Summary of Recent Advances
Recently, stable labeling techniques and use of two-photon microscopy for deep tissue imaging have enabled observation of neuronal structural dynamics within intact cerebral cortical circuits. These studies demonstrate that while neuronal structures are predominantly stable in the adult, a fraction of dendrites and axons are highly dynamic and responsive to experience, remodeling with precise cell type and laminar specificity. The qualitative and quantitative features of dendritic spine, dendritic branch, and axonal remodeling suggest their purpose may be to provide access to and alter connectivity between different circuits in cortical space. The net number of synapses lost or gained during arbor remodeling may not be as important as the change to the circuit diagram resulting from the shuffling of synaptic partners.
I. Introduction
An important feature of neuronal function is the capacity to dynamically adapt in response to changes in input activity. In the adult brain, neuronal plasticity has been shown to take many forms, from changes in intrinsic excitability to alterations in the strength of existing synapses. A long-debated question is to what degree structural changes that result in the formation or elimination of synaptic connections contribute to experience-dependent modifications of functional circuitry. The long term nature of structural changes make them particularly attractive as cellular substrates for persistent changes in connectivity, such as might be required for learning and memory [1] or changes in cortical map representation [2].
Recently, techniques for stable neuronal labeling [3,4] and the implementation of two-photon microscopy for deep tissue imaging [5] have for the first time enabled longitudinal observation of structural dynamics in cerebral cortical neurons embedded within an intact circuit. These studies demonstrate that while neuronal structures are predominantly stable in the adult, a fraction are highly dynamic and responsive to experience. Here we review recent reports of experience-dependent structural plasticity in the adult brain visualized by in vivo two-photon microscopy. While limits in optical penetration has largely confined in vivo imaging to structures extending into layer 1 (L1) and layer 2/3 (L2/3) of the cerebral cortex, it is clear that experience-dependent rearrangements occur in both dendritic and axonal elements with precise cell type and laminar specificity. We speculate as to how these relatively modest dynamics may serve to modulate circuit function.
II. Structural plasticity of neurons in the cerebral cortex is laminar, cell type, and stimulus specific
Cortical circuits are built upon a laminar architecture and consist of excitatory pyramidal neurons and inhibitory interneurons of various cell types [6]. Neurons transmit activity locally or over long distances through axonal arbors containing en passant (EPBs) and terminaux (TBs) boutons, which form synapses at various sites on the post-synaptic cell. A large fraction of excitatory synapses are made on dendritic spines that protrude from the dendrites of pyramidal neurons [7]. For aspiny interneurons, excitatory synapses are largely made on dendritic shafts [8]. Axons of inhibitory neurons form dendritic shaft synapses as well as peri-somatic and axo-axonic synapses [9]. The heterogeneity in synaptic contact types suggests that different modes of structural plasticity may be required to rearrange these different types of connections.
A considerable amount of attention has been focused on the structural dynamics of dendritic spines, as they are thought to provide a one-to-one indicator of excitatory synaptic presence [10-18]. In reality, the one-to-one correspondence between dendritic spine and excitatory synapse is less straightforward. Evidence shows that about 2-4% of spines in the neocortex lack synapses [19-22]. New spines can require a period of up to 4 days to form a synapse [23] suggesting that dendritic spine turnover on the timescale of hours to days may not necessarily reflect synapse turnover. In addition, a spine synapse can share a bouton with multiple partners [23]. Despite these reservations, chronic in vivo imaging of dendritic spine dynamics gives us the closest estimation of excitatory on excitatory synaptic dynamics on the timescale of several days without visualizing synapses directly. Approximately ~5-10% of spines on apical dendrites of layer 5 (L5) and L2/3 pyramidal neurons can form or be eliminated over the course of a week during normal, day-to-day experience [10-18]. The degree of spine dynamics can vary across cortical regions [13,15] suggesting that while the requirement for structural plasticity may be general to the cerebral cortex, specific dynamics may differ according to functional modality.
Sensory manipulations that lead to functional plasticity such as chessboard whisker trimming, monocular deprivation (MD), or retinal lesion are able to enhance the baseline rate of spine turnover by up to three-fold [11,12,14,16]. In the extreme case of a retinal lesion, 90% of initial spines in the silenced region of visual cortex turn over during retinotopic reorganization, an almost complete replacement of the spine population [14]. More recently, behavioral learning through motor training has also been shown to produce increases in spine dynamics of up to 10% in the adult motor cortex, comparable to sensory deprivation [17,18]. Beyond the enhanced spine dynamics seen with these manipulations, a perhaps less anticipated finding is that in some cases the response is specific to both cell type and laminar location. MD increases spine gain on apical dendrites of L5 pyramidal neurons extending into the superficial layers of binocular visual cortex but not on neighboring L2/3 pyramidal neurons [11]. In regions between barrel columns, chessboard whisker deprivation selectively increases the number of new persistent spines on L5B pyramidal neurons with complex, large apical tufts, but decreases previously persistent spines in neurons with simple, small apical tufts [12] (see Figure 1 for summary).
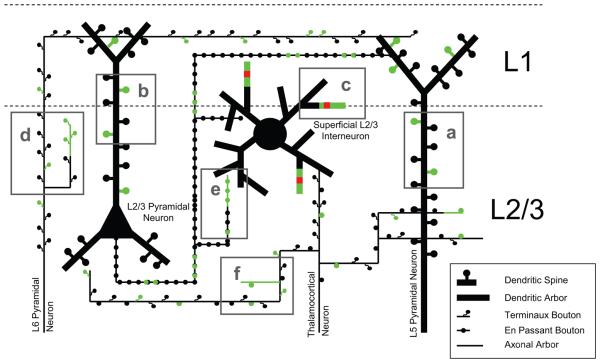
Figure 1. Diversity of Structural Rearrangements in the Adult Brain.
A schematic of the types of structural rearrangements and associated synaptic changes (glutamatergic in green; GABAergic in red) observed by chronic in vivo two-photon imaging for cell types within L1 and L2/3 of the adult cortex during normal experience. This includes: a) L5 pyramidal apical dendritic spines (~5-10% per week) [10-18]. b) L2/3 pyramidal dendritic spines (~5-10% per week) [11,13,14]. c) superficial L2/3 interneuron dendritic arbors (~3% per week, ~10μm per arbor) [26,29,30]. d) L6 pyramidal axonal arbors (~20% per week, ~3μm per arbor) and terminaux boutons (~20% per week) [36]. e) L2/3 pyramidal axonal arbors (tens of microns over weeks, *retinal lesion) and en passant boutons (~7-12% per week) [36-38]. f) thalamocortical axonal arbors (~8% per week, ~2μm per arbor), en passant (~4% per week) and terminaux boutons (~7% per week) [36].
An important aspect of spine remodeling in terms of physiological relevance is that it is not simply activity that drives spine dynamics, it is highly-dependent on the experiential context. Dendritic spine dynamics in the adult motor cortex increase only during naïve training to specific motor tasks as opposed to retraining on the same tasks learned earlier in life [17]. Similarly, dendritic spine gain in the adult visual cortex is observed only during an initial MD and not during a second MD of the same eye [11]. These initial structural changes can persist from weeks to months, long after the functional expression of these modifications become suppressed such as during recovery from MD or post-training [11,18]. It is thought that lasting structural changes can contribute to rapid functional adaptations upon re-exposure to the previous sensory manipulation or during re-learning [24,25]. This highlights the unique feature of structural plasticity that distinguishes it from other forms of neuronal plasticity in the adult brain, it is persistent over significantly longer time intervals.
Aside from their dendritic spines, dendritic arbors of pyramidal neurons are largely stable in the adult cortex [16,26]. However, some mouse mutants show uncharacteristically large dendritic remodeling. Delta-catenin knock out animals show a progressive loss of dendrites and spines concomitant with a decrease in cortical function [27]. In mice with a late onset deletion of the Pten tumor suppressor gene apical dendrites of L2/3 pyramidal neurons continued to grow and expand in the mature cortex while the basal dendrites on the same neurons or neighboring apical dendrites of L5 pyramidal neurons were unaffected [28]. These studies reveal a latent capacity for large-scale dendrite remodeling in the adult cortex that also seems laminar and cell type specific.
Unlike pyramidal neurons, ~3% of inhibitory interneuron dendritic branch tips, which equals to ~1 out of 35-45 dendritic branch tips per cell, can remodel on a weekly basis [26]. Over several months an average of 8 dendritic branches out of 35-45 total are likely to change [29]. These remodeling interneurons are not subtype specific but are spatially restricted to a superficial strip of L2/3, while interneurons above them in L1 and below in deep L2/3 remain stable [30]. Each dynamic branch tip can elongate or retract approximately 10 μm per week on average and up to ~ 90μm over weeks. These can also include the addition and elimination of entire branch tips that primarily occur on higher order branches at the arbor periphery [26]. Serial electron microscopy reconstructions of interneuron dendrites indicate they contain a synaptic density of about one synapse per micron [31]. Thus, while localization and hence synapse gain/loss on these cells is less obvious than on spines of excitatory cells, a significant degree of synaptic reorganization is likely to accompany dendritic rearrangements on inhibitory neurons. Interneuron dendrite remodeling can also be driven by sensory manipulations. Monocular and binocular deprivation increase the fraction of dynamic branch tips in these cells by 3 fold, to ~10% of arbor branch tips per week [29]. The timing of branch tip remodeling and the relative distribution of tips extending into L1 versus L2/3 differs for these two deprivation protocols, indicating that interneuron structural rearrangements in addition to being cell type and lamina specific are also stimulus specific.
Axonal remodeling in response to injury-related perturbations had been demonstrated in the adult cortex by classic histological techniques prior to the advent of chronic in vivo imaging methods [32,33]. In vivo imaging validated these observations, and further revealed more subtle and dynamic aspects of day-to-day axonal arbor and bouton remodeling. Like dendritic spines, bouton turnover is not necessarily indicative of one-to-one synaptic gain or loss, as boutons vary in regards to the number of their synaptic contacts ranging from several to none [34], and multiple boutons, excitatory and inhibitory, can partner with the same dendritic spine [35]. Never-the-less, axonal arbor remodeling and bouton turnover are likely to reflect a change in the synaptic population. In this case too, dynamics seem to reflect cell type and lamina specific rules. Axons of L6 pyramidal neurons and thalamocortical axons that terminate in L1 and L2/3 remodel up to ~30μm over 4 days [36]. The TBs on axons of L6 pyramidal neurons turn over at a rate of ~20% per week, while thalamocortical axons contain a mixture of less dynamic EPBs and TBs that turn over at ~4% and ~7% per week, respectively. In the neocortex of primates, horizontally projecting axons of L2/3 pyramidal neurons contain a high density of EPBs approximately 7-12% of which turn over per week under normal experience but whose arbors appear stable [37]. A retinal lesion can produce a 5-10 fold increase in axonal bouton turnover in these cells [38], accompanied by rapid initial growth of axonal arbors on the order of tens of microns from surrounding horizontal projections into the deprived region followed by a steady period of retractions over the course of weeks.
III. What function can structural plasticity serve in the adult brain?
Quantitatively, the fraction of dynamic dendritic and axonal elements seems modest in relation to their stable cohorts, particularly in the unperturbed cortex. However, their contribution to cortical plasticity may be cumulative if the remodeling of pre and postsynaptic components are at different synapses. Indeed, new dendritic spines preferentially synapse onto existing boutons [23], implying that dendrites and axons that remodel concurrently are not necessarily synaptic partners. In addition, structural dynamics appear far from random. They are driven by specific patterns of activity and are restricted to certain cell types and spatial domains, as if specific circuit features are targeted for remodeling dependent on the required outcome. Below, we consider the potential functional significance of structural dynamics in spines, inhibitory dendrites, and axonal arbors within the cortical circuit.
Based on steric considerations it is estimated that each dendritic spine is capable of making approximately 4 potential synaptic contacts with adjacent axons [39]. This density of potential synapses within a given volume of neuropil suggests that even small structural changes, such as spine additions and retractions, can significantly alter circuit connectivity [40]. Moreover, a typical cortical pyramidal neuron receives only a handful of inputs from each of a thousand or so different excitatory cells [7]. These inputs are not necessarily random as fine-scale excitatory subnetworks can be embedded within the larger circuit [41] and long-range inputs can be segregated into subcellular compartments of pyramidal neurons [42]. A 5-10% rate of dendritic spine turnover would affect the rearrangement of 250-500 synaptic connections or potentially a minimum of 50-100 neuronal partners. These findings support the view that structural plasticity in the adult brain serves to increase the number of potential synaptic contacts by increasing spatial access to distinct circuits within an arbor's vicinity [40].
In the case of interneurons, an estimate of synaptic turnover associated with a baseline dendrite remodeling of 10 μm per cell/week would be on the order of 10 synapses, or in the case of MD, 30 synapses per cell with approximately 10% of branch tips remodeling [29]. This is a minimal estimate of total synaptic turnover per interneuron, not accounting for potential synapse dynamics on stable dendrites. While slightly lower, the estimated synaptic turnover from dendrite remodeling is on the same order of fractional dendritic spine turnover of ~5-10% observed in L5 pyramidal neurons during MD in visual cortex [11] or during motor learning behavior in motor cortex [17,18]. This suggests that the relative scale of structurally-related synaptic remodeling that occurs in the adult cortex during plasticity is comparable between excitatory and inhibitory cell types.
Approximately 10 pre-synaptic, temporally correlated excitatory inputs are thought to be sufficient to trigger an action potential in inhibitory cells [43]. For bitufted interneurons, a train of action potentials from even a single synaptic contact can produce an action potential [44]. Accordingly, a total branch tip length change of ~30 μm (or ~30 synapses) per cell during MD could alter the connectivity of up to 30 excitatory pre-synaptic partners, each with significant ability to initiate interneuron firing. Furthermore, one inhibitory cell makes a large number of synapses (15-20) on to local excitatory cells despite representing only about 20% of cortical neurons [8]. As a result, the activity of a given post-synaptic cell can be more strongly influenced by the minority of inhibitory neurons that synapse onto it than by the excitatory majority. The functional consequences of dendritic spine and interneuron arbor remodeling may be quite different.
About 80% of the synapses on distal interneuron dendrites represent excitatory inputs [31] from a large number of local pyramidal neurons that each contributes only 3-7 synapses [8]. This circuit feature, where only a small fraction of the excitatory drive to a given cell is provided by a single presynaptic partner, is thus common between cortical pyramidal cells and interneurons. In the same way that remodeling of a small number of spines can influence the addition or removal of a specific pre-synaptic input, retraction or addition of only a few interneuron dendrites could serve the same purpose. Monocular deprivation specifically increases the fraction of dynamic branch tips without effecting change per branch tip length, supporting the idea that these changes serve to gain or remove access to different circuits in cortical space [29].
A large fraction of excitatory axons that synapse onto cortical pyramidal neurons arrive from long range intracortical and subcortical origins [45] and take rather direct, near linear trajectories through local cortical areas [46], which in terms of sampling provides only limited access. The flexibility of dendritic spines and inhibitory dendritic branch tips potentially allows them to seek these excitatory inputs. Since the reach of dendritic spines is limited, remodeling of projection axons might be required in situations when the reorganization of long-range excitatory connections is needed. Thalamocortical and L6 pyramidal axon arbors appear to be more dynamic on a day-to-day basis compared to horizontal L2/3 pyramidal axons [36]. The high tortuousity and small branch lengths of inhibitory axons [46] might allow them a high degree of local coverage that would not necessitate substantial structural changes to sample different local partners. Indeed, imaging of GABAergic axons in slice cultures show that GABAergic synapses form in the absence of any morphological protrusions, exclusively appearing at preexisting axon-dendrite crossings of excitatory cells [47].
Conclusion
Even within the narrow portion of neocortex examined by two-photon microscopy, current findings paint a complex and diverse picture of structural plasticity in the adult brain. The qualitative and quantitative features of dendritic spine, interneuron dendritic branch tip, and axonal remodeling suggest they are optimally designed to access and alter connectivity between different circuits in cortical space. The relative number of synapses lost or gained during a branch tip retraction or elongation may not be as important as the change to the circuit diagram resulting from the shuffling of synaptic partners. The cell type and laminar-specificity of both pre- and post-synaptic structural dynamics argue that the partner sampling occurring during synaptic remodeling is circuit specific. An open question is whether this specificity is a matter of circuit hot-spots that allow optimal sampling of many converging inputs, or whether locales permissive to remodeling are dictated by the specific circuits that need to be accessed for a given outcome. Evolution of in vivo imaging technologies may soon allow this question to be addressed through the examination of both pre and post-synaptic structures in the context of a labeled circuit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jerry L. Chen, Picower Institute for Learning and Memory, Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA jerchen@mit.edu
Elly Nedivi, Picower Institute for Learning and Memory, Department of Biology and Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology, Cambridge, MA 02139, USA nedivi@mit.edu.
References
- 1.Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 2.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 3.Dittgen T, Nimmerjahn A, Komai S, Licznerski P, Waters J, Margrie TW, Helmchen F, Denk W, Brecht M, Osten P. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc Natl Acad Sci U S A. 2004;101:18206–18211. doi: 10.1073/pnas.0407976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 5.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 6.Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- 7.Peters A. Examining neocortical circuits: some background and facts. J Neurocytol. 2002;31:183–193. doi: 10.1023/a:1024157522651. [DOI] [PubMed] [Google Scholar]
- 8.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 9.Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 10.Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 11••.Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457:313–317. doi: 10.1038/nature07487. In vivo imaging study showing that new dendritic spines formed during monocular deprivation in adult visual cortex persist after the eye is reopened. A repeated monocular deprivation does not increase spine dynamics suggesting that an initial experience can induce structural changes that can potentially facilitate functional adaptation upon repeated experiences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 13.Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Keck T, Mrsic-Flogel TD, Vaz Afonso M, Eysel UT, Bonhoeffer T, Hubener M. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat Neurosci. 2008;11:1162–1167. doi: 10.1038/nn.2181. [DOI] [PubMed] [Google Scholar]
- 15.Majewska AK, Newton JR, Sur M. Remodeling of synaptic structure in sensory cortical areas in vivo. J Neurosci. 2006;26:3021–3029. doi: 10.1523/JNEUROSCI.4454-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 17••.Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. In vivo imaging study showing that training to novel motor tasks produces an initial period of rapid spine formation followed by steady pruning in motor cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. In vivo imaging study examining the lifetime of dendritic spines formed during motor learning in the motor cortex. New spines can persist for months after training. However, novel experiences can promote the elimination of previously persistent spines, suggesting that only a small fraction of these spines are ultimately maintained through the lifetime of the animal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White EL, Rock MP. Three-dimensional aspects and synaptic relationships of a Golgi-impregnated spiny stellate cell reconstructed from serial thin sections. J Neurocytol. 1980;9:615–636. doi: 10.1007/BF01205029. [DOI] [PubMed] [Google Scholar]
- 20.Arellano JI, Espinosa A, Fairen A, Yuste R, DeFelipe J. Non-synaptic dendritic spines in neocortex. Neuroscience. 2007;145:464–469. doi: 10.1016/j.neuroscience.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Hersch SM, White EL. Quantification of synapses formed with apical dendrites of Golgi-impregnated pyramidal cells: variability in thalamocortical inputs, but consistency in the ratios of asymmetrical to symmetrical synapses. Neuroscience. 1981;6:1043–1051. doi: 10.1016/0306-4522(81)90069-5. [DOI] [PubMed] [Google Scholar]
- 22.Benshalom G, White EL. Quantification of thalamocortical synapses with spiny stellate neurons in layer IV of mouse somatosensory cortex. J Comp Neurol. 1986;253:303–314. doi: 10.1002/cne.902530303. [DOI] [PubMed] [Google Scholar]
- 23.Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- 24.Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Prior experience enhances plasticity in adult visual cortex. Nat Neurosci. 2006;9:127–132. doi: 10.1038/nn1610. [DOI] [PubMed] [Google Scholar]
- 25.Knudsen EI. Instructed learning in the auditory localization pathway of the barn owl. Nature. 2002;417:322–328. doi: 10.1038/417322a. [DOI] [PubMed] [Google Scholar]
- 26.Lee WC, Huang H, Feng G, Sanes JR, Brown EN, So PT, Nedivi E. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS Biol. 2006;4:e29. doi: 10.1371/journal.pbio.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matter C, Pribadi M, Liu X, Trachtenberg JT. Delta-catenin is required for the maintenance of neural structure and function in mature cortex in vivo. Neuron. 2009;64:320–327. doi: 10.1016/j.neuron.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Chow DK, Groszer M, Pribadi M, Machniki M, Carmichael ST, Liu X, Trachtenberg JT. Laminar and compartmental regulation of dendritic growth in mature cortex. Nat Neurosci. 2009;12:116–118. doi: 10.1038/nn.2255. In vivo imaging study in Pten conditional knock out mice demonstrating genetic regulation of dendrite growth specifically in L2/3 pyramidal apical dendrites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Chen JL, Lin WC, Cha JW, So PT, Nedivi E. Structural basis for the role of inhibition in facilitating adult brain plasticity. Under Revision. 2010 doi: 10.1038/nn.2799. In vivo imaging study showing that monocular deprivation produces laminar specific dendritic arbor rearrangements in interneurons in adult visual cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee WC, Chen JL, Huang H, Leslie JH, Amitai Y, So PT, Nedivi E. A dynamic zone defines interneuron remodeling in the adult neocortex. Proc Natl Acad Sci U S A. 2008;105:19968–19973. doi: 10.1073/pnas.0810149105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubota Y, Karube G, Sekigawa A, Nomura M, Aoyagi T, Mochizuki Y, Kawaguchi Y. Dendritic dimensions of cortical nonpyramidal cells. Society for Neuroscience Abstracts. 2007 [Google Scholar]
- 32.Darian-Smith C, Gilbert CD. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- 33.Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- 34.White EL, Weinfeld E, Lev DL. Quantitative analysis of synaptic distribution along thalamocortical axons in adult mouse barrels. J Comp Neurol. 2004;479:56–69. doi: 10.1002/cne.20300. [DOI] [PubMed] [Google Scholar]
- 35.Jones EG, Powell TP. Morphological variations in the dendritic spines of the neocortex. J Cell Sci. 1969;5:509–529. doi: 10.1242/jcs.5.2.509. [DOI] [PubMed] [Google Scholar]
- 36.De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Stettler DD, Yamahachi H, Li W, Denk W, Gilbert CD. Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron. 2006;49:877–887. doi: 10.1016/j.neuron.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Yamahachi H, Marik SA, McManus JN, Denk W, Gilbert CD. Rapid axonal sprouting and pruning accompany functional reorganization in primary visual cortex. Neuron. 2009;64:719–729. doi: 10.1016/j.neuron.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stepanyants A, Hof PR, Chklovskii DB. Geometry and structural plasticity of synaptic connectivity. Neuron. 2002;34:275–288. doi: 10.1016/s0896-6273(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 40.Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimura Y, Dantzker JL, Callaway EM. Excitatory cortical neurons form fine-scale functional networks. Nature. 2005;433:868–873. doi: 10.1038/nature03252. [DOI] [PubMed] [Google Scholar]
- 42.Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buhl EH, Tamas G, Szilagyi T, Stricker C, Paulsen O, Somogyi P. Effect, number and location of synapses made by single pyramidal cells onto aspiny interneurones of cat visual cortex. J Physiol. 1997;500(Pt 3):689–713. doi: 10.1113/jphysiol.1997.sp022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaiser KM, Lubke J, Zilberter Y, Sakmann B. Postsynaptic calcium influx at single synaptic contacts between pyramidal neurons and bitufted interneurons in layer 2/3 of rat neocortex is enhanced by backpropagating action potentials. J Neurosci. 2004;24:1319–1329. doi: 10.1523/JNEUROSCI.2852-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stepanyants A, Martinez LM, Ferecsko AS, Kisvarday ZF. The fractions of short- and long-range connections in the visual cortex. Proc Natl Acad Sci U S A. 2009;106:3555–3560. doi: 10.1073/pnas.0810390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stepanyants A, Tamas G, Chklovskii DB. Class-specific features of neuronal wiring. Neuron. 2004;43:251–259. doi: 10.1016/j.neuron.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Wierenga CJ, Becker N, Bonhoeffer T. GABAergic synapses are formed without the involvement of dendritic protrusions. Nat Neurosci. 2008 doi: 10.1038/nn.2180. [DOI] [PubMed] [Google Scholar]