacetoxymethyl ester (BAPTA-AM) was purchased from Invitrogen.
Antibodies
The anti-p27 and anti-p15 antibodies were purchased from Cell Signaling. The anti-Flag-M2 antibody was purchased from Stratagene; the anti-α-tubulin, anti-p21, anti-p16, and anti-R2 antibodies were purchased from Santa Cruz Biotechnology, Inc. The anti-HBcAg was purchased from DakoCytomation, Inc.
Plasmids
FL1-154 HBx, containing N-terminally Flag-tagged HBx cloned into the pcDNA3.1(-) vector, has been previously described (Clippinger and Bouchard 2008). The p16 over-expression plasmid, containing the p16 sequence cloned into the pBABE vector was a gift from Dr. Joan Brugge, Harvard University.
Recombinant Adenoviruses
Consistent with the observations of others, we have found that some analyses, such as HBV replication, requires that 100% of hepatocytes express replicating HBV, and for studies involving HBV replication, we used HBV-expressing recombinant adenoviruses (Clippinger et al. 2009). Construction and use of these recombinant adenoviruses have been previously described (Clippinger et al. 2009). All the recombinant adenoviruses encode green fluorescent protein (GFP). The recombinant adenoviruses used in this study are referred to as AdHBV, which expresses GFP and HBV from a greater-than-unit length cDNA of HBV, and AdHBV(HBx-), which is identical to AdHBV, but contains a mutation that prevents expression of HBx while not affecting the overlapping polymerase gene (Clippinger et al. 2009). GFP expression is used to monitor adenovirus infection efficiency.
Cell Collection and Western Blot Analysis
24 hours following transfection or recombinant adenovirus infection, cells were washed with 1X phosphate buffered saline (PBS), scraped into 1X PBS and pelleted at 2,000 rpm for 3 minutes at 4°C. Cells were then lysed in 0.5% sodium dodecyl sulfate (SDS) Lysis Buffer (0.5% SDS, 240mM Tris pH 6.8, and 10% glycerol) and protein concentrations were determined using the BioRad Protein Assay (BioRad). Equal amounts of protein were loaded on a 12% SDS-polyacrylamide gel. Proteins were transferred to nitrocellulose membrane (BioRad) and blocked for 2 hours in 5% milk in 1X PBS. After incubation with primary antibody overnight at 4°C, the membrane was washed with 1X Tris buffered saline (TBS) + 0.1% Tween-20 and incubated with an alexafluor-conjugated secondary antibody. Blots were visualized using the quantitative, Odyssey® Infared Imaging System (Licor® Biosciences) according to the manufacturers instructions. Western analysis was conducted with antibodies specific for p21, p27, p15, p16, R2, HBcAg, Flag or α-tubulin. All protein levels were first normalized to the α-tubulin loading control, and then fold differences were determined by dividing the intensity of the protein in either the FL1-154 HBx or AdHBV lanes by the intensity of the protein in the pcDNA3.1(-) or AdHBV(HBx-) lanes, respectively. Statistical significance of fold differences was determined using a Student’s T-test (p≤0.05).
siRNAs
Twenty-four hours after plating, hepatocytes were transfected with siRNAs using the Oligofectamine (Invitrogen) transfection reagent. Two separate siRNA constructs for each intended protein were purchased from Qiagen. A separate negative control siRNA (NEG) was purchased from Qiagen. The NEG siRNA also contained an alexafluor tag (488nm) to determine transfection efficiency, and siRNA transfection efficiency of primary rat hepatocytes was approximately 40%. The siRNA sequences for p21, p27 and R2 are shown in Table 1. To confirm knockdown of the intended protein, hepatocytes were collected 72 hours post-transfection, and analyzed via Western blot analysis as described above.
Table 1.
| siRNA | Sense Strand Sequence | Antisense Strand Sequence |
|---|---|---|
| p21 | 5′-CGGUUAGGACCUAAGCGUATT-3′ | 5′-UACGCUUAGGUCCUAACCGGG-3′ |
| p21b | 5′-GAGCCUUGACAUUUACUCATT-3′ | 5′-UGAGUAAAUGUCAAGGCUCTG-3′ |
| p27 | 5′-GCUCCGAAUUAAGAAUAAUTT-3′ | 5′-AUUAUUCUUAAUUCGGAGCTG-3′ |
| p27b | 5′-GUUAAUUGUUUAGCGGUAATT-3′ | 5′-UUACCGCUAAACAAUUAACTG-3′ |
| R2 | 5′-ACGAAUUGUCGUUAAAUUUTT-3′ | 5′-AAAUUUAACGACAAUUCGUGG-3′ |
| R2b | 5′-CAAUAUUCUGGCUCAAGAATT-3′ | 5′-UUCUUGAGCCAGAAUAUUGAT-3′ |
HBV Replication Assay
For studies with siRNA-mediated knockdown of specific protein expression, primary hepatocytes were infected with AdHBV and simultaneously co-transfected with the specific siRNAs. 72 hours post infection, HBV core particles were isolated, and HBV replication was analyzed via Southern blot as previously described (Bouchard et al. 2001a).
To determine the effect of chemical inhibitors on HBV replication, hepatocytes were infected with AdHBV and treated with 50μM BAPTA-AM, 10mM HU, 4 μM FK506 or 4μM CsA for 48 hours. Hepatocytes were collected 72 hours post-infection, HBV core particles were isolated, and HBV replication was analyzed via Southern blot as previously described (Bouchard et al. 2001b).
5-Bromo-2′-deoxy-uridine (BrdU) Incorporation Studies
Cultured, primary rat hepatocytes were infected with AdHBV 24 hours after plating and simultaneously transfected with siRNAs to p21 (si-p21), p27 (si-p27), both p21 and p27 (si-p21/p27) or a scrambled siRNA sequence (NEG). 72 hours post-infection/transfection, the ability to incorporate BrdU into DNA was examined using immunocytochemistry and the BrdU Labeling and Detection Kit II (Roche) according to the manufacturer’s instructions, to determine the percent of cells that were in S phase of the cell cycle. Briefly, BrdU labeling medium was added to cells for 6 hours, after which the cells were fixed, anti-BrdU monoclonal antibody was added, and the cells were incubated with an anti-mouse-Ig-alkaline phosphatase followed by the substrate reaction. Bound BrdU monoclonal antibody, as evidenced by the substrate reaction, was visualized by light microscopy. BrdU-positive cells were counted, and the percent of positive cells per the total number of cells was determined. At least three fields of approximately 150 cells were counted per experimental condition for each experiment. Each experiment was conducted at least four times and for every experiment, each experimental condition was analyzed in triplicate. Statistical significance was determined using a Student’s T test (p≤0.05).
Northern Blot Analysis
For studies with siRNA knockdown of specific proteins, 24 hours after plating, hepatocytes were infected with AdHBV and co-transfected with specific siRNAs as described above. For studies with chemical inhibitors of HBV replication, 24 hours after plating, hepatocytes were infected with AdHBV and 24 hours post-infection, treated with 50μM BAPTA-AM, 4μM FK506, 4μM CsA, or 10mM HU. For all studies, hepatocytes were collected 72 hours after infection with AdHBV, and total RNA was isolated using TRIzol (Invitrogen) according to the manufacturer’s instructions. Poly(A)+ RNA was isolated using oligo(dT)-cellulose columns (Molecular Research Center, Inc.) according to the manufacturer’s instructions, followed by Northern blot analysis as previously described (Bouchard et al. 2003).
HBV Endogenous Polymerase Assay
For studies with siRNA-mediated knockdown of specific proteins, hepatocytes were infected with the AdHBV 24 hours after plating and co-transfected with specific siRNAs as described above. For studies with chemical inhibitors of HBV replication, hepatocytes were infected with the AdHBV and 24 hours post-infection, treated with 50μM BAPTA-AM, 4μM FK506, 4μM CsA, or 10mM HU. For studies where p16 was over-expressed, hepatocytes were transfected with either pBABE control vector or p16 over-expression plasmid, and infected with AdHBV 6 hours later post-transfection. 72 hours post-infection hepatocytes were collected, HBV core particles were isolated, and endogenous polymerase activity was determined as previously described (Bouchard et al. 2003; Bouchard et al. 2006; Bouchard et al. 2001b). Briefly, HBV core particles were collected from lysed, HBV-infected hepatocytes via ultra-centrifugation for 3 hours at 37,000rpm in an SW41Ti rotor and suspended in Polymerase Reaction Buffer (50mM Tris, pH 8.0, 40mM MgCl2, 50mM NH4Cl, 0.3% β-2-mercaptoethanol). The core particles were then incubated at 37°C with 12.5μM dATP/dGTP/dTTP and 10μCi 32P-dCTP for 2 hours followed by incubation with unlabeled 12.5μM dCTP for an additional 1 hour at 37°C. Capsids were then digested with proteinase K (Roche), and HBV DNA was extracted with phenol: chloroform: isoamyl alcohol. Unincorporated radiolabeled nucleotides were removed using a G-50 column (GE Healthcare). The HBV DNA was ethanol precipitated, separated on a 1% agarose/1% SDS gel, and incorporation of radiolabeled nucleotides was analyzed by autoradiography of the dried gel. To demonstrate that equal levels of HBV core particles were assayed in each experimental condition, a fraction of each sample of isolated core particles was examined by Western blot analysis of the core protein.
Supplementary Material
Supplemental Fig. 1: Confirmation of differentiated primary rat hepatocytes in culture. RNA was extracted from (A) freshly isolated hepatocytes or (B) hepatocytes cultured for 72 hours as described in Materials and Methods. RNA was then reverse transcribed and PCR-amplified using primers specific for transferrin (TFN), albumin (ALB), hepatocyte nuclear factor 4 (HNF4), or connexin 26 (CNX 26). (C) RNA was extracted from freshly isolated hepatocytes, hepatocytes cultured for 72 hours, or RAMEC cells, reverse transcribed, and PCR-amplified using primers specific for connexin 43 (CNX 43). Expected band sizes are 914 base pairs (bp) for ALB, 530 bp for TFN, 770 bp for HNF4, 663 bp for CNX 26, and 642 bp for CNX 43. Note that primers for TFN and ALB were combined in the same reaction due to the substantial difference in PCR product size. (-) signifies samples where isolated RNA was directly PCR amplified to confirm the absence of DNA contamination.
Supplemental Fig. 2: HBV replication requires that cells stall in G1 phase: Hepatocytes were infected with AdHBV 24 hours after plating and transfected with si-p21b, si-27b, both si-p21b and si-p27b (si-p21/p27b), or a negative control siRNA (NEG), or AdHBV(HBx-) + NEG (A only). (A-B) Lysates were collected 72 hours post-infection/transfection, resolved via SDS-PAGE, and subjected to Western analysis for α-tubulin, p21, p27, and HBcAg. (C) RNA was isolated from hepatocytes 72 hours post-infection, and viral RNAs were monitored via Northern blot analysis. (D) Hepatocytes were collected 72 hours post-infection, and the activity of the HBV polymerase was determined via an endogenous polymerase assay (see Materials and Methods). (E) 72 hours after infection, HBV replication was analyzed with a standard HBV replication assay (see Materials and Methods); RC, relaxed circular; DL, double-stranded linear; SS, single stranded). Results shown are representative samples from at least 3 experiments performed in duplicate.
Acknowledgments
We thank Peter Adams, Olimpia Meucci, Eishi Noguchi and Laura Steel for continued discussions and advice. This work was supported by NIH grants R01AI064844 to M.J.B and 1 F31 AA017811-01 to T.L.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn JY, Chung EY, et al. Transcriptional repression of p21(waf1) promoter by hepatitis B virus X protein via a p53-independent pathway. Gene. 2001;275(1):163–8. doi: 10.1016/s0378-1119(01)00604-7. [DOI] [PubMed] [Google Scholar]
- Beasley R, Lin C-C, et al. Hepatocellular carcinoma and hepatitis B virus: a prospective study of 22 707 men in Taiwan. Lancet. 1981:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Benhenda S, Cougot D, et al. Hepatitis B virus X protein molecular functions and its role in virus life cycle and pathogenesis. Adv Cancer Res. 2009;103:75–109. doi: 10.1016/S0065-230X(09)03004-8. [DOI] [PubMed] [Google Scholar]
- Bingham MJ, Ong TJ, et al. Physiologic function of the Wilson disease gene product, ATP7B. Am J Clin Nutr. 1998;67(5 Suppl):982S–987S. doi: 10.1093/ajcn/67.5.982S. [DOI] [PubMed] [Google Scholar]
- Block G, Locker J, et al. Population expression, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGFα in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum H, Zhang ZS, et al. Hepatitis B virus X protein is not central to the viral life cycle in vitro. J Virol. 1992;66:1223–1227. doi: 10.1128/jvi.66.2.1223-1227.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M, Wang LH, et al. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science. 2001a;294:2376–2378. doi: 10.1126/science.294.5550.2376. [DOI] [PubMed] [Google Scholar]
- Bouchard MJ, Puro RJ, et al. Activation and inhibition of cellular calcium and tyrosine kinase signaling pathways identify targets of the HBx protein involved in hepatitis B virus replication. J Virol. 2003;77(14):7713–9. doi: 10.1128/JVI.77.14.7713-7719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MJ, Schneider RJ. The enigmatic X gene of hepatitis B virus. J Virol. 2004;78(23):12725–34. doi: 10.1128/JVI.78.23.12725-12734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MJ, Wang L, et al. Activation of focal adhesion kinase by hepatitis B virus HBx protein: multiple functions in viral replication. J Virol. 2006;80(9):4406–14. doi: 10.1128/JVI.80.9.4406-4414.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MJ, Wang LH, et al. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science. 2001b;294(5550):2376–8. doi: 10.1126/science.294.5550.2376. [DOI] [PubMed] [Google Scholar]
- Buendia MA. Hepatitis B viruses and cancerogenesis. Biomed Pharmacother. 1998;52(1):34–43. doi: 10.1016/s0753-3322(97)86239-7. [DOI] [PubMed] [Google Scholar]
- Chabes A, Thelander L. Controlled protein degradation regulates ribonucleotide reductase activity in proliferating mammalian cells during the normal cell cycle and in response to DNA damage and replication blocks. J Biol Chem. 2000;275(23):17747–53. doi: 10.1074/jbc.M000799200. [DOI] [PubMed] [Google Scholar]
- Chami M, Ferrari D, et al. Caspase-dependent alterations of Ca2+ signaling in the induction of apoptosis by hepatitis B virus X protein. J Biol Chem. 2003;278(34):31745–55. doi: 10.1074/jbc.M304202200. [DOI] [PubMed] [Google Scholar]
- Cheng M, Olivier P, et al. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. Embo J. 1999;18(6):1571–83. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin R, Earnest-Silveira L, et al. Modulation of MAPK pathways and cell cycle by replicating hepatitis B virus: factors contributing to hepatocarcinogenesis. J Hepatol. 2007;47(3):325–37. doi: 10.1016/j.jhep.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Chisari F. Rous-Whipple Award Lecture: Viruses, immunity, and cancer: lessons from hepatitis B. Am J Patho. 2000;156:1118–1132. doi: 10.1016/s0002-9440(10)64980-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis. 2001;21(1):27–41. doi: 10.1055/s-2001-12927. [DOI] [PubMed] [Google Scholar]
- Clapham D. Calcium Signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Clippinger AJ, Bouchard MJ. Hepatitis B virus HBx protein localizes to mitochondria in primary rat hepatocytes and modulates mitochondrial membrane potential. J Virol. 2008;82(14):6798–811. doi: 10.1128/JVI.00154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clippinger AJ, Gearhart TL, et al. Hepatitis B virus X protein modulates apoptosis in primary rat hepatocytes by regulating both NF-kappaB and the mitochondrial permeability transition pore. J Virol. 2009;83(10):4718–31. doi: 10.1128/JVI.02590-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Adamovich Y, et al. Hepatitis B virus activates deoxynucleotide synthesis in nondividing hepatocytes by targeting the R2 gene. Hepatology. 2010;51(5):1538–46. doi: 10.1002/hep.23519. [DOI] [PubMed] [Google Scholar]
- Daub H, Blencke S, et al. Identification of SRPK1 and SRPK2 as the major cellular protein kinases phosphorylating hepatitis B virus core protein. J Virol. 2002;76:8124–8137. doi: 10.1128/JVI.76.16.8124-8137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diot C, Gripon P, et al. Replication of hepatitis B virus in differentiated adult rat hepatocytes transfected with cloned viral DNA. J Med Virol. 1992;36:93–100. doi: 10.1002/jmv.1890360206. [DOI] [PubMed] [Google Scholar]
- Duclos-Vallee JC, Capel F, et al. Phosphorylation of the hepatitis B virus core protein by glyceraldehyde-3-phosphate dehydrogenase protein kinase activity. J Gen Virol. 1998;79(Pt 7):1665–70. doi: 10.1099/0022-1317-79-7-1665. [DOI] [PubMed] [Google Scholar]
- Gearhart TL, Bouchard MJ. The Hepatitis B Virus X Protein Modulates Hepatocyte Proliferation Pathways to Stimulate Viral Replication. J Virol. 2010;84(6):2675–86. doi: 10.1128/JVI.02196-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti L, Matze B, et al. Hepatitis B virus replication is cell cycle independent during liver regeneration in transgenic mice. J Virol. 1997;71:4804–4808. doi: 10.1128/jvi.71.6.4804-4808.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HJ, Jung EY, et al. Cooperative repression of cyclin-dependent kinase inhibitor p21 gene expression by hepatitis B virus X protein and hepatitis C virus core protein. FEBS Lett. 2002;518(1–3):169–72. doi: 10.1016/s0014-5793(02)02694-7. [DOI] [PubMed] [Google Scholar]
- Harper JV, Brooks G. The mammalian cell cycle: an overview. Methods Mol Biol. 2005;296:113–53. doi: 10.1385/1-59259-857-9:113. [DOI] [PubMed] [Google Scholar]
- Huang YQ, Wang LW, et al. Effects of cell cycle on telomerase activity and on hepatitis B virus replication in HepG2 2.2.15 cells. Hepatobiliary Pancreat Dis Int. 2004;3(4):543–7. [PubMed] [Google Scholar]
- Huh KW, Siddiqui A. Characterization of the mitochondrial association of hepatitis B virus X protein, HBx. Mitochondrion. 2002;1(4):349–59. doi: 10.1016/s1567-7249(01)00040-x. [DOI] [PubMed] [Google Scholar]
- James SJ, I, Pogribny P, et al. Mechanisms of DNA damage, DNA hypomethylation, and tumor progression in the folate/methyl-deficient rat model of hepatocarcinogenesis. J Nutr. 2003;133(11 Suppl 1):3740S–3747S. doi: 10.1093/jn/133.11.3740S. [DOI] [PubMed] [Google Scholar]
- Kann M, Gerlich WH. Effect of core protein phosphorylation by protein kinase C on encapsidation of RNA within core particles of hepatitis B virus. J Virol. 1994;68(12):7993–8000. doi: 10.1128/jvi.68.12.7993-8000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keasler VV, Hodgson AJ, et al. Enhancement of hepatitis B virus replication by the regulatory X protein in vitro and in vivo. J Virol. 2007;81(6):2656–62. doi: 10.1128/JVI.02020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Koike K, et al. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;353:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- Klein N, Bouchard M, et al. Src kinases involved in hepatitis B virus replication. EMBO J. 1999;18:5019–5027. doi: 10.1093/emboj/18.18.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach JK, Qiao L, et al. Regulation of p21 and p27 expression by the hepatitis B virus X protein and the alternate initiation site X proteins, AUG2 and AUG3. J Gastroenterol Hepatol. 2003;18(4):376–85. doi: 10.1046/j.1440-1746.2003.02990.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Tarn C, et al. Hepatitis B virus X protein differentially regulates cell cycle progression in X-transforming versus nontransforming hepatocyte (AML12) cell lines. J Biol Chem. 2002;277(10):8730–40. doi: 10.1074/jbc.M108025200. [DOI] [PubMed] [Google Scholar]
- Leupin O, Bontron S, et al. Hepatitis B virus X protein stimulates viral genome replication via a DDB1-dependent pathway distinct from that leading to cell death. J Virol. 2005;79(7):4238–45. doi: 10.1128/JVI.79.7.4238-4245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden C, Finegold M, et al. Hepatitis B virus X protein acts a a tumor promoter in development of diethylnitrosamine-induced preneoplastic lesions. J Virol. 2001;75:3851–3858. doi: 10.1128/JVI.75.8.3851-3858.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain SL, Clippinger AJ, et al. Hepatitis B virus replication is associated with an HBx-dependent mitochondrion-regulated increase in cytosolic calcium levels. J Virol. 2007;81(21):12061–5. doi: 10.1128/JVI.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melegari M, Scaglioni PP, et al. Cloning and characterization of a novel hepatitis B virus x Binding protein that inhibits viral replication. J Virol. 1998;72:1737–1743. doi: 10.1128/jvi.72.3.1737-1743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melegari M, Wolf SK, et al. Hepatitis B virus DNA replication is coordinated by core protein serine phosphorylation and HBx expression. J Virol. 2005;79(15):9810–20. doi: 10.1128/JVI.79.15.9810-9820.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherji A, V, Janbandhu C, et al. HBx-dependent cell cycle deregulation involves interaction with cyclin E/A-cdk2 complex and destabilization of p27Kip1. Biochem J. 2007;401(1):247–56. doi: 10.1042/BJ20061091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherji A, Kumar V. J Kobarg. Kerala, India: Research Signpost; 2008. Regulation of the cell cycle by the Hepatitis B virus X protein. The pleiotropic functions of the viral protein HBx in hepatitis B virus infection and the development of liver cancer; pp. 165–177. [Google Scholar]
- Oh JC, Jeong DL, et al. Activation of calcium signaling by hepatitis B virus-X protein in liver cells. Exp Mol Med. 2003;35(4):301–9. doi: 10.1038/emm.2003.41. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, et al. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4(7):552–65. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Ozer A, Khaoustov V, et al. Effect of hepatocyte proliferation and cellular DNA synthesis on hepatitis B virus replication. Gastroenterology. 1996;110:1519–1528. doi: 10.1053/gast.1996.v110.pm8613059. [DOI] [PubMed] [Google Scholar]
- Park SG, Chung C, et al. Up-regulation of cyclin D1 by HBx is mediated by NF-kappaB2/BCL3 complex through kappaB site of cyclin D1 promoter. J Biol Chem. 2006;281(42):31770–7. doi: 10.1074/jbc.M603194200. [DOI] [PubMed] [Google Scholar]
- Park US, Park SK, et al. Hepatitis B virus-X protein upregulates the expression of p21waf1/cip1 and prolongs G1-->S transition via a p53-independent pathway in human hepatoma cells. Oncogene. 2000;19(30):3384–94. doi: 10.1038/sj.onc.1203674. [DOI] [PubMed] [Google Scholar]
- Piechocki MP, Burk RD, et al. Regulation of connexin32 and connexin43 gene expression by DNA methylation in rat liver cells. Carcinogenesis. 1999;20(3):401–6. doi: 10.1093/carcin/20.3.401. [DOI] [PubMed] [Google Scholar]
- Qiao L, Leach K, et al. Hepatitis B virus X protein increases expression of p21Cip-1/WAF1/MDA6 and p27Kip-1 in primary mouse hepatocytes, leading to a reduced cell cycle progression. Hepatology. 2001;34:906–917. doi: 10.1053/jhep.2001.28886. [DOI] [PubMed] [Google Scholar]
- Rahmani Z, Huh KW, et al. Hepatitis B virus X protein colocalizes to mitochondria with human voltage -dependent anion channel, HVDAC3, and alters its transmembrane potential. J Virol. 2000;74:2840–2846. doi: 10.1128/jvi.74.6.2840-2846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard P. Regulation of deoxyribotide synthesis. Biochemistry. 1987;26(12):3245–8. doi: 10.1021/bi00386a001. [DOI] [PubMed] [Google Scholar]
- Rezvan A, Allen FD, et al. Steady unidirectional laminar flow inhibits monolayer formation by human and rat microvascular endothelial cells. Endothelium. 2004;11(1):11–6. doi: 10.1080/10623320490432443. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Garay EA. Cholestasis: human disease and experimental animal models. Ann Hepatol. 2003;2(4):150–8. [PubMed] [Google Scholar]
- Runge D, Runge DM, et al. Serum-free, long-term cultures of human hepatocytes: maintenance of cell morphology, transcription factors, and liver-specific functions. Biochem Biophys Res Commun. 2000;269(1):46–53. doi: 10.1006/bbrc.2000.2215. [DOI] [PubMed] [Google Scholar]
- Seeger C, Zoulim F, et al. Hepadnaviruses. In: Knipe D, Howley P, editors. Fields Virology. Vol. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 2977–3029. [Google Scholar]
- Seglen P. Isolation of hepatocytes by collagenase perfusion. In: Tyson C, Frazier J, editors. Methods in Toxicology. Vol. 1. New York: Academic Press; 1993. pp. 231–243. [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13(12):1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Sitterlin D, Bergametti F, et al. Correct binding of viral X protein to UVDDB-p127 cellular protein is critical for efficient infection by hepatitis B viruses. Oncogene. 2000;19(38):4427–31. doi: 10.1038/sj.onc.1203770. [DOI] [PubMed] [Google Scholar]
- Slagle BL, Lee TH, et al. Increased sensitivity to the hepatocarcinogen diethylnitrosamine in transgenic mice carrying the hepatitis B virus X gene. Mol Carcinog. 1996;15(4):261–9. doi: 10.1002/(SICI)1098-2744(199604)15:4<261::AID-MC3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Tang H, Delgermaa L, et al. The transcriptional transactivation function of HBx protein is important for its augmentation role in hepatitis B virus replication. J Virol. 2005;79(9):5548–56. doi: 10.1128/JVI.79.9.5548-5556.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terradillos O, Billet O, et al. The hepatitis B virus X gene potentiates c-myc-induced liver oncogenesis in transgenic mice. Oncogene. 1997;14:395–404. doi: 10.1038/sj.onc.1200850. [DOI] [PubMed] [Google Scholar]
- Thelander L, Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–58. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Tsuge M, Hiraga N, et al. HBx protein is indispensable for development of viremia in human hepatocyte chimeric mice. J Gen Virol. 2010 doi: 10.1099/vir.0.019224-0. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Emerman M. Retroviral infection of non-dividing cells: old and new perspectives. Virology. 2006;344(1):88–93. doi: 10.1016/j.virol.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Yeh CT, Wong SW, et al. Cell cycle regulation of nuclear localization of hepatitis B virus core protein. Proc Natl Acad Sci U S A. 1993;90(14):6459–63. doi: 10.1073/pnas.90.14.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DY, Moon HB, et al. Incidence of hepatocellular carcinoma in transgenic mice expressing the hepatitis B virus X-protein. J Hepatol. 1999;31(1):123–32. doi: 10.1016/s0168-8278(99)80172-x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Protzer U, et al. Inhibition of Cellular Proteasome Activities Enhances Hepadnavirus Replication in an HBX-Dependent Manner. J Virol. 2004;78(9):4566–4572. doi: 10.1128/JVI.78.9.4566-4572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1: Confirmation of differentiated primary rat hepatocytes in culture. RNA was extracted from (A) freshly isolated hepatocytes or (B) hepatocytes cultured for 72 hours as described in Materials and Methods. RNA was then reverse transcribed and PCR-amplified using primers specific for transferrin (TFN), albumin (ALB), hepatocyte nuclear factor 4 (HNF4), or connexin 26 (CNX 26). (C) RNA was extracted from freshly isolated hepatocytes, hepatocytes cultured for 72 hours, or RAMEC cells, reverse transcribed, and PCR-amplified using primers specific for connexin 43 (CNX 43). Expected band sizes are 914 base pairs (bp) for ALB, 530 bp for TFN, 770 bp for HNF4, 663 bp for CNX 26, and 642 bp for CNX 43. Note that primers for TFN and ALB were combined in the same reaction due to the substantial difference in PCR product size. (-) signifies samples where isolated RNA was directly PCR amplified to confirm the absence of DNA contamination.
Supplemental Fig. 2: HBV replication requires that cells stall in G1 phase: Hepatocytes were infected with AdHBV 24 hours after plating and transfected with si-p21b, si-27b, both si-p21b and si-p27b (si-p21/p27b), or a negative control siRNA (NEG), or AdHBV(HBx-) + NEG (A only). (A-B) Lysates were collected 72 hours post-infection/transfection, resolved via SDS-PAGE, and subjected to Western analysis for α-tubulin, p21, p27, and HBcAg. (C) RNA was isolated from hepatocytes 72 hours post-infection, and viral RNAs were monitored via Northern blot analysis. (D) Hepatocytes were collected 72 hours post-infection, and the activity of the HBV polymerase was determined via an endogenous polymerase assay (see Materials and Methods). (E) 72 hours after infection, HBV replication was analyzed with a standard HBV replication assay (see Materials and Methods); RC, relaxed circular; DL, double-stranded linear; SS, single stranded). Results shown are representative samples from at least 3 experiments performed in duplicate.