Abstract
Opioids have been widely applied in clinics as one of the most potent pain relievers for centuries, but their abuse has deleterious physiological effects beyond addiction. We previously reported that opioids inhibit cell growth and trigger apoptosis in lymphocytes. However, the underlying mechanism by which microglia apoptosis in response to opioids is not yet known. In this study, we show that morphine induces microglia apoptosis and caspase-3 activation in an opioid-receptor dependent manner. Morphine decreased the levels of microglia phosphorylated Akt (p-Akt) and p-GSK-3β (glycogen synthase kinase 3 beta) in an opioid receptor dependent manner. More interestingly, GSK-3β inhibitor SB216763 significantly increases morphine-induced apoptosis in both BV-2 microglia and mouse primary microglial cells. Moreover, co-treatment of microglia with SB216763 and morphine led to a significant synergistic effect on the level of phospho-p38 mitogen-activated protein kinase (MAPK). In addition, inhibition of p38 MAPK by its specific inhibitor SB203580 significantly inhibited morphine-induced apoptosis and caspase-3 activation. Taken together, our data clearly demonstrates that morphine induced apoptosis in microglial cells, which is mediated via GSK-3β and p38 MAPK pathways.
Keywords: Opioids, microglia, Akt, GSK3β, p38, apoptosis
1. Introduction
Opioids are a standard for treatment of chronic pain, however, their efficacy is limited by side effects including dependence, tolerance, hyperalgesia, mental clouding, and immune modulation (Matthes et al., 1996; Roy et al., 1998). Opioids, including morphine, cause apoptosis in various systems. We have previously reported that chronic morphine exposure, such as occurs in drug abuse, promotes apoptosis both in vitro and in vivo (Yin et al., 1999a; Yin et al., 2006; Li et al., 2009; Moorman et al., 2009; Li et al., 2010). In the central nervous system (CNS), we and others have previously shown that the importance of opioid-induced apoptosis in neurons (Svensson et al., 2008; Li et al., 2010). Opioid receptors play critical roles in the processes of opioids-induced effects. All three opioid receptor types, μ, δ, and κ have been identified on microglia (Mazumder et al., 2008; Horvath and DeLeo, 2009).
Glia, once thought to be merely supporting cells of the CNS, are now recognized to play a critical role in the formation and maintenance of opioid dependence and tolerance (Mazumder et al., 2008; Song and Zhao, 2001; Horvath and DeLeo, 2009). Microglial cells represent the resident immune host defense and are considered the major immune inflammatory cells of the CNS (Reed-Geaghan et al., 2009). Previous studies by Hu et al. (Hu et al., 2002) have shown that chronic morphine treatment induces microglia apoptosis. Naloxone (an opioid receptor antagonist) blocked this effect, implicating involvement of an opioid receptor-mediated apoptosis. Based on these findings, we investigated the molecular mechanisms by which morphine primes microglia apoptosis. Specifically we determined the involvement of the Akt/GSK-3β (glycogen synthase kinase-3β), and the pro-apoptotic p38 mitogen-activated protein kinase (MAPK) pathways.
Serine/threonine kinase Akt (also known as protein kinase B, PKB) modulates cellular activation, inflammatory response, and apoptosis (Fruman and Cantley, 2002; Cantley, 2002; Zhang et al., 2008a; Shi et al., 2007). Activated Akt in turn phosphorylates a variety of proteins involved in survival and apoptotic pathways leading to diminished cell apoptosis (Cantley, 2002; Fukao and Koyasu, 2003; Fukao et al., 2002). Activated Akt phosphorylates several downstream targets of the PI3K pathway including GSK3β (Jope and Johnson, 2004; Martin et al., 2005). GSK3β is a constitutively active enzyme that is inactivated by Akt through phosphorylation of serine 9 (Jope and Johnson, 2004; Martin et al., 2005). GSK3β is a crucial regulator of cell survival and apoptosis (Jope and Johnson, 2004). Growing evidence supports that GSK3 modulates key steps in each of the two major apoptotic signaling pathways (the mitochondrial intrinsic apoptotic and the death receptor-mediated extrinsic apoptotic signaling pathways) (Beurel and Jope, 2006). We have recently revealed that the Akt/GSK3β signaling plays an important role in opioid-mediated apoptosis in neuronal and breast cancer cells (Zhao et al., 2009; Li et al., 2010). However, the precise role of the Akt/GSK3β signaling in microglia is unknown. One of three major subfamilies of MAPKs described, p38 MAPK plays a pro-apoptotic role (Chang and Karin, 2001;Ichijo, 1999;Tegeder and Geisslinger, 2004). Chronic morphine administration enhances the phosphorylation of p38 MAPK in dorsal root ganglion neurons (Ma et al., 2001; Macey et al., 2006). Inhibition of p38 MAPK by p38 inhibitor SB203580 significantly attenuated tolerance to morphine analgesia (Cui et al., 2006). P38 MAPK seems to sensitize cells to apoptosis by up-regulating Bax (Porras et al., 2004; Chang et al., 2009), a pro-apoptotic member of Bcl-2 family (Porras et al., 2004; Chang et al., 2009). But the role of p38 in morphine-promoted microglia apoptosis is unknown.
In this study, we show that morphine induces cell apoptosis in an opioid receptor-dependent manner in brain microglia as well as in the microglial cell line BV-2. We report for the first time that morphine promotes microglia apoptosis through Akt/GSK3β and p38 MAPK signaling pathways. Thus, our results provide an important insight into the mechanism of microglia apoptosis in response to opioids.
2. Materials and methods
2.1. Reagents
Morphine sulfate and naloxone were obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies, including total and phospho-GSK-3β (serine 9), total and phospho-Akt (serine 473), total and phospho-p38, total and cleaved caspase-3 and- 8, were purchased from Cell Signal Technology (Beverly, MA). The antibodies of GAPDH, Bax, and Bcl-2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). GSK-3β inhibitor SB216763 and p38 inhibitor SB203580 were obtained from Tocris Bioscience (Bristol, UK).
2.2. Cell culture
Microglial cultures
BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). Mice were housed, bred, and euthanized in accordance with protocols reviewed and approved by the East Tennessee State University Institutional Animal Care and Use Committee. Mouse primary microglial cells were isolated from mixed glial cultures, as described previously (Lee et al., 2001). Briefly, primary mixed glial cultures were prepared from postnatal day 1–2 mice. Primary microglia were co-cultured with astrocytes in poly-D-lysine-coated 75-cm2 culture flasks in DMEM (Gibco BRL, Gaithersburg, MD) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA) and 1% Penicillin/Streptomycin. On days 10-14, microglia were harvested by shaking the cultures (180 rpm) and collecting the floating cells. These cells were seeded into plastic tissue culture flasks. After incubation at 37 °C for 1 h, non-adherent cells were removed by replacing culture medium. The cells were grown in DMEM with 10% FBS and maintained at 37 °C and 5% CO2. The purity of microglia was verified > 95% by ricinus communis agglutinin-1 (RCA-1, a microglia marker) immunostaining.
BV-2 cell culture
The BV-2 mouse microglial cell line (Mandrekar et al., 2009) was a gift from Dr. Gary Landreth (Case Western Reserve University School of Medicine, OH). The cells were cultured in DMEM medium (Invitrogen Corporation, Carsbad, CA), supplemented with sodium pyruvate, l-glutamate, 10 % FBS (Sigma, St. Louis, MO), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cultures were incubated at 37 °C and 5% CO2 in a fully humidified incubator.
2.3. Quantification of apoptosis by TUNEL assay
The experimental cells were treated with different concentrations of morphine in the presence or absence of naloxone, SB216763, or SB203580 for 24 h. Apoptotic cells were determined by terminal deoxynucleotidyl transferase biotin-d UTP nick end labeling (TUNEL) assay using a situ cell death detection kit (Roche Diagnostic, Indianpolis, IN) as described in our previous publications (Yin et al., 2000; Chen et al., 2009). Briefly, the 3′-OH ends of fragmented nucleosomal DNA were specifically labeled in situ in the presence of exogenously added terminal transferase biotin labeled dUTP, and were detected with anti-fluorescein antibody alkaline peroxidase conjugated. Cells were fixed on coverslips with ice cold 4% PFA (paraformaldehyde) for 30 min and exposed for appropriate time to a permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate). Coverslips were coated by poly-D-lysine. After washing, 50 μl of TUNEL reaction mixture was placed on the cells and incubated in a humidified atmosphere for 60 min at 37 °C. 50 μl substrate solution was added onto coverslips following convert-AP incubation. Finally coverslips were washed with PBS and mounted with citiflor. The percentage of apoptotic cells was calculated by counting approximately 500 cells.
2.4. Cell viability assay
Cell viability was performed as described previously (Li et al., 2009). The experimental cells were seeded in 96-well plates at an initial cell density of 1 × 104/well. Cell viability was assessed by the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Briefly, 20 μl MTT (5 mg/ml, Sigma–Aldrich, St. Louis, MO) was added to each well, and plates were incubated at 37 °C for 4 hours. The color formation was quantified by means of an ELISA plate reader (Dynotech Instruments, New Delhi, India) at 570 nm wavelength using 100 μl MTT solubilization solution. All of the experiments were conducted at least three times.
2.5. Western blot analysis
Western blot was performed as described previously (Yin et al., 1999b; Yin et al., 2006; Zhang et al., 2008b). Briefly, the cellular proteins were separated by SDS–polyacrylamide gel electrophoresis and transferred onto Hybond ECL membranes (Amersham Pharmacia, Piscataway, NJ). The membrane was then incubated at room temperature in a blocking solution composed of 5% skim milk powder dissolved in 1× TBS (10 mM Tris, pH 8.0, and 140 mM NaCl) for 1 h. The membrane was then incubated with the blocking solution containing the first antibody overnight at 4°C. After washing three times with TBS for 5 min, the blot was then incubated with a second antibody. The blot was again washed three times with TBS before being exposed to the SuperSignal West Dura Extented Duration substrate (Pierce Biotechnology, Rockford, IL). Band intensity was quantified by densitometric analyses using a densitometer.
2.6. Reverse transcription–PCR (RT-PCR)
Total RNA was extracted from the cells by use of the VERSA GENE™ RNA Tissue Kit (Gentra SYSTEMS; Minnesota) and the RT-PCR detection technique was performed as described previously (Liu et al., 2005; Li et al., 2010). Briefly, first-strand cDNA was synthesized from 1μg of total RNA using a Reaction Ready™ first strand cDNA synthesis kit (SABioscience Corporation, Frederick, MD). After incubation at 70 °C for 3 min and cooling down to 37 °C for 10 min, RT cocktail was added to the annealing mixture and further incubated at 37 °C for 60 min. The following primer pairs were used: for Bax: GGTTTCATCCAGG ATCGAGACGG (forward) and ACAAAGATGGTCACGGTCTGCC (reverse); Bcl-2: AGAGGGGCTACGAGTGGGAT (forward) and CTCAGTCATCCACAGGGCGA (reverse); Fas: TCTGGTGCTTGCTGGCTCAC (forward) and CCATAGGCG ATTTCTGGGAC (reverse); FasL: ATAGGTCTTAAGAAGACTCTCATTCAAG (forward) and TGATCAATTTTGAGGAATCTAAGGCC (reverse).
2.7. Statistical analysis
The results were presented as mean ± S.D. The data were analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni tests to determine where differences among groups existed. A value of p < 0.05 was considered statistically significant.
3. Results
3. 1. Morphine induces microglia apoptosis in an opioid-receptor dependent manner
We have reported that morphine induces lymphocyte apoptosis (Yin et al., 1999a; Yin et al., 2006; Yin et al., 2000). To examine whether morphine has an effect on cell death of microglia, BV-2 microglia were cultured in the absence (control) or presence of morphine at different concentrations, and cell viability was determined by the MTT assay. As shown in Fig. 1A, morphine caused significant decreases in cell viability in a dose-dependent manner. This process is dependent on functional opioid receptors since morphine-decreased cell viability could be inhibited by naloxone, an antagonist of the opioid receptor. The inhibitory effect of morphine was dose-dependent. Morphine treatment at 10 μM for 24 h led to more than 40% inhibition and was used in the subsequent experiments. Similar results were observed in mouse primary microglial cells (data not shown). We next examined whether morphine-attenuated cell viability is mediated cell apoptosis. Apoptotic cells were determined using TUNEL assay. We found that morphine significantly induces cell apoptosis both in BV-2 and primary mouse microglia (Fig. 1B). In addition, opioid receptor antagonist naloxone blocked morphine-primed apoptosis (Fig. 1B), suggesting involvement of an opioid receptor mechanism.
Fig. 1.
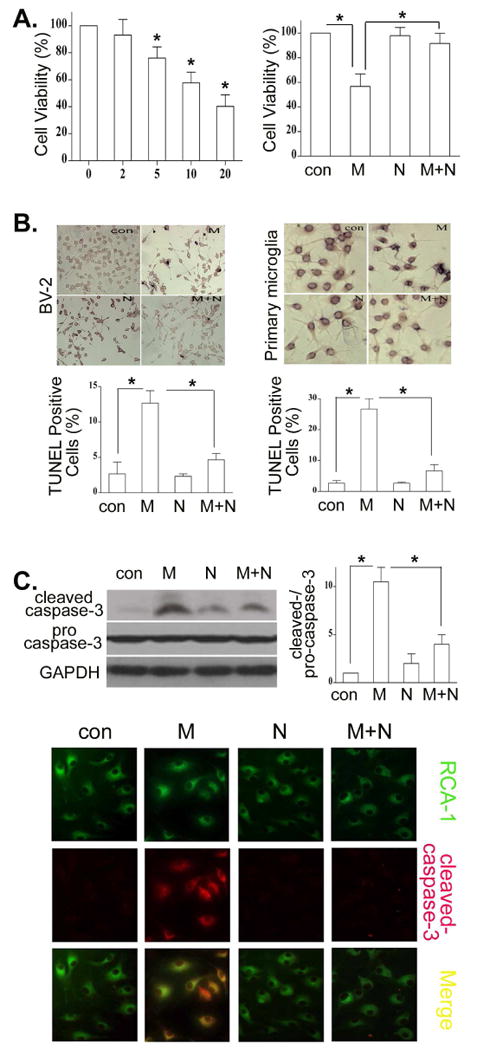
Morphine promotes microglia apoptosis through an opioid receptor. (A). BV-2 microglia were incubated in the presence or absence of morphine at indicated concentrations for 24 h or cultured with morphine at 10 μM with or without 10 μM naloxone for 24h. The cell viability was examined by the MTT assay. * p < 0.01 compared with control or with indicated groups. (B). Naloxone inhibits morphine-induced microglia apoptosis. BV-2 and mouse primary microglial cells were treated with 10μM morphine with or without 10μM naloxone for 24 h. Apoptotic cells (dark cells) were determined by TUNEL assay. Photographs of representative TUNEL-stained cells are shown at the top. Magnification 40 ×. The bar graph at the bottom shows the percentage of apoptotic cells. (C). BV-2 and primary microglia were treated with morphine and naloxone as in B. Cleaved caspase-3 was determined by Western blot in BV-2 cells or immunostaining in primary microglia with cleaved caspase-3 antibody (Red) and (RCA-1 (Green). Merged images displayed co-localizations of cleaved caspase 3 and RCA-1 (Yellow). All data are representative of three independent experiments. * p < 0.01.
The cleaved caspase-3 is an established specific marker for apoptosis (Mazumder et al., 2008). Thus, we examined caspase-3 activation in microglia. As shown in Fig. 1C, after exposure to morphine for 24 h, BV-2 and mouse primary microglia have a significantly higher level of cleaved caspase-3 than control cells. Moreover, morphine- induced activation of caspase-3 can be blocked by naloxone in both BV-2 and mouse primary microglia.
3.2. Morphine decreased the levels of microglia phospho-Akt and phospho-GSK-3β through an opioid receptor
We have observed previously that morphine attenuated the levels of phospho-Akt in cancer cells (Zhao et al., 2009). In the present study, we examined the levels of phospho-Akt (Thr473) in BV-2 cells in the presence or absence of morphine. The levels of phospho-Akt in morphine treated cells were significantly lower compared to control cells in a dose- and time-dependent manner (Fig. 2A). Furthermore, pretreatment of naloxone to BV-2 cells blocked morphine-decreased the levels of phospho-Akt (Fig. 2B).
Fig. 2.
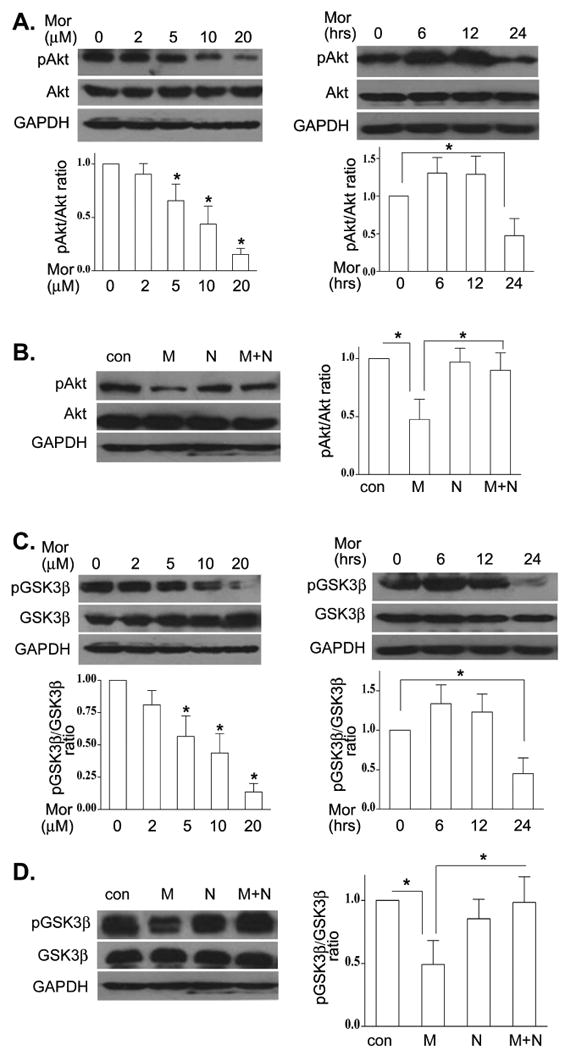
Morphine decreased microglia phospho-Akt and phospho-GSK-3β lelvels through an opioid receptor. (A). BV-2 cells were incubated in various concentrations of morphine for 24 h or treated with 10μM morphine for different time periods. Total and phospho-Akt (p-Akt) were determined by Western blot. Western blots are representative, and the densitometry data represent the mean ± SEM from at least three independent experiments. (B). BV-2 cells were treated with morphine at 10 μM in the presence or absence of 10 μM naloxone. The levels of total and p-Akt were examined as in A. (C). BV-2 cells were incubated with morphine as in A. The levels of total and p-GSK-3β were determined by Western blot. (D). BV-2 cells were subjected to morphine and naloxone as in B. The levels of total and p-GSK-3β were examined as in C. Mean values were derived from three independent experiments. * p <0.01 compared with indicated groups.
GSK-3β is an important downstream target of Akt (Martin et al., 2005; Beurel and Jope, 2006). GSK-3β is a constitutively active enzyme (Martin et al., 2005). Phosphorylation of GSK-3β by Akt results in GSK-3β inactivation (Martin et al., 2005). We investigated the effect of morphine on phosphorylation of GSK-3β in microglial cells. Fig. 2C showed that the microglia levels of phospho-GSK-3β are significantly lower in the presence of morphine and in dose- and time-dependent mechanism. This effect was inhibited by naloxone (Fig. 2D). Similar results were observed in mouse primary microglia treated with morphine and naloxone (data not shown). These data demonstrate that morphine decreased phospho-Akt and phospho-GSK-3β levels via an opioid receptor.
3.3. GSK-3β inhibition increases morphine-induced microglia apoptosis
Recent studies report that GSK3β plays a pivotal role in regulating many cellular functions, including cell survival and apoptosis (Beurel and Jope, 2006). How GSK3β affects microglia apoptosis in the setting of opioids, however, remains unknown. We examined whether GSK3β is required for morphine-induced microglia apoptosis. BV-2 and mouse primary microglial cells were pretreated with the GSK3β inhibitor SB216763 (Martin et al., 2005; Abell et al., 2007), and then treated with morphine for 24 h. We found that treatment with SB216763 significantly increased morphine-induced apoptosis in both BV-2 (Fig. 3A) and mouse primary microglia (Fig. 3B). SB216763 alone did not alter the percentage of apoptotic cells compared to control (Figs.3A and 3B).
Fig. 3.
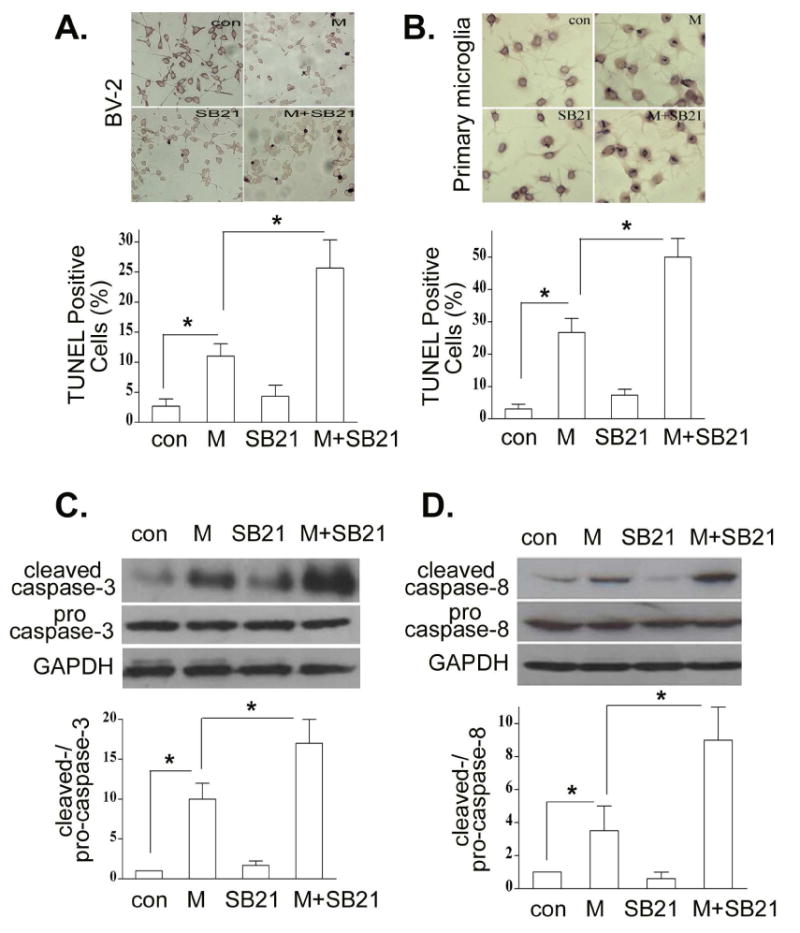
Inhibition of GSK-3β enhances morphine-primed microglia apoptosis. BV-2 (A) and primary microglia (B) cells were subjected to SB216763 at 10μM for 1 h and then treated with or without 10 μM morphine for 24 h. Apoptotic cells were determined as in Fig. 1B. BV-2 cells were incubated with morphine and SB216763 as in A. The levels of total and cleaved caspase-3 (C), and total and cleaved capspase-8 (D) were determined by Western blot analysis. All data are representative of three independent experiments. * p < 0.01.
To determine whether GSK3β affects the activation of caspase-3 and caspase-8, BV-2 cells were treated with SB216763 in the presence or absence of morphine. Intriguingly, significantly greater activation of casapse-3 (Fig. 3C) and caspase-8 (Fig. 3D) was observed when BV-2 cells were exposed to both SB216763 and morphine than they were exposed to SB216763 or morphine alone. SB216763 alone did not induce the activation of caspase-3 and caspase-8. Similar results were observed in primary microglia (data not shown).
3.4. Effect of GSK3β on the levels of Bcl-2/Bax and p38 MAPK following morphine treatment
Growing evidence suggests that Bcl-2 family proteins may be the functional downstream target(s) of GSK3β (Juhaszova et al., 2009; Kotliarova et al., 2008). We determined the expression of an anti-apoptotic protein Bcl-2 and Bax, a pro-apoptotic member of the Bcl-2 family, following morphine treatment with or without inhibition of GSK3β by SB216763. Treatment of morphine with SB216763 significantly decreased the levels of Bcl-2 and increased the levels of Bax (Fig. 4A). SB216763 alone did not alter the expression of Bcl-2 and Bax. Taken together, our results suggest that morphine-induced apoptosis is through GSK3β-mediated mitochondrial pathway.
Fig. 4.
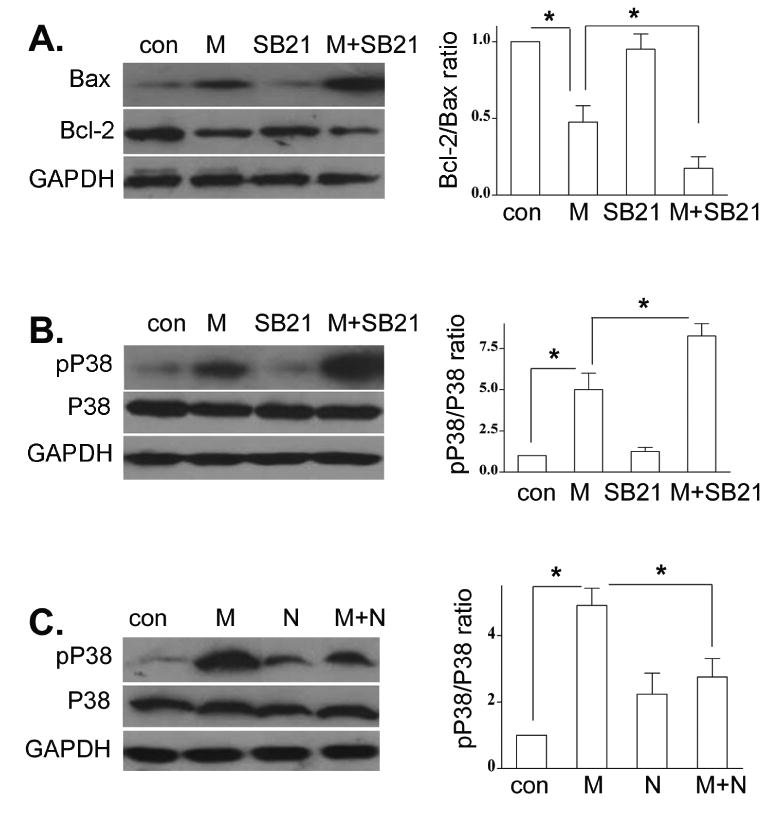
Effect of GSK-3β on morphine-induced changes of Bax/Bcl-2 and p38 MAPK. BV-2 cells were subjected to SB216763 and morphine as in Fig. 3A. The levels of Bax and Bcl-2 (A), total and p-p38 MAPK (B), were determined by Western blot analysis. (C). BV-2 cells were treated with naloxone and morphine as in Fig. 2B. Total and p-p38 MAPK were examined by Western blot analysis. Mean values were derived from three independent experiments. * p <0.01 compared with indicated groups.
Recently, it has been reported that GSK3β negatively regulates p38 MAPK signaling (Abell et al., 2007). In addition, chronic morphine treatment enhances the phosphorylation of p38 MAPK in neurons (Ma et al., 2001; Macey et al., 2006). We next evaluated whether GSK3β plays a role in morphine-induced phosphorylation of p38 MAPK. BV-2 cells were pretreated with SB216763 and then treated with morphine. Morphine alone significantly increased the level of phospho-p38 MAPK (Fig. 4B). Of great significance, combination of SB216763 and morphine treatment has a significant synergistic effect on the level of phospho-p38 MAPK (Fig. 4B). Moreover, naloxone significantly attenuated morphine-induced the level of phospho-p38 MAPK (Fig. 4C). These data clearly show that morphine primes GSK3β-mediated phospho-p38 MAPK in an opioid receptor mechanism.
3.5. Inhibition of p38 MAPK inhibits morphine-induced microglia apoptosis
It has been shown that morphine induces macrophage apoptosis through p38 MAPK phosphorylation (Singhal et al., 2002). Since morphine could effectively increase the level of phospho-p38 MAPK in microglia (Fig. 4C), its consequence on the p38 MAPK function was next examined in BV-2 and mouse primary microglial cells. We pretreated BV-2 and mouse primary microglial cells with the specific p38 MAPK inhibitor SB 203580 (Chang et al., 2009) and then treated the cells in the presence or absence of morphine. Apoptotic cells were examined by TUNEL assay. We showed that SB203580 significantly blocked morphine-induced apoptosis in both BV-2 (Fig. 5A) and mouse primary microglial cells (Fig. 5B). SB203580 alone did not induce apoptosis in both BV-2 and mouse primary microglial cells (Figs. 5A and 5B). In addition, inhibition of p38 by SB203580 dramatically inhibited morphine-induced activation of caspase-3 (Fig. 5C) and caspase-8 (Fig. 5D). The similar results were observed when mouse primary microglial cells were treated with SB203580 and morphine (data not shown).
Fig. 5.
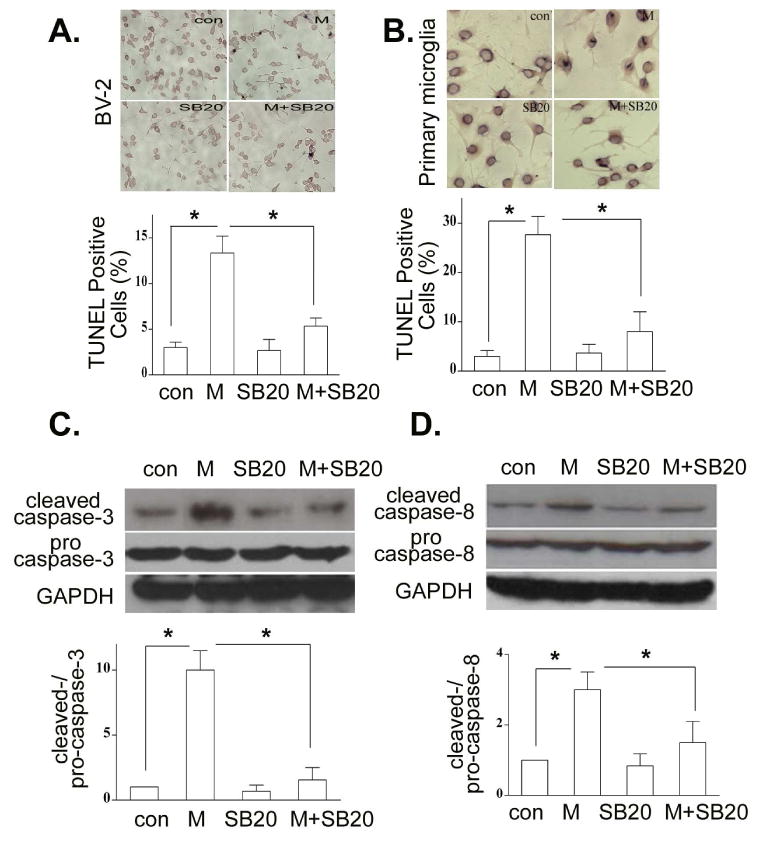
Inhibition of p38 MAPK attenuates morphine-induced microglia apoptosis. BV-2 (A) and mouse primary microglia (B) cells were pretreated with SB203850 at 10 μM for 1 h and then treated with morphine at 10 μM for 24 h. Apoptotic cells were examined by TUNEL assay as in Fig. 3A. Total and cleaved caspase-3 (C), and total and cleaved capspase-8 (D) were determined by Western blot analysis. All data are representative of three independent experiments. * p < 0.01 compared with indicated groups.
3.6. Inhibition of p38 attenuates morphine-induced alteration of Bcl-2 and Bax expression
It has been reported that p38 MAPK modulates Bcl-2/Bax-mediated apoptosis in neuroblastoma cells (Gomez-Lazaro et al., 2007). In the present study, to examine whether p38 MAPK modulates Bcl-2 signaling following morphine treatment in BV-2 cells, we determined the expression of Bcl-2 and Bax. BV-2 cells were treated with SB203580 for 1 h and then treated with or without morphine for 24 h. As shown in Fig. 6, inhibition of p38 MAPK by SB203580 significantly inhibited morphine-induced changes of Bcl-2 and Bax compared with the morphine treatment alone. SB203580 alone did not alter the levels of Bcl-2 and Bax. Similar results were observed in mouse primary microglia (data not shown). Collectively, our data indicate that morphine induces microglia apoptosis via p38 MAPK-mediated mitochondrial pathway.
Fig. 6.
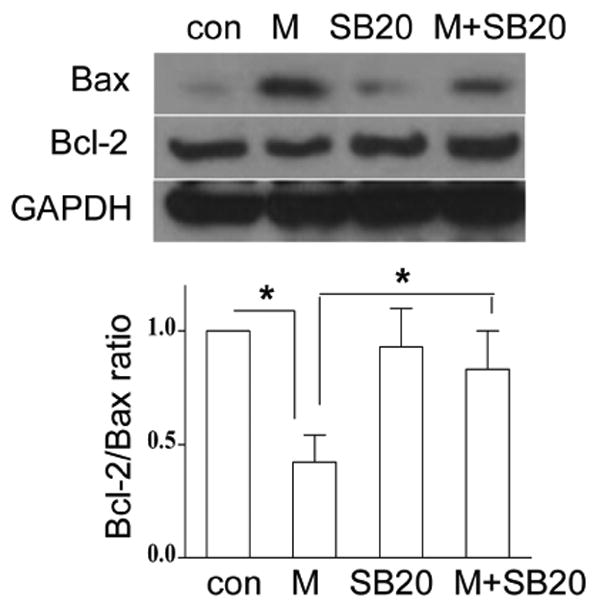
Inhibition of p38 MAPK attenuates morphine-induced changes of Bax and Bcl-2 expression. BV-2 cells were treated with SB203850 and morphine as in Fig. 5A. The expression of Bax and Bcl-2 was determined by Western blot analysis. Mean values were derived from three independent experiments. * p <0.01 compared with indicated groups.
3.7. Effect of p38 MAPK on the level of GSK-3β following morphine treatment
P38 MAPK can phosphorylate GSK3β at serine 389 in the brain and thymocytes (Thornton et al., 2008) but whether p38 MAPK work as an upstream regulator of GSK3β in morphine induced microglia apoptosis is unknown. To determine whether GSK3β can be phosphorylated by p38 MAPK, BV-2 cells were pretreated with p38 MAPK inhibitor SB203580 and then treated with morphine. As shown in Fig. 7, inhibition of p38 MAPK by p38 MAPK inhibitor SB203580 combined with morphine treatment did not alter the level of phosphor-GSK-3β at serine 9 compared to the cells treated with morphine alone, indicating that p38 MAPK is not an upstream regulator of GSK-3β in morphine-mediated microglia apoptosis.
Fig. 7.
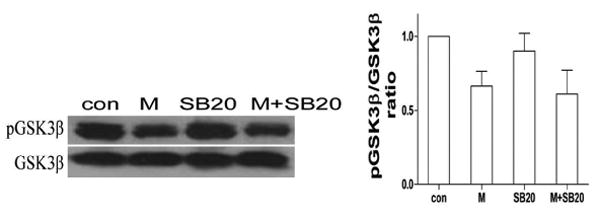
Effect of p38 MAPK on morphine-induced changes of GSK-3β. BV-2 cells were subjected to SB203850 and morphine as in Fig. 5A. The levels of total and p- GSK-3β were determined by Western blot analysis. Mean values were derived from three independent experiments.
3.8. Morphine could not alter the levels of Fas and FasL in microglia
Our previous studies reported that morphine increases the expression of Fas and promotes FasL-mediated apoptosis in lymphocytes (Yin et al., 1999a). To examine whether morphine induces the expression of Fas and FasL in microglia, we determined the expression of FasL and Fas by RT-PCR analysis in microglia cells following morphine treatment. As shown in Fig. 8, morphine could not induce the expression of Fas and FasL in microglial cells.
Fig. 8.
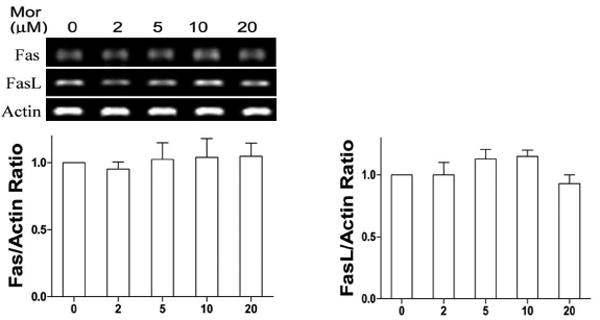
Morphine does not alter the expression of Fas and FasL. BV-2 cells were incubated in various concentrations of morphine as indicated for 12 h. The expression of Fas and FasL was determined by RT-PCR analysis. RT-PCR is representative, and the densitometry data represent the mean ± SEM from three independent experiments.
3.9. GSK-3β and p38 MAPK modulate the levels of Bax and Bcl-2 at the transcriptional level
To investigate whether GSK-3β and p38 MAPK regulate the levels of Bax and Bcl-2 at transcription or post-transcription in our morphine treatment model, we determined the levels of Bax and Bcl-2 by RT-PCR. We found that inhibition of GSK-3β by GSK-3β inhibitor SB216763 increased morphine induced changes of Bax and Bcl-2 (Fig. 9). However, inhibition of p38 MAPK by p38 MAPK inhibitor SB203580 attenuated morphine induced changes of Bax and Bcl-2 (Fig. 9). These results are consistent with the Western blot analysis. Therefore, our data suggest that GSK-3β and p38 MAPK modulate the expression levels of Bax and Bcl-2 at the transcriptional level.
Fig. 9.
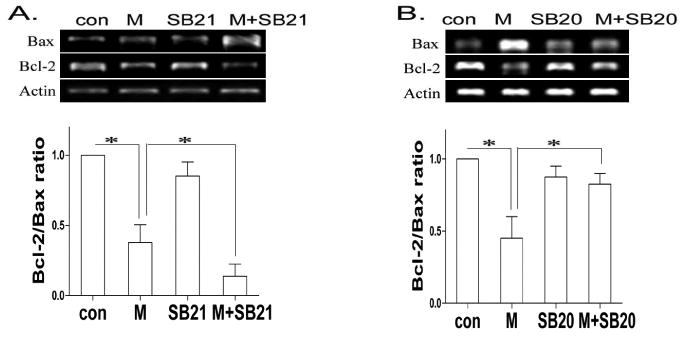
Mophine regulates the levels of Bax and Bcl-2 at the transcriptional level. BV-2 cells were treated with SB216763 or SB203850 in the absence or presence of morphine as in Figs. 4 and 5. The expression of Bax and Bcl-2 was determined by RT-PCR analysis. Mean values were derived from three independent experiments. * p < 0.01 compared with indicated groups.
4. Discussion
Our previous studies have shown that opioids promote cell apoptosis both in vitro and in vivo (Yin et al., 1999a; Yin et al., 2000; Yin et al., 2006; Moorman et al., 2009). Recently, we and others have revealed that the Akt/GSK3β signaling pathway plays an important role in opioid-induced apoptosis (Zhao et al., 2009; Li et al., 2010; Boronat et al., 2001). Apoptotic pathways induced by opioids seem to be mediated by the mitochondrial apoptotic pathway, associated with a decrease in Bcl-2 levels (Lin et al., 2009). The effects of opioids on neurons are well known. However, very little is known about the effect of opioids on microglia. Microglia is an important glial cells that function as the major innate immune cells in the brain (Olson and Miller, 2004;Hertz et al., 1990; Mandrekar et al., 2009).
Previous studies (Hu et al., 2002) have shown that morphine induces apoptosis of human microglia. We found that morphine induces microglia cell death and apoptosis in a dose- and time-dependent manner. These data are consistent with the findings of Hu (Hu et al., 2002). However, the mechanisms by which morphine induces microglia apoptosis remain elusive. In the current study, we investigated the mechanisms in morphine-induced apoptosis in microglial BV-2 and mouse primary microglial cells. We observed that morphine primed microglia apoptosis in an opioid receptor dependent mechanism. Our evidences have already shown that caspase-3 activity was significantly increased by chronic morphine exposure in neurons (Li et al., 2010). The morphine-induced activation of caspase-3 could be blocked by naloxone, a specific opioid receptor antagonist, indicating a pivotal role of an opioid receptor in this process. The data presented herein demonstrated for the first time, to our best knowledge, a key role for microglial GSK3β and p38 MAPK in the induction of morphine-mediated apoptosis. Our studies show that chronic morphine treatment modulates Akt/GSK3β signaling and results in microglia apoptosis.
We have previously reported that opioids activate phosphorylation of Akt and GSK3β in neuron and cancer cells (Zhao et al., 2009; Li et al., in press). Akt is an important physiologic mediator of the PI3K pathway (Martin et al., 2005; Cantley, 2002). Phosphorylation of Akt activates the enzyme, which modulates cell survival and apoptosis (Cantley, 2002). Activated Akt phosphorylates several downstream targets of the PI3K pathway including GSK3 (Jope and Johnson, 2004; Martin et al., 2005). GSK3 is a constitutively active enzyme that is inactivated by Akt through phosphorylation of serine 9 (Jope and Johnson, 2004). GSK3 is a crucial regulator of many cellular functions, including cell survival and apoptosis (Jope and Johnson, 2004). The role of Akt/GSK3 in the response to opioids in microglia is only now coming to light. We showed that morphine-induced dephosphorylation of Akt/GSK3β in an opioid receptor dependent mechanism (Figs. 2 and 3). Furthermore, we found that inhibition of GSK3β significantly increased morphine-induced apoptosis in both BV-2 and mouse primary microglia cells (Fig. 3). Taken together, our data clearly demonstrate that GSK3β is required for morphine-induced microglia apoptosis.
It is important to point out that not all reports have demonstrated GSK3β plays an anti-apoptotic role (Beurel and Jope, 2006; Zhang et al., 2008b; Kotliarova et al., 2008). Recent evidence revealed that GSK-3β plays both pro- and anti-apoptotic effects (Zhang et al., 2008b; Kotliarova et al., 2008; Beurel and Jope, 2006; Kotliarova et al., 2008). GSK3β promotes apoptosis mainly through the mitochondria-mediated intrinsic apoptotic pathway (Beurel and Jope, 2006). It is also established that GSK3 inhibits the death receptor-mediated extrinsic apoptosis pathways (Beurel and Jope, 2006; Kotliarova et al., 2008). We therefore evaluated the effects of GSK3β on mitochondrial-mediated apoptotic pathway following morphine treatment. We observed that inhibition of GSK3β and morphine treatment significantly decreased the levels of anti-apoptotic protein Bcl-2 but increased the levels of pro-apoptotic protein Bax (Fig. 4A). Thus, morphine promotes microglia apoptosis through GSK3β-mediated mitochondria pathway. We found that morphine could not induce the expression of Fas and Fas ligand (FasL) in microglia cells. Therefore, we do not have evidence to support that morphine also activates the extrinsic apoptotic pathway.
It has been reported that inhibition of GSK3β kinase activity with SB216763 results in increased p38 MAPK activation in COS-7 cells (Abell et al., 2007). However, the role of GSK3β in regulating p38 MAPK in microglia following morphine treatment was unclear. Here we show that co-treatment of microglia with GSK3β SB216763 and morphine significantly enhanced the levels of p38 MAPK activation compared with morphine treatment alone (Fig. 4B). Morphine-enhanced p38 MAPK activation was mediated through an opioid receptor (Fig. 4C). However, the exact mechanism of how GSK3β inhibits p38 MAPK activity is unclear. Recent evidence revealed that p38 MAPK can phosphorylate GSK3β at serine 389 in the brain and thymocytes (Thornton et al., 2008). Our studies support that p38 MAPK is not an upstream regulator of phosphor-GSK-3β at serine 9 in morphine-mediated microglia apoptosis (Fig. 7). The role of phosphorylation of GSK-3β at serine 389 in p38 MAPK mediated signaling will be investigated in future studies.
MAPKs are a family of serine/threonine kinases that perform important functions as mediators of cellular responses to various extracellular stimuli, including cell survival and apoptosis. MAPKs consist of three major subfamilies in mammalian cells (Chang and Karin, 2001), including p38 MAPK and the extracellular regulated kinases (ERK1/2, also p42/44). The activation of ERK1/2 kinase is generally associated with inhibition of apoptosis, while p38 activity promotes apoptosis (Chang and Karin, 2001; Xia et al., 1995). Chronic morphine treatment increases the phosphorylation of p38 MAPK and ERK1/2 in dorsal root ganglion neurons (Ma et al., 2001;Macey et al., 2006). We found in this study that morphine induces microglia p38 MAPK activation (Fig. 4C) and ERK1/2 activation (data not shown). Moreover, our results showed that inhibition of p38 MAPK dramatically inhibited morphine-induced microglia apoptosis (Fig. 5). The molecular mechanism of morphine enhancement of p38 MAPK activation is currently unclear, but it seems to be mediated by MEKK4, the upstream kinase of p38 MAPK. However, more study is required to reveal the detailed pathway from an opioid receptor to p38 MAPK.
Recently, it has been shown that p38 is necessary for Bcl-2-induced inhibition of apoptosis in fibroblasts (Nelyudova et al., 2007). The role of Bcl-2 and Bax in p38 MAPK-mediated microglia apoptosis was investigated in our studies. We found that inhibition of p38 MAPK and morphine treatment significantly increased Bcl-2 expression and decreased Bax expression (Fig. 6). Thus, our studies demonstrate that Bcl-2 and Bax, members of the Bcl-2 family, participate in p38 MAPK-mediated microglia apoptosis induced by morphine treatment. Further, we identified that downstream of p38 MAPK, caspase-8 as a crucial factor for morphine-induced microglia apoptosis. Our data showed that inhibition of GSK-3β enhanced morphine induced changes of Bax and Bcl-2. However, inhibition of p38 MAPK decreased morphine induced changes of Bax and Bcl-2 (Fig. 9). Thus, our studies suggest that GSK-3β and p38 MAPK modulate the expression levels of Bax and Bcl-2 at the transcriptional level.
In summary, to our best knowledge, this study is the first report in elucidating the molecular mechanisms that GSK3β and p38 MAPK are required for morphine-induced microglia apoptosis. Further understanding the mechanisms mediated by GSK3β and p38 MAPK may provide insights into potential therapeutic interventions opioid-related side effects.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grant DA020120-03A1 and the ETSU grant to D. Yin. The authors wish to express their appreciation to Dr. Gary Landreth (Case Western Reserve University School of Medicine, OH), for providing BV-2 mouse microglia cell line.
Footnotes
Disclosures: The authors have no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abell AN, Granger DA, Johnson GL. MEKK4 stimulation of p38 and JNK activity is negatively regulated by GSK3beta. J Biol Chem. 2007;282:30476–30484. doi: 10.1074/jbc.M705783200. [DOI] [PubMed] [Google Scholar]
- Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronat MA, Garcia-Fuster MJ, Garcia-Sevilla JA. Chronic morphine induces up-regulation of the pro-apoptotic Fas receptor and down-regulation of the anti-apoptotic Bcl-2 oncoprotein in rat brain. Br J Pharmacol. 2001;134:1263–1270. doi: 10.1038/sj.bjp.0704364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Chang CW, Tsai WH, Chuang WJ, Lin YS, Wu JJ, Liu CC, Tsai PJ, Lin MT. Procaspase 8 and Bax are up-regulated by distinct pathways in Streptococcal pyrogenic exotoxin B-induced apoptosis. J Biol Chem. 2009;284:33195–33205. doi: 10.1074/jbc.M109.020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang Y, Sun X, Li H, LeSage G, Javer A, Zhang X, Wei X, Jiang Y, Yin D. Synthetic resveratrol aliphatic acid inhibits TLR2-mediated apoptosis and an involvement of Akt/GSK3beta pathway. Bioorg Med Chem. 2009;17:4378–4382. doi: 10.1016/j.bmc.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069:235–243. doi: 10.1016/j.brainres.2005.11.066. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Cantley LC. Phosphoinositide 3-kinase in immunological systems. Semin Immunol. 2002;14:7–18. doi: 10.1006/smim.2001.0337. [DOI] [PubMed] [Google Scholar]
- Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- Gomez-Lazaro M, Galindo MF, Melero-Fernandez de Mera RM, Fernandez-Gomez FJ, Concannon CG, Segura MF, Comella JX, Prehn JH, Jordan J. Reactive oxygen species and p38 mitogen-activated protein kinase activate Bax to induce mitochondrial cytochrome c release and apoptosis in response to malonate. Mol Pharmacol. 2007;71:736–743. doi: 10.1124/mol.106.030718. [DOI] [PubMed] [Google Scholar]
- Hertz L, McFarlin DE, Waksman BH. Astrocytes: auxiliary cells for immune responses in the central nervous system? Immunol Today. 1990;11:265–268. doi: 10.1016/0167-5699(90)90106-j. [DOI] [PubMed] [Google Scholar]
- Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009;29:998–1005. doi: 10.1523/JNEUROSCI.4595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Ichijo H. From receptors to stress-activated MAP kinases. Oncogene. 1999;18:6087–6093. doi: 10.1038/sj.onc.1203129. [DOI] [PubMed] [Google Scholar]
- Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotliarova S, Pastorino S, Kovell LC, Kotliarov Y, Song H, Zhang W, Bailey R, Maric D, Zenklusen JC, Lee J, Fine HA. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-kappaB, and glucose regulation. Cancer Res. 2008;68:6643–6651. doi: 10.1158/0008-5472.CAN-08-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Lee J, Kim S, Lee MS, Yagita H, Kim SY, Kim H, Suk K. NO as an autocrine mediator in the apoptosis of activated microglial cells: correlation between activation and apoptosis of microglial cells. Brain Res. 2001;892:380–385. doi: 10.1016/s0006-8993(00)03257-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Sun X, Zhang Y, Huang J, Hanley G, Ferslew KE, Peng Y, Yin D. Morphine promotes apoptosis via TLR2, and this is negatively regulated by beta-arrestin 2. Biochem Biophys Res Commun. 2009;378:857–861. doi: 10.1016/j.bbrc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Li Y, Li H, Zhang Y, Sun X, Hanley GA, Zhang Y, Sun S, Peng Y, Yin D. Toll-like receptor 2 is required for opioids-induced neuronal apoptosis. Biochem Biophys Res Commun. 2010;391:426–430. doi: 10.1016/j.bbrc.2009.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Wang YJ, Li Q, Hou YY, Hong MH, Cao YL, Chi ZQ, Liu JG. Chronic high-dose morphine treatment promotes SH-SY5Y cell apoptosis via c-Jun N-terminal kinase-mediated activation of mitochondria-dependent pathway. FEBS J. 2009;276:2022–2036. doi: 10.1111/j.1742-4658.2009.06938.x. [DOI] [PubMed] [Google Scholar]
- Liu K, Chi DS, Li C, Hall K, Milhorn D, Krishnaswamy G. HIV-1 Tat protein-induced VCAM-1 expression in human pulmonary artery endothelial cells and its signaling. Am J Physiol Lung Cell Mol Physiol. 2005;289:L252–L260. doi: 10.1152/ajplung.00200.2004. [DOI] [PubMed] [Google Scholar]
- Ma W, Zheng WH, Powell K, Jhamandas K, Quirion R. Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons: an in vitro and in vivo study. Eur J Neurosci. 2001;14:1091–1104. doi: 10.1046/j.0953-816x.2001.01731.x. [DOI] [PubMed] [Google Scholar]
- Macey TA, Lowe JD, Chavkin C. Mu opioid receptor activation of ERK1/2 is GRK3 and arrestin dependent in striatal neurons. J Biol Chem. 2006;281:34515–34524. doi: 10.1074/jbc.M604278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci. 2009;29:4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Mazumder S, Plesca D, Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol. 2008;414:13–21. doi: 10.1007/978-1-59745-339-4_2. [DOI] [PubMed] [Google Scholar]
- Moorman J, Zhang Y, Liu B, LeSage G, Chen Y, Stuart C, Prayther D, Yin D. HIV-1 gp120 primes lymphocytes for opioid-induced, beta-arrestin 2-dependent apoptosis. Biochim Biophys Acta. 2009;1793:1366–1371. doi: 10.1016/j.bbamcr.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelyudova A, Aksenov N, Pospelov V, Pospelova T. By blocking apoptosis, Bcl-2 in p38-dependent manner promotes cell cycle arrest and accelerated senescence after DNA damage and serum withdrawal. Cell Cycle. 2007;6:2171–2177. doi: 10.4161/cc.6.17.4610. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Porras A, Zuluaga S, Black E, Valladares A, Alvarez AM, Ambrosino C, Benito M, Nebreda AR. P38 alpha mitogen-activated protein kinase sensitizes cells to apoptosis induced by different stimuli. Mol Biol Cell. 2004;15:922–933. doi: 10.1091/mbc.E03-08-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Barke RA, Loh HH. MU-opioid receptor-knockout mice: role of mu-opioid receptor in morphine mediated immune functions. Brain Res Mol Brain Res. 1998;61:190–194. doi: 10.1016/s0169-328x(98)00212-5. [DOI] [PubMed] [Google Scholar]
- Shi Y, Feng Y, Kang J, Liu C, Li Z, Li D, Cao W, Qiu J, Guo Z, Bi E, Zang L, Lu C, Zhang JZ, Pei G. Critical regulation of CD4+ T cell survival and autoimmunity by beta-arrestin 1. Nat Immunol. 2007;8:817–824. doi: 10.1038/ni1489. [DOI] [PubMed] [Google Scholar]
- Singhal PC, Bhaskaran M, Patel J, Patel K, Kasinath BS, Duraisamy S, Franki N, Reddy K, Kapasi AA. Role of p38 mitogen-activated protein kinase phosphorylation and Fas-Fas ligand interaction in morphine-induced macrophage apoptosis. J Immunol. 2002;168:4025–4033. doi: 10.4049/jimmunol.168.8.4025. [DOI] [PubMed] [Google Scholar]
- Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- Svensson AL, Bucht N, Hallberg M, Nyberg F. Reversal of opiate-induced apoptosis by human recombinant growth hormone in murine foetus primary hippocampal neuronal cell cultures. Proc Natl Acad Sci U S A. 2008;105:7304–7308. doi: 10.1073/pnas.0802531105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder I, Geisslinger G. Opioids as modulators of cell death and survival--unraveling mechanisms and revealing new indications. Pharmacol Rev. 2004;56:351–369. doi: 10.1124/pr.56.3.2. [DOI] [PubMed] [Google Scholar]
- Thornton T, Pedraza-Alva G, Deng B, Wood C, Aronshtam A, Clements J, Sabio G, Davis R, Matthews D, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yin D, Mufson RA, Wang R, Shi Y. Fas-mediated cell death promoted by opioids. Nature. 1999a;397:218. doi: 10.1038/16612. [DOI] [PubMed] [Google Scholar]
- Yin D, Tuthill D, Mufson RA, Shi Y. Chronic restraint stress promotes lymphocyte apoptosis by modulating CD95 expression. J Exp Med. 2000;191:1423–1428. doi: 10.1084/jem.191.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D, Woodruff M, Zhang Y, Whaley S, Miao J, Ferslew K, Zhao J, Stuart C. Morphine promotes Jurkat cell apoptosis through pro-apoptotic FADD/P53 and anti-apoptotic PI3K/Akt/NF-kappaB pathways. J Neuroimmunol. 2006;174:101–107. doi: 10.1016/j.jneuroim.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Yin D, Zhang L, Wang R, Radvanyi L, Haudenschild C, Fang Q, Kehry MR, Shi Y. Ligation of CD28 in vivo induces CD40 ligand expression and promotes B cell survival. J Immunol. 1999b;163:4328–4334. [PubMed] [Google Scholar]
- Zhang Y, Foster R, Sun X, Yin Q, Li Y, Hanley G, Stuart C, Gan Y, Li C, Zhang Z, Yin D. Restraint stress induces lymphocyte reduction through p53 and PI3K/NF-kappaB pathways. J Neuroimmunol. 2008a;200:71–76. doi: 10.1016/j.jneuroim.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y, Miao J, Hanley G, Stuart C, Sun X, Chen T, Yin D. Chronic restraint stress promotes immune suppression through toll-like receptor 4-mediated phosphoinositide 3-kinase signaling. J Neuroimmunol. 2008b;204:13–19. doi: 10.1016/j.jneuroim.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Zhou G, Zhang Y, Chen T, Sun X, Stuart C, Hanley G, Li J, Zhang J, Yin D. beta-arrestin2 inhibits opioid-induced breast cancer cell death through Akt and caspase-8 pathways. Neoplasma. 2009;56:108–113. doi: 10.4149/neo_2009_02_108. [DOI] [PubMed] [Google Scholar]


