Abstract
We investigated the nuclear import of low risk HPV11 E7 protein using 1) transfection assays in HeLa cells with EGFP fusion plasmids containing 11E7 and its domains and 2) nuclear import assays in digitonin-permeabilized HeLa cells with GST fusion proteins containing 11E7 and its domains. The EGFP-11E7 and EGFP-11cE739–98 localized mostly to the nucleus. The GST-11E7 and GST-11cE739–98 were imported into the nuclei in the presence of either Ran-GDP or RanG19V-GTP mutant and in the absence of nuclear import receptors. This suggests that 11E7 enters the nucleus via a Ran-dependent pathway, independent of nuclear import receptors, mediated by a nuclear localization signal located in its C-terminal domain (cNLS). This cNLS contains the zinc-binding domain consisting of two copies of Cys-X-X-Cys motif. Mutagenesis of Cys residues in these motifs changed the localization of the EGFP-11cE7/-11E7 mutants to cytoplasmic, suggesting that the zinc-binding domain is essential for nuclear localization of 11E7.
Introduction
Human papillomaviruses (HPVs) infections cause hyperproliferative lesions of mucosal and cutaneous epithelial tissues. Some 200 HPV genotypes have been identified and 30 of them infect anogenital mucosal tissues. Depending on their oncogenic potential mucosal HPVs have been classified into high risk, such as HPV types 16, 18, 31 and 45, and low risk, such as HPV types 6 and 11. The high risk HPVs have been frequently detected in cervical cancers and also in anal, perianal, vulvar, penile and oropharyngeal cancers. The low risk HPV types are associated with benign anogenital warts which can affect 1–2% of young adults in many countries (Doorbar, 2006; zur Hausen, 2000; zur Hausen, 2009).
The replication cycle of HPVs is connected to the differentiation program of host epithelial cells. Productive viral DNA amplification occurs in the upper layers of the postmitotic, differentiated epithelial cells and depends on the host DNA replication machinery. Consequently, HPVs have evolved the E7 proteins to induce reentry into the S phase of the differentiated epithelial cells and establish the appropriate environment necessary to support viral DNA amplification. The high risk HPV16/18 oncoproteins bind and destabilize nuclear proteins involved in the control of the cell cycle, like the retinoblastoma protein (pRB) and the RB-related pocket proteins, p130 and p107 and this binding is mediated by the LxCxE motif in the CR2 domain. The low risk HPV E7 proteins bind pRB with approx. 10-fold lower efficiency and destabilize it inefficiently (McLaughlin-Drubin and Munger, 2009). More recently it has been shown that both the high risk HPV18 E7 and the low risk HPV11 E7 bind and target nuclear p130 for degradation and this induces S-phase entry in the differentiated human keratinocytes. This activity is dependent on an intact pocket protein binding domain and phosphorylation by casein kinase II (CK II) of serine residues located in the CR2 domain (Genovese et al., 2008).
Both HPV16 E7 and HPV11 E7 have also targets in the cytoplasm, such as the microtubule-associated N-end rule ubiqutin ligase p600 (Huh et al., 2005) and can cause the relocalization of steroid receptor coactivator (SRC-1) from the nucleus to the cytoplasm (Baldwin, Huh, and Munger, 2006). More recently, it was reported that HPV16 E7 and HPV11 E7 can associate with the nuclear mitotic apparatus protein (NuMA) and this correlates with induction of defects in chromosome alignment during prometaphase (Nguyen and Munger, 2009).
High risk HPV16 E7 is predominantly a nuclear protein in invasive cervical carcinoma in situ, in the CaSki cervical carcinoma cell line and also when expressed transiently in different cell lines such as HaCaT and U2OS (Guccione et al., 2002). We have previously analyzed the nuclear import of HPV16 E7 and discovered that it enters the nucleus via a novel Ran-dependent pathway that is independent of karyopherin β import receptors/ importins and this pathway is mediated by cNLS located in the C terminal domain (Angeline, Merle, and Moroianu, 2003; Knapp et al., 2009). We discovered that HPV16 E7 contains also a leucine-rich nuclear export signal (NES) in the C terminal domain which can mediate nuclear export of 16E7 (Knapp et al., 2009). The low risk HPV11 E7 protein is localized mostly to the nucleus when expressed in different cell lines such as U2OS and HaCaT (Guccione et al., 2002). However, virtually nothing was known about the nuclear import pathways and the NLS(s) mediating its entry into the nucleus.
In this study we investigated the nuclear import pathway for the low risk HPV11 E7 protein and found that it enters the nucleus via a Ran-dependent pathway and independent of nuclear import receptors belonging to the karyopherin β/importin β family. We also identified a cNLS located in the C-terminal domain, which mediates this Ran-dependent nuclear import pathway for HPV11 E7 protein. Both the full length HPV11 E7 and its cNLS can bind to Phenyl-Sepharose beads, which mimic the specificity of phenylalanine-glycine (FG)-containing nucleoprins at the nuclear pore complex. These data support a model in which HPV11 E7 protein may enter the nucleus via direct low affinity hydrophobic interactions with FG-containing nucleoporins in a similar manner as the nuclear import receptors belonging to the karyopherin β/importin β family. Indeed, both Kap β1 and Kap β2 receptors in excess inhibited nuclear import mediated by HPV11 E7' cNLS in competition nuclear import assays. Mutagenesis of Cys residues in the two CysXXCys motifs involved in zinc binding changed the localization of the resultant EGFP-11cE7 and EGFP-11E7 to mostly cytoplasmic suggesting that the integrity of the zinc binding domain is essential for the activity of the cNLS driving the nuclear localization of 11E7 protein.
Results
HPV11 E7 enters the nucleus via a Ran dependent pathway and independent of nuclear import receptors
In order to investigate the intracellular localization of low risk HPV11 E7 we used transient transfections in HeLa cells with a plasmid containing EGFP fused to the N-terminus of HPV11 E7. Confocal fluorescence microscopy analysis revealed that the expressed EGFP-11E7 fusion protein was localized predominantly in the nucleus (Fig. 1, panel A), whereas the EGFP itself was as expected pancellular (data not shown). An HA-tagged 11E7 protein was previously found localized mostly to the nucleus when expressed in different cell lines including U2OS and HaCaT (Guccione et al., 2002). To examine if CKII phosphorylation plays a role in nuclear localization of low risk HPV11 E7 we analyzed the localization of an EGFP-11E7 32SS/AA mutant, which cannot be phosphorylated by CKII, in comparison with the EGFP-11E7 wild type. Transient transfection experiments in HeLa cells revealed that the EGFP-11E7SS/AA mutant is mostly nuclear in the majority of cells (Fig. 1, panel C and Fig. 4B) similar with the EGFP-11E7 wild type (Fig. 1, panel A and Fig. 4B), suggesting that CKII phosphorylation is not required for the nuclear localization of 11E7 protein. We previously obtained similar results regarding the nuclear localization of high risk HPV16 E7 (Knapp et al., 2009).
Fig. 1. HPV11 E7 wild type and a variant deficient in CKII phosphorylation are predominantly nuclear.
HeLa cells were transfected with either EGFP-11E7 wild type or the EGFP-11E7SS/AA mutant and examined by fluorescence microscopy at 24 h post transfection. Panels A and C represent the fluorescence of the EGFP and panels B and D the DAPI staining of the nuclei.
Fig. 4B. Quantitative analysis of the intracellular localization of EGFP fusions with 11E7 full length, and its N and C domains.
The data from experiments using EGFP-11E7, EGFP-11E7SS/AA mutant, EGFP-11nE71–38 and EGFP-11cE739–98 plasmids have been used for the quantification graphic representation. Black bars, predominant nuclear localization; gray bars, pancellular localization; dotted bars, predominant cytoplasmic localization.
To dissect the nuclear import pathway for HPV11 E7 protein we generated a GST-11E7 fusion protein and tested it in nuclear import assays in digitonin-permeabilized HeLa cells. The GST-11E7 protein entered into the nucleus in the presence of exogenous cytosol, whereas the GST negative control did not (Fig. 2, panels B and D). In previous studies we discovered that the high risk HPV16 E7 oncoprotein enters the nucleus via a Ran-dependent import pathway and independent of nuclear import receptors belonging to the karyopherin/importin family (Angeline, Merle, and Moroianu, 2003). Therefore, we analyzed the nuclear import of GST-11E7 in the presence of either Ran-GDP or a RanG19V-GTP mutant deficient in GTP hydrolysis. The RanG19V-GTP mutant is an inhibitor of karyopherin/importin-mediated pathways as it binds to the Kap β import receptors and prevents formation of the import complexes. As a positive control we used the GST-M9 fusion protein, where M9 is the NLS of hnRNP A1 using the Kap β2/transportin-mediated pathway (Bonifaci et al., 1997; Chook and Blobel, 1999). As expected, the positive control GST-M9 was imported in the presence of Ran-GDP plus Kap β2 whereas the RanG19V-GTP mutant inhibited its nuclear import (Fig. 3, panels B and C). The GST-11E7 protein was imported into the nucleus in the presence of either Ran-GDP and the RanG19V-GTP mutant (Fig. 3, panels E and F) whereas the GST negative control was not imported in any condition (Fig. 3, panels G–I). These data suggest that the low risk HPV11 E7, like the high risk HPV16 E7 oncoprotein, enters the nucleus via a Ran-dependent pathway and independent of karyopherin β nuclear import receptors.
Fig. 2. Low risk HPV11 E7 protein is imported into the nuclei of digitonin-permeabilized HeLa cells in the presence of exogenous cytosol.
Digitonin-permeabilized HeLa cells were incubated with either GST-11E7 (A and B), or GST (C and D) in the presence of either transport buffer (A and C) or exogenous HeLa cytosol (B and D). Protein localization was detected with an anti-GST antibody. Note the nuclear import in panel B.
Fig. 3. HPV11 E7 nuclear import is mediated by either Ran-GDP or the RanG19V-GTP mutant deficient in GTP hydrolysis.
Digitonin-permeabilized HeLa cells were incubated with either GST-M9 (A, B, C), GST-11E7 (D, E, F) or GST (G, H, I) in the presence of either transport buffer (A, D, G), recombinant Ran-GDP (B, E, H) or recombinant RanG19V-GTP (C, F, I). Import reactions contained also Kap ß2 import receptor in B and C. Protein localization was detected with an anti-GST antibody. Note the nuclear import in panels B, E and F.
HPV11 E7 has a cNLS which mediates nuclear import via a Ran-dependent pathway and independent of import receptors
To analyze which domains can mediate the nuclear localization of HPV11 E7 in vivo we made EGFP-E7 domains fusion plasmids and transfected HeLa cells. Analysis of the localization of the translated EGFP-E7 fusion proteins using confocal fluorescence microscopy revealed that the EGFP-11cE739–98 was mostly nuclear in like the EGFP-11E7 (Fig. 4A, panels A and C; Fig. 4B), whereas the EGFP-11nE71–38 showed a pancellular localization in the majority of cells (Fig. 4A, panel B; Fig. 4B) with some 30% of cells having cytoplasmic localization (Fig. 4B). Immunoblot analysis with an anti-GFP Ab of the different EGFP-11E7 translated proteins indicated that they are intact, expressed at the correct molecular weight (data not shown). The data suggest that HPV11 E7 protein contains a potential NLS in the C-terminal domain (cNLS) mediating the nuclear localization of EGFP-11cE7.
Fig. 4A. EGFP-11E7 and EGFP-11cE739–98 are predominantly nuclear in HeLa cells.
HeLa cells were transfected with EGFP-11E7, EGFP-11nE71–38 and EGFP-11cE739–98 fusion plasmids as indicated in the figure. Panels A, B and C show the GFP staining and panels D, E and F the DAPI staining of the nuclei.
To analyze the nuclear import mediated by this potential cNLS we generated GST-11cE7 fusion protein and tested it in nuclear import assays in digitonin-permeabilized HeLa cells in the presence of either HeLa cytosol, Ran-GDP or the RanG19V-GTP mutant. As a positive control we used GST-M9 and as a negative control GST itself. The GST-11cE7 was imported in the presence of either Ran-GDP or RanG19V-GTP (Fig. 5, panels E and F) or HeLa cytosol (data not shown). As expected the GST-M9 entered the nuclei in the presence of RanGDP + Kap β2 and the RanG19V-GTP mutant inhibited its nuclear import (Fig. 5, panels B and C). The GST negative control was not imported into the nuclei in any conditions (Fig. 5, panels G–I). These data suggest that the cNLS of 11E7 mediates nuclear import via a Ran dependent pathway and independent of Kap β nuclear import receptors. To note that a GST-n11E7 fusion protein was not imported into the nuclei in any of these conditions in agreement with the localization data of the EGFP-11E7 (data not shown).
Fig. 5. GST-11cE7 enters the nucleus in the presence of either Ran-GDP or the RanG19V-GTP mutant.
Digitonin-permeabilized HeLa cells were incubated with either GST-M9 (A, B, C), GST-11cE7 (D, E, F) or GST (G, H, I) in the presence of either transport buffer (A, D, G), recombinant Ran-GDP (B, E, H) or recombinant RanG19V-GTP (C, F, I). Import reactions contained also Kap ß2 in B and C. Protein localization was detected with an anti-GST antibody. Note the nuclear import in panels B, E and F.
One possible mechanism for 11E7 nuclear import is via direct hydrophobic low affinity interactions with nucleoporins containing FG (phenylalanine-glycine) repeats and bypassing the requirement for karyopherins/importins. Previously it has been shown that a simple Phenyl-Sepharose column mimics the specificity of the FG-containing nucleoporins and binds all the nuclear import and export receptors belonging to the karyopherin superfamily (Ribbeck and Gorlich, 2002). Therefore we analyzed the interactions of GST-11E7 and GST-11cE7 with a Phenyl-Sepharose matrix. As a positive control we used GST-Kap β2 and as a negative control GST itself. Both the 11E7 and the 11cE7 bound to Phenyl-Sepharose as did the Kap β2 import receptor (Fig. 6, lanes 1–3), whereas the GST showed only a low background (Fig. 6, lane 4). These data suggest that HPV11 E7 may indeed interact directly with FG-nucleoporins via its cNLS and use these interactions to enter the nucleus in a similar manner as the nuclear import receptors.
Fig. 6. GST-11E7 and GST-11cE7 bind to Phenyl-Sepharose, which mimics the specificity of FG-containing nucleoporins.
Phenyl-Sepharose beads were incubated separately with either GST-11E7 (lanes 1 and 5), GST-11cE7 (lanes 2 and 6), GST-Kap β2 (lanes 3 and 7) or GST (lanes 4 and 8) for 1hr at RT. Lanes 1–4 show the bound proteins and lanes 5–8 the input used in the binding reactions.
If HPV11 E7 interacts directly with FG-containing nucleoporins via low affinity hydrophobic interactions than it would be expected that some of the nuclear import receptors would compete with E7 for binding to FG-containing nucleoporins and nuclear import. To test this we performed competition assays with Kap β1 and Kap β2 receptors in nuclear import of GST-11cE7. Digitonin-permeabilized HeLa cells were incubated with GST-11cE7 in the presence of Ran-GDP and in the absence or presence of excess of either Kap β1, Kap β2 or Kap β3. Indeed, we found that all three nuclear import receptors, Kap β1, Kap β2 and Kap β3, inhibited the Ran-dependent nuclear import of GST-11cE7 (Fig. 7, compare panels C and D with panel B, and data not shown). These data support a model where HPV11 E7 enters the nucleus via direct hydrophobic interactions of its cNLS with FG-nucleoporins at the nuclear pore complex.
Fig 7. Kap β1 and Kap β2 nuclear import receptors efficiently compete for nuclear import with GST-11cE7.
Digitonin-permeabilized HeLa cells were incubated with GST-11cE7 in only transport buffer (A), Ran-GDP (B), Ran-GDP plus Kap β1 in excess (C), or Ran-GDP plus Kap β2 in excess (D). Note the nuclear import in panel B and its inhibition in panels C and D.
Our results showed that either Ran-GDP or the RanG19V-GTP mutant can stimulate nuclear import of GST-11E7 or GST-11cE7 in digitonin-permeabilized HeLa cells. To get insight into the role of Ran in nuclear import of HPV11 E7 we analyzed if there is a direct interaction with Ran in the GDP or GTP bound conformation. GST-11E7, GST-11cE7, or GST immobilized on Glutathione-Sepharose beads were incubated separately with either Ran-GDP or RanG19V-GTP and the bound proteins were analyzed by SDS-PAGE. Neither Ran-GDP nor RanG19V-GTP bound to GST-11E7 or GST-11cE7 (Fig. S1, A and B, lanes 1 and 2).
The zinc-binding domain is essential for the nuclear import activity of HPV11 E7's cNLS
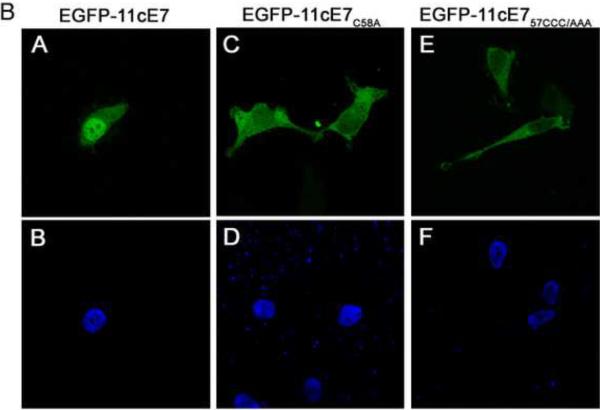
The cNLS of 11E7 located in the C terminal domain contains the zinc-binding domain consisting of two copies of Cys-X-X-Cys sequence motif separated by 29 amino acids (where all four Cys residues interact with Zn). To investigate if the zinc binding domain is essential for the nuclear import activity of the cNLS we mutated Cys residues in each of the two copies of Cys-X-X-Cys, and analyzed the localization of the resultant EGFP-11cE7 mutants in comparison with the wild type. The EGFP-11cE7C91A mutant was cytoplasmic (Fig. 8A, panel B and Fig. 8C) in contrast with the mostly nuclear localization of the EGFP-11cE7 wild type (Fig. 8A, panel A and Fig. 8C) in the great majority of cells. A similar cytoplasmic localization was observed for the EGFP-11cE7C91_ (containing a stop codon at Cys 91 and lacking both Cys91 and Cys93) (Fig. 8A, panel C). The C58A mutation also affected the nuclear localization of EGFP-11cE7C58A; however, the effect was less dramatic than the C91A mutation, with 24.5+/− 7.9% cells having a cytoplasmic localization (Fig. 8B, panel C) and 75.5+/− 7.9% cells a pancellular localization (Fig. 8C). This could be due to the fact that there are three CC58C residues and when Cys58 is mutated to Ala, one of the other Cys residues could potentially substitute for the interaction with zinc. Therefore we generated a CCC/AAA triple mutant and analyzed the localization of the EGFP-11cE7CCC/AAA mutant after transfection of HeLa cells. Indeed, the localization of the EGFP-11cE7CCC/AAA mutant was cytoplasmic in 97.9 +/− 1.3% of cells (Fig. 8B, panel E and Fig. 8C), like the localization of the EGFP-11cE7C91A and EGFP-11cE7C91_ mutants (Fig. 8A, panels B and C). Immunoblot analysis with an anti-GFP Ab of all the EGFP-11cE7 mutant proteins indicated that they are intact, expressed at the correct molecular weight (data not shown).
Fig. 8A. The EGFP-11cE7(C91A) and EGFP-11cE7(C91_) mutants are localized in the cytoplasm, in contrast with the mostly nuclear localization of the EGFP-11cE7 wild type.
HeLa cells were transfected with either EGFP-11cE7 wild type (panels A and D), EGFP-11cE7C91A (panels B and E), or EGFP-11cE7 C91_ (panels C and F) plasmids and examined by fluorescence microscopy at 24 h post transfection. Panels A, B and C represent the fluorescence of the EGFP and panels D, E and F the DAPI staining of the nuclei.
Fig. 8C. Quantitative analysis of the intracellular localization of C58A, 57CCC/AAA, and C91A mutants in comparison with the wild type EGFP-11cE7.
The data from four experiments using EGFP-11cE7, EGFP-11cE7C58A, EGFP-11cE7CCC/AAA, and EGFP-11cE7C91A plasmids have been used for the quantification graphic representation. Black bars, predominant nuclear localization; gray bars, pancellular localization; dotted bars, predominant cytoplasmic localization.
Fig. 8B. The effect of C58A and 57CCC/AAA mutations on the localization of EGFP-11cE7.
HeLa cells were transfected with either EGFP-11cE7 wild type (panels A and B), EGFP-11cE7C58A (panels C and D), and EGFP-11cE7CCC/AAA (panels E and F) plasmids and examined by fluorescence microscopy at 24 h post transfection. Panels A, C and E represent the fluorescence of the EGFP and panels B, D and F the DAPI staining of the nuclei.
In addition, in nuclear import assays in digitonin-permeabilized cells the C58A, 57CCC/AAA and C91A mutations inhibited the nuclear import of the resultant GST-11cE7 mutants (Fig. S2 and data not shown). Moreover, the 57CCC/AAA and C91A mutations in the context of EGFP-11E7 containing the 11E7 full length also led to a cytoplasmic localization (Fig. 9A, panels C and E and Fig. 9B) in the great majority of cells in contrast with the mostly nuclear localization of EGFP-11E7 wild type (Fig. 9, panel A and Fig. 9B). The C58A mutation in the context of EGFP-11E7 resulted in a mixed phenotype with both pancellular and cytoplasmic localization (Fig. 9B). Together these data suggest that the integrity of the zinc-binding domain is essential for the nuclear import activity of the 11E7' cNLS and the nuclear localization of HPV11 E7 protein.
Fig. 9A. The 57CCC/AAA and the C91A mutations led to cytoplasmic localization of 11E7 full length.
HeLa cells were transfected with EGFP-11E7 (panels A and B), EGFP-11E7CCC/AAA (panels C and D), and EGFP-11E7C91A (panels E and F) plasmids and examined by fluorescence microscopy at 24 h post transfection. Panels A, C and E represent the fluorescence of the EGFP and panels B, D and F the DAPI staining of the nuclei. Note the mostly nuclear localization of EGFP-11E7 wild type (panel A) and the cytoplasmic localization of EGFP-11E7CCC/AAA (panel C) and EGFP-11E7C91A (panel E).
Fig. 9B. Quantitative analysis of the intracellular localization of C58A, 57CCC/AAA, and C91A mutants in comparison with the wild type EGFP-11E7.
The data from four experiments using EGFP-11E7, EGFP-11E7C58A, EGFP-11E7ccc/AAA, and EGFP-11E7C91A have been used for the quantification graphic representation. Black bars, predominant nuclear localization; gray bars, pancellular localization; dotted bars, predominant cytoplasmic localization.
Cys59 is conserved in the E7 proteins of many HPVs, although it is not involved in zinc binding. To analyze if Cys59 plays a role in the nuclear localization of 11E7 we mutated Cys59 to Ala both in the EGFP-11cE7 and EGFP-11E7 and analyzed their localization using confocal fluorescence microscopy. Both the EGFP-11cE7C59A and EGFP-11E7C59A had a pancellular localization (Fig. 10A, panels C and G) in a high percent of cells (Fig. 10B) and a mostly nuclear localization only in a low percent of cells (Fig. 10B). This is in contrast with the mostly nuclear localization of the wild type proteins (Fig. 10A, panels A and E) in the majority of cells (Fig. 10B). Mutation of Cys59 to an acidic amino acid had the same effect leading to a pancellular localization of EGFP-11cE7C59D and EGFP-11E7C59D in the majority of the cells (Fig. 10B). In nuclear import assays in digitonin-permeabilized cells the nuclear import of GST-11cE7C59A and GST-11cE7C59D was also reduced in comparison with that of the GST-11cE7 wild type (Fig. S3).
Fig. 10A. The effect of C59A mutation on the localization of EGFP-c11E7 and EGFP-11E7.
HeLa cells were transfected with EGFP-11cE7 (panels A and B), EGFP-11cE7C59A (panels C and D), EGFP-11E7 (panels E and F) and EGFP-11E7C59A (panels G and H) plasmids and examined by fluorescence microscopy at 24 h post transfection. Panels A, C, E and G represent the fluorescence of the EGFP and panels B, D, F and H the DAPI staining of the nuclei. Note the mostly nuclear localization of EGFP-11cE7 and EGFP-11E7 wild type (panels A and E) and pancellular localization of the C59A mutants (panels C and G).
Fig. 10B. Quantitative analysis of the intracellular localization of C59A and C59D mutants in comparison with the wild type EGFP-11E7 and EGFP-11cE7.
The data from experiments using EGFP-11E7, EGFP-11E7C59A, EGFP-11E7C59D, EGFP-11cE7, EGFP-11cE7C59A, and EGFP-11cE7C59D plasmids have been used for the quantification representation. Black bars, predominant nuclear localization; gray bars, pancellular localization; dotted bars, predominant cytoplasmic localization.
Discussion
In this study we investigated the nuclear import pathway and the NLSs of the low risk HPV11 E7 protein using both transfection assays in HeLa cells with EGFP fusions containing 11E7 or its domains and nuclear import assays in digitonin-permeabilized cells with GST fusions to 11E7 and its domains. We used EGFP and GST tags instead of a small HA tag to increase the molecular weight of the fusion proteins containing 11E7 and its domains so that we could distinguish between active nuclear import mediated by an NLS versus passive diffusion through the nuclear pore complex coupled with nuclear retention. In addition, the use of the EGFP tag allowed us to avoid the immunostaining step with an HA antibody. The EGFP-11E7 protein was mostly nuclear in HeLa cells, as has been previously shown for the HA-11E7 protein (containing the HA tag at the N terminus) in U2OS and HaCaT cells (Guccione et al., 2002). However, there was a small fraction of the protein localized to the cytoplasm, which was resistant to the treatment with Leptomycin B (LMB), an inhibitor of nuclear export (data not shown). This suggests that this fraction of 11E7 is most likely retained in the cytoplasm via interaction with a cytoplasmic target protein(s) as we found before for 16E7 (Knapp et al., 2009). It has been previously shown that CKII phosphorylation of HPV11 E7 takes place in vivo and it is necessary for efficient binding and degradation of p130 and S-phase reentry (Genovese et al., 2008). Analysis of the localization of an 11E7SS/AA mutant deficient in CKII phosphorylation revealed that it is localized mostly to the nucleus similar to the 11E7 wild type, suggesting that CKII phosphorylation is not required for 11E7 nuclear localization.
In nuclear import assays we found that HPV11 E7 enters the nuclei of digitonin-permeabilized HeLa cells in the presence of either Ran-GDP or the RanG19V-GTP mutant deficient in GTP hydrolysis, an inhibitor of Kap β/importin-mediated pathways. These data suggest that 11E7 nuclear import requires the GTPase Ran and it is independent of the Kap β nuclear import receptors.
Analysis of the localization of EGFP fusions with 11E7 and its domains revealed that EGFP-11cE739–98 is localized mostly to the nucleus in the transfected HeLa cells indicating the presence of a cNLS in the C terminal domain of 11E7 and mediating its nuclear localization. Further investigation in nuclear import assays in digitonin-permeabilized cells showed that GST-11cE739–98 can enter the nucleus in the presence of either Ran-GDP or the RanG19V-GTP mutant deficient in GTP hydrolysis. These data suggest that the cNLS mediates nuclear import of 11E7 via a Ran-dependent pathway and independent of the nuclear import receptors. This nuclear import pathway is in common with that used by the high risk HPV16 E7 oncoprotein (Knapp et al., 2009).
The cNLS of HPV11 E7 in the C terminal domain contains a unique zinc binding domain consisting of two CysXXCys motifs separated by 29 amino acids, present in both the high and low risk HPVs (Liu et al., 2006; Ohlenschlager et al., 2006). The zinc binding domain is involved in dimerization, and mutagenesis of Cys residues affecting zinc binding also interfere with dimerization and for the high risk types with transformation (Clemens et al., 1995; McIntyre et al., 1993). We discovered that mutations of Cys residues in the two CysXXCys motifs which affect the zinc binding, clearly disrupted the nuclear localization of the resultant EGFP-11cE7 and EGFP-11E7 mutants and the nuclear import of the GST-11cE7 mutants. These data suggest that the integrity of the zinc binding domain is essential for the nuclear import activity of 11E7's cNLS and the nuclear localization of 11E7 protein. The Cys residues involved in zinc binding are the most conserved Cys residues among the different E7 proteins. It will be interesting to investigate in the future if the zinc binding domain of other HPV E7 proteins is essential for the nuclear import and localization of these proteins. Preliminary data suggest that the zinc binding domain is essential for the nuclear localization of high risk HPV16 E7 oncoprotein (Jeremy Eberhardt and Junona Moroianu). Another interesting result was the change from predominantly nuclear to pancellular localization when the conserved Cys59 residue, not involved in zinc binding, was mutated to Ala or Asp in the context of either 11E7 full length or 11cE7. These data suggest that this conserved Cys residue is involved in the nuclear import and localization of 11E7 protein.
One possible mechanism for nuclear import of 11E7 is via direct low affinity hydrophobic interactions between E7 and nucleoporins containing phenylalanine-glycine (FG) repeats, in a similar manner as the nuclear import receptors. Our results showing that 11E7 and its cNLS can bind to a Phenyl-Sepharose matrix, which mimics the selectivity of the hydrophobic interactions of FG-nucleoporins and binds all karyopherin β nuclear import receptors (Ribbeck and Gorlich, 2002), support this model. Also in agreement with this model, Kap β1, Kap β2 and Kap β3 nuclear import receptors efficiently competed with 11E7's cNLS for binding to FG-nucleoporins in nuclear import assays in digitoninpermeabilized cells and consequently inhibited its nuclear import. It has been shown that receptor surface hydrophobicity is sufficient to provide access to the nuclear pore complex and the translocation process involves non-specific interactions between hydrophobic patches on the surface of the receptors/Kaps and FG-nucleoporins (Naim et al., 2009). The zinc binding domain of E7 proteins is very rich in nonpolar, hydrophobic residues, with the majority of them arranged in hydrophobic patches, which could mediate the low affinity hydrophobic interactions with the FG-nucleoporins at the nuclear pore complex. A previous report showed that another viral protein, the TAX protein of human T lymphotropic virus type 1 enters the nucleus via direct interactions of its zinc-binding domain with FG-nucleoporins at the nuclear pore complex (Tsuji et al., 2007).
Although nuclear import of 11E7 is independent of the karyopherins/importins it is dependent on the GTPase Ran. As we found that 11E7 and 11cE7 do not interact with RanGDP, this excludes the possibility of 11E7 entering the nucleus via piggyback on RanGDP, which is imported via a specific import receptor, p10/NTF2. As RanG19V-GTP mutant can support nuclear import of 11E7 one possible role of RanGTP would be to dissociate 11E7 from its interactions with nucleoplasmic FG-nucleoporins like Nup 153, and release 11E7 into the nucleus. Future detailed biochemical analysis will test this model.
Interestingly, there is a difference between the localization of EGFP-16cE7, which is mostly cytoplasmic (Knapp et al., 2009) and EGFP-11cE7, which is predominantly nuclear (this report). The cytoplasmic localization of EGFP-16cE7 and also 2xEGFP-16cE7 is due to the activity of a leucine-rich nuclear export signal (NES) located in the C-terminal domain of 16E7. Inactivation of this NES via mutagenesis of critical residues or the use of specific inhibitors of nuclear export like leptomycin B (LMB) or ratjadone A (RJA) lead to a predominantly nuclear localization of the mutant proteins mediated by the cNLS located in the C terminal domain of 16E7 (Knapp et al., 2009). Interestingly, in the presence of the RJA nuclear export inhibitor, the localization of the EGFP-11cE7C59A mutant changes from pancellular to mostly nuclear (preliminary data), suggesting that the corresponding NES of 11E7 is also functional. The predominant nuclear localization of EGFP-11cE7 wild type could be due to the fact that either the potential corresponding NES of 11E7 is weaker than the NES of 16E7 or/and the cNLS of 11E7 is stronger than the cNLS of 16E7 and consequently, the balance between nuclear import and export is in favor of nuclear import and localization of 11cE7. Future detailed analysis will examine this difference in the nucleocytoplasmic traffic between the high risk HPV16 E7 oncoprotein and the low risk HPV11 E7.
Materials and Methods
Generation of EGFP fusion plasmids with 11E7 wild type, 11E7 domains and 11E7 and cE7 containing different mutations
The enhanced green fluorescent protein (EGFP) expression plasmid pEGFP-C1 (Clontech, Inc.) was used to generate the EGFP fusion protein expression plasmids (EGFP-11E7, EGFP-11E71–38, EGFP-11E739–98) as follows. The pEGFP-C1 plasmid was double digested with EcoR1 and BamH1, run on a 0.7% agarose gel and the digested vector was extracted using the protocol from the QIAquick Gel Extraction Kit (Qiagen). DNA fragments spanning the full length HPV11 E7 or the CR3 domain (aa 39–98) were amplified using PCR oligonucleotides that added EcoR1 and BamH1 restriction endonuclease sites and the PCR products were digested with EcoR1 and BamH1 restriction enzymes and then ligated using T4 DNA ligase into the EcoR1 and BamH1 cloning sites of pEGFP-C1. The EGFP-E71–38 was generated using the QuikChange™ Site-Directed Mutagenesis Kit from Stratagene with EGFP-E7 as a template together with mutated primers containing a stop codon at aa 39. The EGFP-11cE7C58A, EGFP-11cE7C59A, EGFP-11CE7C91D, EGFP-11cE7CCC/AAA, EGFP-11cE7C91A and EGFP-11cE7C91_mutant plasmids were generated using the QuikChange™ Site-Directed Mutagenesis Kit from Stratagene with EGFP-cE7 as a template together with the corresponding mutated primers. The same mutations were also introduced in the context of EGFP-11E7 using the corresponding mutated primers. All the resulting plasmids were used to transform XL1-Blue cells and the purified plasmids were confirmed by sequencing (MGH DNA Sequencing Department).
Transient expressions of EGFP fusion proteins and confocal fluorescence microscopy analysis
HeLa cells (ATCC) were plated on 12 mm poly-L-lysine-coated glass coverslips to 50–70 % confluency 24 hours prior to transfection. Cells in each well were transfected with the corresponding EGFP fusion plasmid (as indicated in the Fig. legends) and the FuGENE 6 reagent (Roche Applied Science, IN). Media was changed to DMEM with 10% FBS and pen-strep after 5 hours and the cells were fixed 24 hours after the initial transfection with 10% formaldehyde in PBS (10 min). Coverslips were mounted using Vectashield-Dapi mounting medium (Vector Labs, CA) to identify the nuclei by DAPI staining and examined by fluorescence microscopy using a Leica TCS Sp5 broadband confocal microscope. Quantification of the intracellular localization phenotypes of the different EGFP fusion proteins (mostly nuclear, pancellular and mostly cytoplasmic) was performed on all transfection assays: data from at least four experiments were used for the quantifications.
Preparation of GST fusion proteins with E7 wild type, E7 domains and cE7 mutants
The GST-11E7 plasmid was kindly provided by Dr. David Pim (ICGEB, Trieste, Italy). The GST-11E71–38 construct was generated using the QuikChange™ Site-Directed Mutagenesis Kit with the GST-11E7 plasmid as a template together with mutated primers containing a stop codon at aa 39. The GST-11E71–38 plasmid was transformed in XL1 blue bacteria and the construct was confirmed by automated sequence analysis (MGH DNA sequencing department). The GST-11cE739–98 was generated as follows.
The GST-containing plasmid was double digested with EcoR1 and Xho1, run on a 0.7% agarose gel and the digested vector was extracted using the protocol from the QIAquick Gel Extraction Kit (Qiagen). The DNA fragment spanning the C domain of 11E7 (aa 39–98) were amplified using PCR oligonucleotides that added EcoR1 and Xho1 restriction endonuclease sites and the PCR product was digested with EcoR1 and Xho1 restriction enzymes and then ligated using T4 DNA ligase into the EcoR1 and Xho1 cloning sites of the pGEX -4T-1. The GST-11cE739–98 plasmid was transformed in XL1 blue bacteria and the construct was confirmed by automated sequence analysis (MGH DNA sequencing department). The C91A, C58A, and 57CCC/AAA mutations were introduced in the GST-11cE7 using QuikChange™ Site-Directed Mutagenesis Kit with the GST-11cE7 plasmid as a template together with the corresponding mutated primers.
For protein expression, the different GST-11E7 plasmids were used to transform E.coli BL21 CodonPlus. After induction of the bacteria with 0.5 mM IPTG for 3 hours at 300, the GST-11E7, GST-11nE71–38, and GST-11cE739–98 and the different mutant proteins were purified in their native state on glutathione-Sepharose beads using a standard procedure. The GST-M9 positive control containing the NLS of hnRNPA1 was prepared as previously described (Angeline, Merle, and Moroianu, 2003). All the proteins were checked for purity and lack of proteolytic degradation by SDS-PAGE and Coomassie Blue staining. All the purified proteins were dialyzed in transport buffer (20 mM HEPES-KOH, pH 7.3, 110 mM potassium acetate, 2 mM magnesium acetate, 1 mM EGTA, 2 mM DTT, plus protease inhibitors) (Moroianu, Blobel, and Radu, 1995) and stored in aliquots at −80°C.
Preparation of recombinant human nuclear transport factors
GST-RanG19V (Lounsbury et al., 1996) was expressed in E.coli BL21 CodonPlus by induction with 1mM IPTG at 37°C and the soluble protein was bound in its native state on gluthatione-Sepharose beads. RanG19V was obtained by cleaving the GST-RanG19V with biotinylated thrombin directly from the beads, followed by removal of the biotinylated thrombin by binding to Streptavidin-containing beads. During the purification 1mM GTP was added to the bacterial lysate to obtain the RanG19V in the GTP bound form. The complete removal of the GST was checked-up by immunoblot with an anti-GST antibody. Human Ran in the GDP bound state (Coutavas et al., 1993) was obtained as described (Floer and Blobel, 1996). GST-Kap β2 (Chook and Blobel, 1999), cleaved Kap β2 and His-tagged Kap β1 were obtained as we previously described (Nelson, Rose, and Moroianu, 2002). The proteins were checked for purity and lack of proteolytic degradation by SDS-PAGE and Coomassie Blue staining and then dialyzed in transport buffer and stored in aliquots at −80°C.
In solution binding assays
GST and different GST-E7 fusion proteins immobilized on Glutathione-Sepharose beads (2 μg protein/10 μl beads) were incubated with 2 μg of either Ran-GDP or RanG19V-GTP for 30 min at RT in transport buffer with 0.01% Tween and protease inhibitors. After incubation and three washes the bound proteins were eluted with SDS sample buffer and analyzed by SDS-PAGE and Coomassie Blue staining.
Phenyl-Sepharose beads (10 μl) were incubated with either GST-11E7, or GST-11cE7, or GST-Kap β2 (as a positive control) or GST (as a negative control) for 30 min at RT in transport buffer with 0.01% Tween. After incubation and three washes the bound proteins were eluted with SDS sample buffer and analyzed by SDS-PAGE.
Nuclear import assays
The nuclear import assays were carried out as previously described (Angeline, Merle, and Moroianu, 2003). Briefly, subconfluent HeLa cells, grown on poly-L-lysine coated glass coverslips for 24 h, were permeabilized with 70 μg/μl digitonin for 5 min on ice, and washed with cold transport buffer. All import reactions contained an energy regenerating system (1 mM GTP, 1mM ATP, 5 mM phosphocreatine, and 0.4 U creatine phosphokinase) (5 μl) and the different GST-fusion proteins (as indicated in the Fig. legends). Some import reactions contained HeLa cytosol (5 μl), or 2μg Ran-GDP, or 2μg RanG19V-GTP. In the competition experiments 5 μg Kap β21 or Kap β2 were used per assay. The final import reaction volume was adjusted to 20 μl with transport buffer. For visualization of nuclear import the GST fusion proteins were detected by immunofluorescence with an anti-GST antibody, as previously described. Coverslips were mounted using Vectashield-DAPI mounting medium (Vector Labs, CA) to identify the nuclei by DAPI staining. Nuclear import was analyzed with a Nikon Eclipse TE 300 Microscope that has a fluorescence attachment and photos were taken with a SPOT RT3 camera with an Advanced SPOT computer software.
Supplementary Material
A. GST-11E7 (lane 1), GST-11cE7 (lane 2) and GST (lane 3) immobilized on Glutathione-Sepharose beads were incubated with Ran-GDP (input [I]: lane 4) for 30 min at RT. B. GST-11E7 (lane 1), GST-11cE7 (lane 2) and GST (lane 3) immobilized on Glutathione-Sepharose beads were incubated with RanG19V-GTP (input [I]: lane 4) for 30 min at RT. Bound (B) proteins in A and B were eluted with SDS sample buffer and analyzed by PAGE and Coomassie Blue staining.
Digitonin-permeabilized HeLa cells were incubated with either GST-11cE7 (A and B), GST-11cE7C58A (C and D), or GST-11cE7CCC/AAA (E and F) or GST (G and H) in the presence of only transport buffer (A, C, E and G) or HeLa cytosol (B, D, F and H). Detection of the localization of the GST fusion proteins was performed with an anti-GST antibody. Note the nuclear import in panel B.
Digitonin-permeabilized HeLa cells were incubated with either GST-11cE7 (A and B), GST-11cE7C59A (C and D), or GST-11cE7C59D (E and F) or GST (G and H) in the presence of only transport buffer (A, C, E and G) or HeLa cytosol (B, D, F and H). Detection of the localization of the GST fusion proteins was performed with an anti-GST antibody. Note the nuclear import in panel B.
Acknowledgments
We thank Dr. David Pim (ICGEB, Trieste, Italy), for his generous gift of GST-11E7 expression plasmid and Drs. Yuh-Min Chook and Steve Adam for the GST-Kap β2 and Kap β1-His expression plasmids. We thank Patrick McManus for initial transfection assays with EGFP-11E7 and its domains. We also thank Dr. Joshua Rosenberg for excellent technical assistance with confocal fluorescence microscopy. Courtney McKee was a Beckman Scholar and she was supported by a Beckman fellowship. This work was supported by a grant from the National Institutes of Health (R01 CA94898) to Junona Moroianu.
Abbreviations
- HPV
human papillomavirus
- NLS
nuclear localization signal
- NES
nuclear export signal
- EGFP
enhanced green fluorescent protein
- GST
glutathione-S-transferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angeline M, Merle E, Moroianu J. The E7 oncoprotein of high-risk human papillomavirus type 16 enters the nucleus via a nonclassical Ran-dependent pathway. Virology. 2003;317(1):13–23. doi: 10.1016/j.virol.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Baldwin A, Huh KW, Munger K. Human papillomavirus E7 oncoprotein dysregulates steroid receptor coactivator 1 localization and function. J Virol. 2006;80(13):6669–77. doi: 10.1128/JVI.02497-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifaci N, Moroianu J, Radu A, Blobel G. Karyopherin beta2 mediates nuclear import of a mRNA binding protein. Proc Natl Acad Sci U S A. 1997;94(10):5055–60. doi: 10.1073/pnas.94.10.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chook YM, Blobel G. Structure of the nuclear transport complex karyopherin-beta2-Ran x GppNHp. Nature. 1999;399(6733):230–7. doi: 10.1038/20375. see comments. [DOI] [PubMed] [Google Scholar]
- Clemens KE, Brent R, Gyuris J, Munger K. Dimerization of the human papillomavirus E7 oncoprotein in vivo. Virology. 1995;214(1):289–93. doi: 10.1006/viro.1995.9926. [DOI] [PubMed] [Google Scholar]
- Coutavas E, Ren M, Oppenheim JD, D'Eustachio P, Rush MG. Characterization of proteins that interact with the cell-cycle regulatory protein Ran/TC4. Nature. 1993;366(6455):585–7. doi: 10.1038/366585a0. [DOI] [PubMed] [Google Scholar]
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006;110(5):525–41. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- Floer M, Blobel G. The nuclear transport factor karyopherin beta binds stoichiometrically to Ran-GTP and inhibits the Ran GTPase activating protein. J Biol Chem. 1996;271(10):5313–6. doi: 10.1074/jbc.271.10.5313. [DOI] [PubMed] [Google Scholar]
- Genovese NJ, Banerjee NS, Broker TR, Chow LT. Casein kinase II motif-dependent phosphorylation of human papillomavirus E7 protein promotes p130 degradation and S-phase induction in differentiated human keratinocytes. J Virol. 2008;82(10):4862–73. doi: 10.1128/JVI.01202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guccione E, Massimi P, Bernat A, Banks L. Comparative analysis of the intracellular location of the high- and low- risk human papillomavirus oncoproteins. Virology. 2002;293(1):20–5. doi: 10.1006/viro.2001.1290. [DOI] [PubMed] [Google Scholar]
- Huh KW, DeMasi J, Ogawa H, Nakatani Y, Howley PM, Munger K. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc Natl Acad Sci U S A. 2005;102(32):11492–7. doi: 10.1073/pnas.0505337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp AA, McManus PM, Bockstall K, Moroianu J. Identification of the nuclear localization and export signals of high risk HPV16 E7 oncoprotein. Virology. 2009;383(1):60–8. doi: 10.1016/j.virol.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Clements A, Zhao K, Marmorstein R. Structure of the human Papillomavirus E7 oncoprotein and its mechanism for inactivation of the retinoblastoma tumor suppressor. J Biol Chem. 2006;281(1):578–86. doi: 10.1074/jbc.M508455200. [DOI] [PubMed] [Google Scholar]
- Lounsbury KM, Richards SA, Carey KL, Macara IG. Mutations within the Ran/TC4 GTPase. Effects on regulatory factor interactions and subcellular localization. J Biol Chem. 1996;271(51):32834–41. doi: 10.1074/jbc.271.51.32834. [DOI] [PubMed] [Google Scholar]
- McIntyre MC, Frattini MG, Grossman SR, Laimins LA. Human papillomavirus type 18 E7 protein requires intact Cys-X-X-Cys motifs for zinc binding, dimerization, and transformation but not for Rb binding. J Virol. 1993;67(6):3142–50. doi: 10.1128/jvi.67.6.3142-3150.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Munger K. The human papillomavirus E7 oncoprotein. Virology. 2009;384(2):335–44. doi: 10.1016/j.virol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroianu J, Blobel G, Radu A. Previously identified protein of uncertain function is karyopherin alpha and together with karyopherin beta docks import substrate at nuclear pore complexes. Proc Natl Acad Sci U S A. 1995;92(6):2008–11. doi: 10.1073/pnas.92.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim B, Zbaida D, Dagan S, Kapon R, Reich Z. Cargo surface hydrophobicity is sufficient to overcome the nuclear pore complex selectivity barrier. Embo J. 2009;28(18):2697–705. doi: 10.1038/emboj.2009.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LM, Rose RC, Moroianu J. Nuclear import strategies of high risk HPV16 L1 major capsid protein. J Biol Chem. 2002;23:23. doi: 10.1074/jbc.M200724200. [DOI] [PubMed] [Google Scholar]
- Nguyen CL, Munger K. Human papillomavirus E7 protein deregulates mitosis via an association with nuclear mitotic apparatus protein 1. J Virol. 2009;83(4):1700–7. doi: 10.1128/JVI.01971-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlenschlager O, Seiboth T, Zengerling H, Briese L, Marchanka A, Ramachandran R, Baum M, Korbas M, Meyer-Klaucke W, Durst M, Gorlach M. Solution structure of the partially folded high-risk human papilloma virus 45 oncoprotein E7. Oncogene. 2006;25(44):5953–9. doi: 10.1038/sj.onc.1209584. [DOI] [PubMed] [Google Scholar]
- Ribbeck K, Gorlich D. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. Embo J. 2002;21(11):2664–71. doi: 10.1093/emboj/21.11.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Sheehy N, Gautier VW, Hayakawa H, Sawa H, Hall WW. The nuclear import of the human T lymphotropic virus type I (HTLV-1) tax protein is carrier- and energy-independent. J Biol Chem. 2007;282(18):13875–83. doi: 10.1074/jbc.M611629200. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92(9):690–8. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384(2):260–5. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. GST-11E7 (lane 1), GST-11cE7 (lane 2) and GST (lane 3) immobilized on Glutathione-Sepharose beads were incubated with Ran-GDP (input [I]: lane 4) for 30 min at RT. B. GST-11E7 (lane 1), GST-11cE7 (lane 2) and GST (lane 3) immobilized on Glutathione-Sepharose beads were incubated with RanG19V-GTP (input [I]: lane 4) for 30 min at RT. Bound (B) proteins in A and B were eluted with SDS sample buffer and analyzed by PAGE and Coomassie Blue staining.
Digitonin-permeabilized HeLa cells were incubated with either GST-11cE7 (A and B), GST-11cE7C58A (C and D), or GST-11cE7CCC/AAA (E and F) or GST (G and H) in the presence of only transport buffer (A, C, E and G) or HeLa cytosol (B, D, F and H). Detection of the localization of the GST fusion proteins was performed with an anti-GST antibody. Note the nuclear import in panel B.
Digitonin-permeabilized HeLa cells were incubated with either GST-11cE7 (A and B), GST-11cE7C59A (C and D), or GST-11cE7C59D (E and F) or GST (G and H) in the presence of only transport buffer (A, C, E and G) or HeLa cytosol (B, D, F and H). Detection of the localization of the GST fusion proteins was performed with an anti-GST antibody. Note the nuclear import in panel B.