Abstract
Hypertrophic pachymeningitis is rarely observed in inflammatory bowel disease. We report a woman with ulcerative colitis whose biopsy-confirmed hypertrophic pachymeningitis was complicated by cerebral venous sinus thrombosis and intracranial hypertension and required ventriculostomy and steroid therapy. This report highlights the challenge facing the diagnosis and management of hypertrophic pachymeningitis from an unusual primary cause.
Keywords: Cerebral venous sinus thrombosis, Hypertrophic pachymeningitis, Inflammatory bowel disease, Intracranial hypertension, Neuroimmunology
1 Introduction
First described by Charcot1, hypertrophic pachymeningitis is a rare condition that causes chronic inflammation and progressive fibrosis of the cerebral or spinal dura mater. While severe headache and vision deterioration are the classic presenting symptoms, additional manifestations include papilledema, cranial neuropathies, cerebellar involvement, seizure and myelopathy.2 The pathogenic mechanism underlying hypertrophic pachymeningitis is unknown, but it is considered as an autoimmune disorder.3 It can be associated with trauma, hemodialysis, intracranial hypotension, intrathecal drug administration, infection (tuberculosis, syphilis, borrelia, viral and fungal), systemic inflammation (sarcoidosis, rheumatoid arthritis, Wegener’s granulomatosis, Sjögren’s, giant cell arteritis, multifocal fibrosclerosis), or neoplasm (meningioma, carcinomatosis meningitis, metastasis).2 To our knowledge, hypertrophic pachymeningitis in the setting of inflammatory bowel disease (IBD) has not been described.
2 Case Report
A 42-year-old woman presented with progressively a debilitating headache that commenced 1 year earlier. She had long-standing ulcerative colitis (UC) in remission for at least 2 years on sulfasalazine, no history of substance abuse, and no pertinent family history. Superior sagittal sinus thrombosis was found on cerebral angiogram 6 months earlier, and the presumed etiology was systemic inflammation secondary to UC. Despite adequate anticoagulation, cerebral venous sinus thrombosis (CVST) progressed.
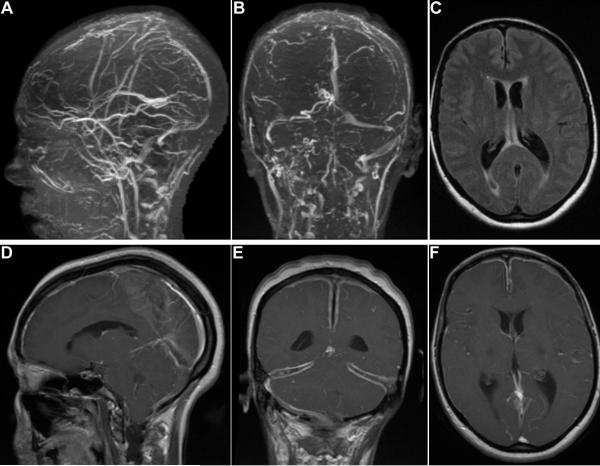
In the preceding 2 months, headache began to involve frontal and bitemporal regions with associated blurry vision, intractable nausea and weight loss. On exam, she appeared cachectic. Other than bilateral papilledema and impaired visual acuity (20/200 oculus dexter, 20/100 oculus sinister), there was no focal neurologic deficit. Brain MRI with gadolinium showed increasing clot burden (Fig. 1A–B). Ventricular enlargement (Fig. 1C) and cerebellar tonsillar herniation were noted as well as marked leptomeningeal enhancement and diffuse dural thickening without arachnoid or pial involvement (Fig. 1D–F).
Figure 1.
(A) Sagittal and (B) coronal head magnetic resonance venogram showing extensive thrombosis in the superior sagittal, straight, transverse (right > left) and sigmoid (right > left) sinuses. (C) Axial fluid-attenuated inversion recovery showing ventricular enlargement. (D) sagittal, (E) coronal and (F) axial gadolinium-enhanced MRI showing diffuse leptomeningeal enhancement and dural thickening consistent with hypertrophic pachymeningitis.
Further investigation for a hypercoagulable state uncovered a heterozygous genotype for the C677T mutation in methylene tetrahydrofolate reductase (MTHFR) but without hyperhomocysteinemia. Patient had normal levels of antithrombin III, protein C, free protein S, and negative lupus anticoagulant, anti-beta2 glycoprotein 1, anti-prothrombin and anti-cardiolipin antibodies. Mutations in Factor II (G20210A) and Factor V Leiden (G1691A) were absent.
Concern for malignancy was raised due to the weight loss and history of UC, but the patient declined colonoscopy. There was no clinical sign of UC flare. Non-contrast computerized tomography of the body was unrevealing; the patient was allergic to iodinated contrast. Body positron emission tomography showed diffuse uptake within the spinal canal from mid thoracic to lower lumbar spine. Spine MRI showed dural nodular enhancement from T4 to L5, but there were no clinical signs of myelopathy or radiculopathy. Cerebrospinal fluid (CSF) cytology and flow cytometry were unrevealing.
Lumbar puncture (LP) from 6 months earlier was notable for opening pressure of 30 cm H2O, and the CSF contained 8 red blood cells/mm3, 64 white blood cells/mm3 (62% lymphocytes), 140 mg/dL protein and 50 mg/dL glucose. Because of cerebellar tonsil herniation, LP was contraindicated on this admission. CSF was obtained by a ventricular approach with an opening pressure exceeding the maximum measurement. CSF contained fewer white blood cells (4/mm3) and lower protein (7 mg/dL). Serum inflammatory markers were elevated (erythrocyte sedimentation rate 55 mm/hour, C-reactive protein 33 mg/L). Anti-nuclear antibody screen was positive at 1:80 with a speckled pattern. Rheumatoid factor, anti-cyclic citrullinated peptide antibody, and angiotensin-converting enzyme were negative. Enzyme-linked immunosorbent assay was negative for anti-proteinase 3 and anti-myeloperoxidase. Indirect immunofluorescence for anti-neutrophil cytoplasmic antibodies showed a nuclear staining pattern atypical for vasculitis but consistent with IBD.
Infectious evaluation included negative serology for Lyme, rapid plasma reagin, human immunodeficiency virus, human T-cell lymphotrophic virus (HTLV), and negative CSF for bacterial culture, Lyme, Epstein-Barr virus (EBV), varicella zoster virus, Venereal Disease Research Laboratory test, fluorescent treponemal antibody absorption, and fungal smear. Tuberculosis evaluations, including CSF mycobacterial culture, acid-fast bacilli from CSF and 3 induced sputums, and purified protein derivative were all negative.
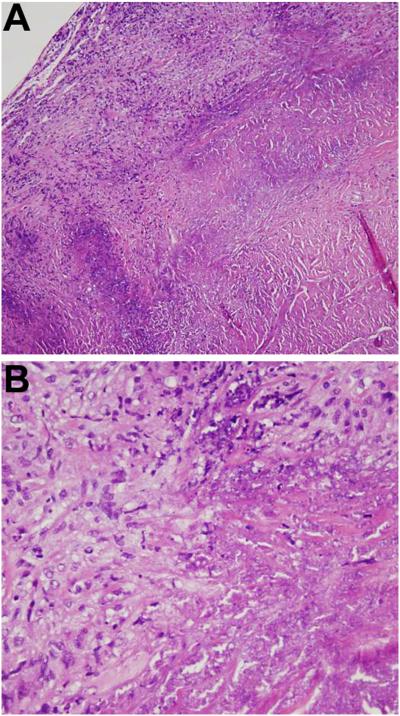
Because of failed medical therapy for intracranial hypertension, the patient underwent ventriculoperitoneal (VP) shunt placement. Dural biopsy did not show malignancy, granuloma, vasculitis or any infectious etiology, but established the diagnosis of hypertrophic pachymeningitis (Fig. 2). VP shunt provided immediate relief for nausea and produced improvement in vision. Given that CVST was refractory to enoxaparin and warfarin, fondaparinux was started 2 weeks after VP shunt placement and there was no further progression. Headache responded only to steroid therapy (1 mg/kg prednisone for 10 weeks), and worsened with each attempted steroid taper to below 10 mg daily. Switches to steroid-sparing agents, including azathioprine, mycophenolate, adalimumab and methotrexate were unsuccessful. Three years after the initial onset of symptoms, she regained weight. Serial follow-up MRI scans remained stable without further deterioration.
Figure 2.
Hematoxylin and eosin stained tissue biopsy sections of the dura and meninges showing multifocal necrosis surrounded by inflammatory foamy histiocytes and mature lymphocytes without granuloma; (A) ×10 and (B) ×40.
3 Discussion
We report a patient with hypertrophic pachymeningitis in the setting of IBD, complicated by CVST and intracranial hypertension. MRI findings of dural thickening and diffuse leptomeningeal enhancement suggested hypertrophic pachymeningitis. Elevated protein and lymphocytosis in CSF were non-specific, but the dural biopsy confirmed the diagnosis. CVST and intracranial hypertension are known complications of dural inflammation.4, 5
We excluded many primary causes for which hypertrophic pachymeningitis is a secondary manifestation. These included neurosarcoidosis, neurosyphillis, tuberculosis, EBV, HTLV, bacterial and fungal infection, rheumatoid arthritis, Wegener’s granulomatosis, metastatic or primary brain tumor, and trauma. We could not definitively conclude that our case was idiopathic because the long-standing UC raised the possibility that chronic inflammation directed against antigens shared between dural meninges and gastrointestinal mucosa contributed to the neurologic manifestations. Although patient’s UC was stable at the course of hypertrophic pachymeningitis, the lack of clinical flare does not rule out low-grade mucosal inflammation. We were unable to test this immune hypothesis because colonoscopy to evaluate mucosal inflammation could not be obtained.
An alternative but less unifying hypothesis is that a hypercoagulable state secondary to UC led to CVST while hypertrophic pachymeningitis was idiopathic and coincidental. Chronic IBD, including UC, increases the risk of systemic thrombosis, but whether the prothrombotic predilection depends on the clinical activity of IBD remains controversial.6 The pathogenesis likely involves an inflammatory response against the reactive fibrinolytic pathway, platelet dysfunction, and hyperhomocysteinemia. Our patient was a carrier for the MTHFR C677T mutation, a common heterozygous genotype associated with decreased homocysteine remethylation, but there was no hyperhomocysteinemia. That CVST in our patient progressed despite adequate anticoagulation but stabilized after treatment for hypertrophic pachymeningitis argues against the hypothesis that CVST and pachymeningitis were coincidental.
Immunosuppression has high success in treating hypertrophic pachymeningitis. Although our patient initially demonstrated a dramatic response to steroid therapy, she continued to suffer symptomatic recurrence with steroid taper or attempts to switch to steroid-sparing agents, a pattern consistent with previous case reports.2 Steroid dependence may reflect an ongoing chronic inflammation. It is unclear whether more aggressive therapy for IBD would impact the clinical course of hypertrophic pachymeningitis.
Complications of hypertrophic meningitis require additional therapeutic interventions. Symptoms stemming from intracranial hypertension were remarkably responsive to VP shunt here. Recommendation for anticoagulation therapy in CVST associated with pachymeningitis is unclear. Bhatia et al. proposed the use of anticoagulation in patients with a short history and definitive thrombosis, but long-term anticoagulation in the presence of vessel fibrosis from chronic pachymeningitis would be difficult to justify.4 Our patient was treated for 2 years with fondaparinux.
Acknowledgements
We thank the patient and her family. Dr Xia is supported by NIH (AI074549-02). Chen-Plotkin is supported by a Burroughs Wellcome Fund Career Award for Medical Scientists and NIH (K08 AG033101). Schmahmann is supported in part by the Birmingham Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Charcot JM, Joffroy A. Deux cas d’atrophie musculaire progressive avec lésions de la substance gris et des faisceaux antériolatéraux de la melle épinière. Arch Physiol Norm Pathol. 1869;2:354–367. [Google Scholar]
- 2.Kupersmith MJ, Martin V, Heller G, Shah A, Mitnick HJ. Idiopathic hypertrophic pachymeningitis. Neurology. 2004;62:686–694. doi: 10.1212/01.wnl.0000113748.53023.b7. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman AP. Inflammatory dural masses: if it’s not one thing, it’s another. J Neuroophthalmol. 2007;27:89–90. doi: 10.1097/WNO.0b013e318064c484. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia R, Tripathi M, Srivastava A, et al. Idiopathic hypertrophic cranial pachymeningitis and dural sinus occlusion: two patients with long-term follow up. J Clin Neurosci. 2009;16:937–942. doi: 10.1016/j.jocn.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Malik A, Mishra NK, Gaikwad SB. Idiopathic hypertrophic pachymeningitis: spectrum of the disease. Neuroradiology. 1997;39:619–623. doi: 10.1007/s002340050479. [DOI] [PubMed] [Google Scholar]
- 6.Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol. 2007;102:174–186. doi: 10.1111/j.1572-0241.2006.00943.x. [DOI] [PubMed] [Google Scholar]