Abstract
Purpose
To investigate the effect of a metronomic (low dose, high frequency) small molecule inhibitor of Bcl-2 (TW-37) in combination with radiotherapy on microvascular endothelial cells in vitro and in tumor angiogenesis in vivo.
Methods and materials
Primary human dermal microvascular endothelial cells (HDMEC) were exposed to ionizing radiation and/or TW-37, and colony formation as well as capillary sprouting in 3-D collagen matrices, was evaluated. Xenografts vascularized with human blood vessels were engineered by co-transplantation of human squamous cell carcinoma cells (OSCC3) and HDMEC seeded in highly porous biodegradable scaffolds into the subcutaneous space of immunodeficient mice. Mice were treated with metronomic TW-37 and/or radiation, and tumor growth was evaluated.
Results
Low dose TW-37 sensitized primary endothelial cells to radiation-induced inhibition of colony formation. Low dose TW-37 or radiation partially inhibited endothelial cell sprout formation, while in combination these therapies abrogated new sprouting. Combination of metronomic TW-37 and low dose radiation inhibited tumor growth and resulted in significant increase in time to failure as compared to controls, whereas single agents did not. Notably, histopathological analysis revealed that tumors treated with TW-37 (with or without radiation) are more differentiated and showed more cohesive invasive fronts, which is consistent with less aggressive phenotype.
Conclusions
These results demonstrate that metronomic TW-37 potentiates the anti-tumor effects of radiotherapy, and suggest that patients with head and neck cancer might benefit from the combination of small molecule inhibitor of Bcl-2 and radiation therapy.
Keywords: Developmental therapeutics, Radiotherapy, Head and Neck Cancer, Apoptosis, Neovascularization
Introduction
Head and neck cancer has a generally aggressive phenotype and is particularly resistant to therapy with a 5-year survival rate of around 60% (1,2). Platinum-based chemotherapeutic drugs and radiation are the primary interventions for head and neck cancers, however tumors frequently generate resistance to these treatments and relapse. This has raised interest in molecularly targeted drugs aimed at tumor specific pathways (3). Tumors overexpressing Bcl-2 are protected from chemotherapy or radiotherapy. Indeed Bcl-2 expression is highly predictive of local relapse in early stage breast cancer after surgery and radiotherapy (4). Abnormal expression of Bcl-2 is found in a number of cancers including head and neck cancer, breast cancer, prostate cancer and rectal cancer (5-8). Notably, Bcl-2 expression is highly elevated in the endothelial cells lining head and neck tumor blood vessels, as compared to the endothelial cells of normal oral mucosa (9). We have shown that therapeutic inhibition of Bcl-2 results in an anti-tumor cell and anti-angiogenic effects (10,11), however it is not known if it sensitizes head and neck tumors to radiation therapy.
Combination regimens with radiation and molecularly targeted drugs have shown encouraging efficacy in an increasing number of cancers (12). One such new class of small molecule inhibitors, targeted to Bcl-2, is showing efficacy in the laboratory and in phase I trials (13). Recent studies have also presented data for the in vitro efficacy of small molecule inhibitors of Bcl-2 used in high concentration in combination with radiation, showing inhibition of tumor cell growth (14-16). TW-37 is a novel small molecule inhibitor drug derived from gossypol but possessing a greater specificity for Bcl-2 (17). It is designed to occupy the BH3 domain of Bcl-2, preventing its interaction with other members of the Bcl-2 family and therefore its pro-survival effect. Inhibition of Bcl-2 function results in the induction of cell death by default. TW-37 was shown to inhibit tumor cell growth in vitro and in vivo (10,11,17-19).
An emerging concept is that low dose daily administration (metronomic administration) of chemotherapeutic drugs is less toxic than traditional dosing while maintaining similar efficacy (20,21). The present study was designed to investigate the effect of metronomic TW-37 in combination with radiation therapy in vitro and in xenograft models of head and neck cancer
Methods and Materials
Irradiation
Irradiations were carried out using a Pantak Therapax DXT 300 Model X-ray unit (PANTAK, East Haven, CT) at a dose rate of approximately 3 Gy/min. Dosimetry was carried out using an ionization chamber connected to an electrometer system that is directly traceable to a National Institute of Standards and Technology calibration.
Sulphorhodamine B assay
HDMEC (Lonza, Walkersville, MD, USA) were treated with TW-37 diluted in EGM2-MV (Lonza) and irradiated. Cellular protein was stained by addition of 0.4% Sulphorhodamine B (Sigma/Aldrich, St. Louis, MO, USA) and absorbance was determined on a microplate reader at 560 nm (Genius; Tecan, Graz, Austria), as described (11,22). Results were normalized against initial plating density and drug-free radiation-free controls. Here and throughout this manuscript, experiments were performed in triplicates and repeated at least 3 independent times.
Clonogenic assay
After TW-37 treatment and/or irradiation, HDMEC were plated at clonal densities, as previously described (23). Fourteen days later, cells were fixed and stained with crystal violet. Colony counting was done using an automated counter. The mean inactivation dose (MED) (24) was calculated for control and each dose of TW-37, and the enhancement ratio (ER) was calculated as the MED in the control curve divided by the MED in the TW-37 curve. An enhancement ratio greater than one indicates radiosensitization, while a ratio less than one conveys resistance to radiation.
Flow cytometry
Cells were treated for 24 hours with TW-37 then exposed to ionizing radiation (6 Gy). Total cell population was assessed for apoptosis by hypotonic lysis and staining with propidium iodide, as described (25). Apoptotic levels and cell cycle status were determined by flow cytometry (FACSCalibur, BD Biosciences, San Jose, USA).
Endothelial cell sprouting assay
5-8 × 105 HDMEC were added to each well of 6-well plates pre-coated with Vitrogen 100 collagen (AngioTech BioMaterials, Palo Alto, CA) and allowed to adhere overnight. Cells were treated daily with 50 ng/ml rhVEGF165 (R & D Systems, Minneapolis, MN) to induce sprouting, as described (26). Cells were then treated with 0.5 μM TW-37 for three consecutive days with or without 1 Gy radiation on the second treatment day. Data are displayed as difference in sprout number from start of therapeutic treatment.
SCID Mouse Model of Human Tumor Angiogenesis
Porous poly L-lactic acid (PLLA) scaffolds (6 × 6 × 1 mm) were fabricated, as described (26). Before implantation, scaffolds were seeded with a mixture of 1 × 105 oral squamous cell carcinoma (OSCC3) cells and 9 × 105 HDMEC. Male severe combined immunodeficient (SCID) mice (CB.17.SCID, Charles Rivers) were anesthetized, and two scaffolds were implanted bilaterally subcutaneously in the dorsal region of each mouse. For co-treatment experiments, drug treatment groups were given 15-21 mg/kg TW-37 i.p. (in vehicle: PBS/Tween 80/ethanol) and two groups received vehicle alone i.p. for 7-10 consecutive days. For groups receiving radiation, 0.8-1 Gy was given on the second day of TW-37 treatment, and continued for 3-5 consecutive days. Radiation therapy was routinely given 4-6 hours after drug treatment. Tumor volume was determined by caliper measurement (length × breadth2). The pathology of tumors was evaluated by a trained pathologist blinded to the treatment conditions, as described (27). Treatment of animals was in accordance with University of Michigan institutional guidelines.
Immunohistochemistry
Immunohistochemistry for identification of blood vessels was performed with rabbit anti-Von Willebrand factor polyclonal (1:500 dilution; Thermo Fisher Scientific Inc, Fremont, USA), as described (11). Microvessel density was determined by the Chalkley count method (28).
Statistical analysis
Statistical significance was determined by one-way ANOVA followed by post-hoc tests (SigmaStat 2.0 software; SPSS; Chicago, IL, USA). Kaplan-Meier curves were analyzed with the Gehan-Breslow-Wilcoxon test (GraphPad Software, La Jolla, CA, USA).
Results
Cooperative effects of ionizing radiation and TW-37 on endothelial cell proliferation
The proliferation of primary human endothelial cells was examined in the presence of varying doses of ionizing radiation and/or TW-37, using the SRB assay. Radiation alone inhibited HDMEC proliferation (Figure 1A). No difference was seen in proliferative function, relative to vehicle treatment, when HDMEC were treated with a standard range of TW-37 doses (0.023-50 μM) and exposed to radiation (2-20 Gy), compared with non-irradiated controls over a 72-hour period (Figure 1B). The clonogenic assay revealed modest but significant (p<0.05; radiation + TW-37 curve versus each alone and the control curve) radiosensitizing effects for low concentrations of TW-37 in inhibition of individual endothelial cell proliferation (Figure 1C). The enhancement ratios for TW37 + RT compared with RT alone was 1.2 +/- 0.05. The plating efficiency for 0.1 μM TW-37 was 11% +/- 0.3% while for no drug was 10% +/- 1% (P>0.05). Notably, the concentration of TW-37 used in the clonogenic assay has been shown to have no effect on endothelial cell viability (11).
Figure 1. TW-37 potentiates the effects of radiation on microvascular endothelial cells.
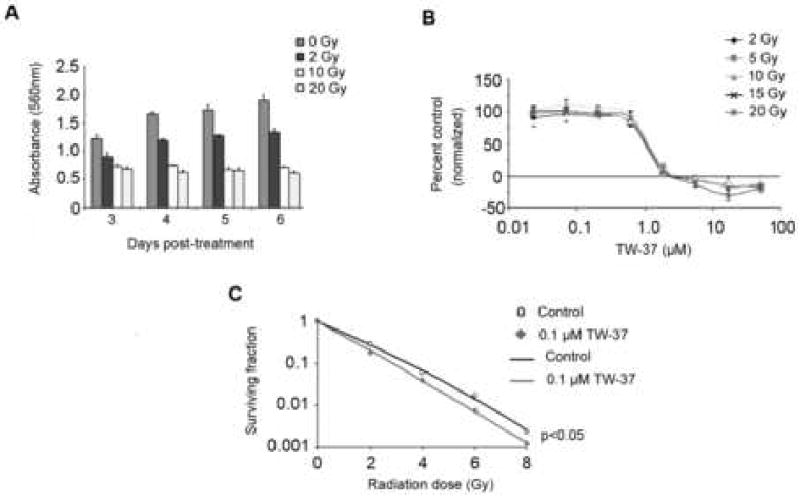
Human dermal microvascular endothelial cells (HDMEC) were exposed to increasing doses of radiation, as indicated. Effects of treatment on cell density were evaluated by the SRB assay. The anti-proliferative effects of radiation persisted over 7 days and are dose dependent (A). Radiation did not affect dose response, of total cell population, to TW-37 induced inhibition of proliferation as determined by SRB assay (B). A radiosensitizing effect was displayed by low dose TW-37 on radiation-treated endothelial cells (C).
Effects of ionizing radiation and TW-37 on endothelial cell cycle
Radiation (6 Gy) alone induced a G2 cell cycle block in endothelial cells, when compared to non-irradiated controls (Figure 2A). Consistently, there was little effect from concentrations of TW-37 up to 0.5 μM, however 5 μM TW-37 caused a cell cycle accumulation in S-phase in both radiation-treated (Figure 2B) and untreated cells (Figure 2C). S-phase accumulation was associated with a reduction in the proportion of cells in both, G1 and G2 phase (Figure 2B and 2C). Combination therapy had no effect on endothelial cell apoptosis levels when compared to TW-37 alone, at the concentrations used here (Figure 2D). For both irradiated and non-irradiated samples, apoptosis levels were unchanged up to 0.5 μM TW-37, when compared to controls. Apoptosis increased to approximately 18% for cells treated with 5 μM TW-37 irrespective of exposure to RT. Importantly the conditions used in this assessment for apoptosis are equivalent to, or exceed, those used to successfully induce radiosensitization in the clonogenic assay (Figure 1C), and have no additive or synergistic effect on apoptotic fraction of HDMEC (Figure 2D).
Figure 2. Radiation does not potentiate the pro-apoptotic effects of TW-37.
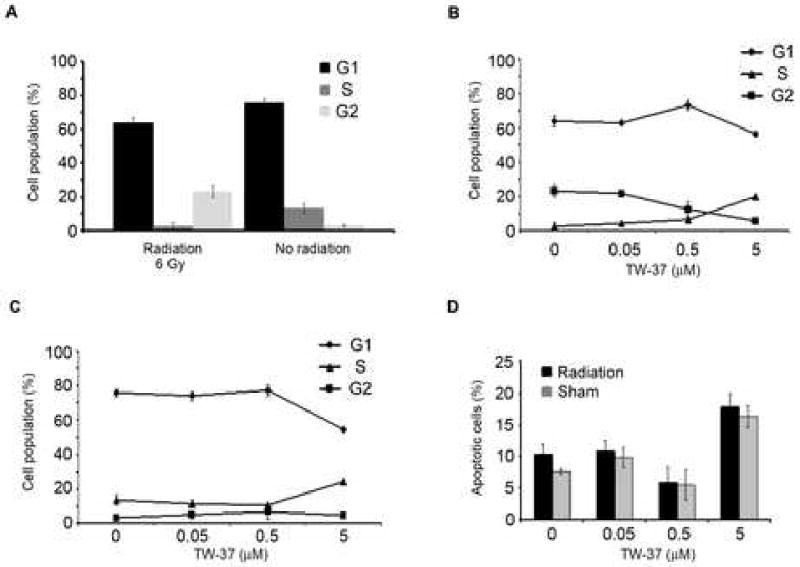
HDMEC were exposed to radiation (6 Gy) and/or TW-37 (0.05-5 μM) in various combinations for 48 hours. Propidium iodide staining followed by flow cytometric analysis was used to evaluate cell cycle. Radiation alone induces an accumulation of HDMEC in G2-phase (A). In HDMEC exposed to radiation (6 Gy), TW-37 led to an accumulation of cells in S-phase (B). TW-37 also induced accumulation of cells in S-phase in the absence of radiation (C). TW-37 induced apoptosis, shown by the Sub-G0/G1 fraction, was unaffected by exposure to radiation with 6 Gy (D).
Combination therapy inhibits angiogenic sprouting in vitro
In a previous study, we determined that persistent inhibition of sprouting occurs from 1 Gy to 5 Gy, with no change in effect between 2 Gy and 5 Gy (23). As a dose of 1 Gy gave a sub-maximal effect, this dose was used in the combination studies performed here. Both treatments caused a significant attenuation in sprouting, but only combination treatment with radiation and TW-37 abolished new sprout formation (Figure 3). Notably, the concentration of TW-37 used here does not induce significant apoptosis or, inhibit proliferation of, endothelial cells (11).
Figure 3. TW-37 and radiation cooperatively inhibit endothelial cell sprouting.
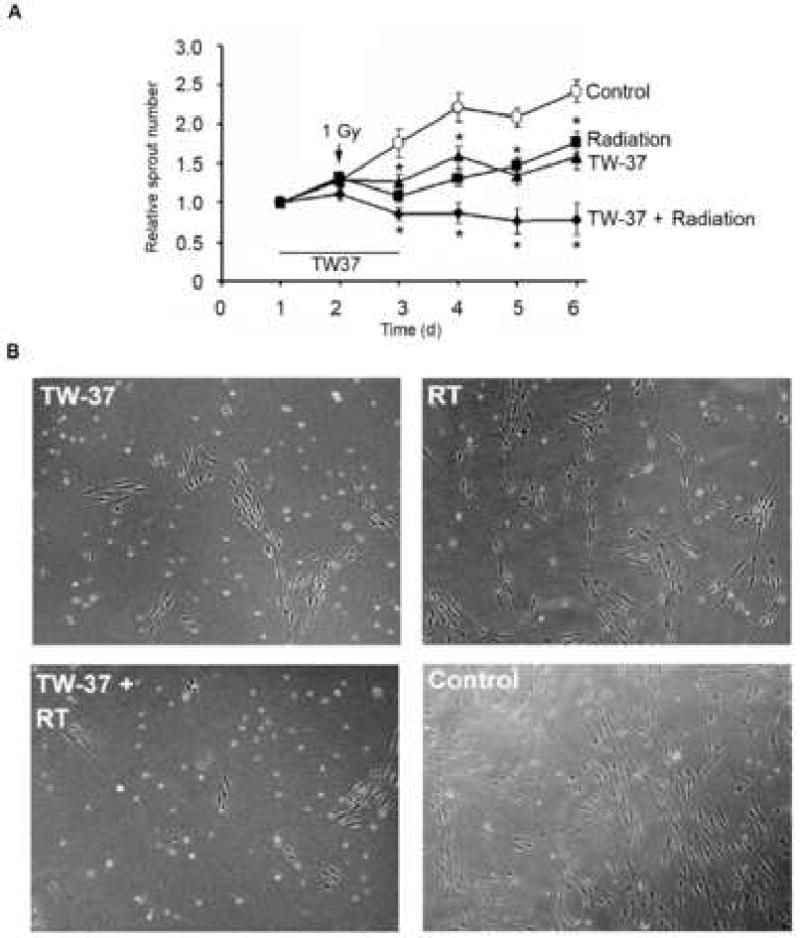
HDMEC were cultured on 3-D collagen gels, and capillary sprouting was induced by treatment with 50 ng/ml rhVEGF165 for 4 days. Cells were treated concurrently with drug and/or radiation in presence of 50 ng/ml rhVEGF165 thereafter. Sprouting structures were counted in 6 high power (200×) fields per well, from 3 wells per experimental condition. Graph depicts the quantification of sprout number per microscopic field (A). Photomicrographs show representative images for each treatment regimen (B).
Effects of ionizing radiation and TW-37 on tumor angiogenesis and growth
To determine appropriate radiation dose for our humanized model of tumor angiogenesis (29), we performed a limited dosing study. Radiation at either 0.8 Gy or 1 Gy, administered for 5 consecutive days, inhibited tumor growth (p<0.05) up to one week after treatment (Figure 4A). Both doses resulted in significantly smaller tumor weights at the end of the study (Figure 4B). However, there was no significant difference in tumor weight between either 0.8 Gy or 1 Gy. No significant dose limiting toxicity was observed as determined by changes in body weight (Figure 4C).
Figure 4. Calibration of radiation dosing for PLLA scaffold model of humanized tumor vasculature.
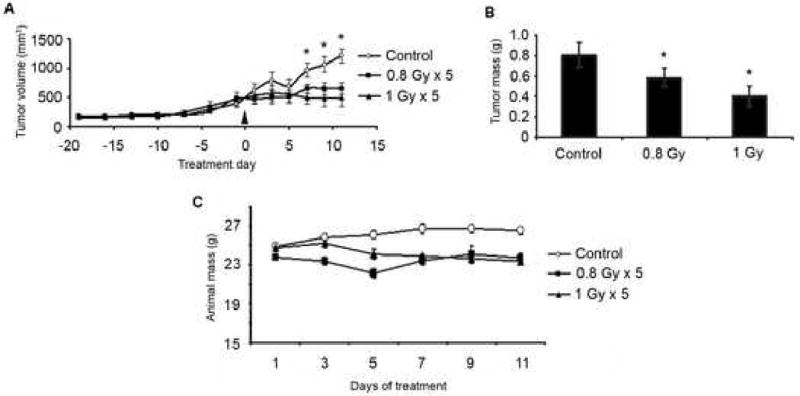
Mice were implanted with PLLA scaffolds bearing 1×105 OSCC3 tumor cells and 9×105 HDMEC. Mice were submitted to radiation (0.8 or 1 Gy) for 5 days. Xenograft tumor growth was evaluated by caliper measurement every other day for the duration of the experimental period (A). End-point tumor weight (B) and body weight over the experimental period (C) were determined and analyzed.
Administration of TW-37 was performed daily for 10 days in order to mimic a metronomic regime (21), while radiation therapy was performed for 3 consecutive days. Evaluation of tumor volume over time showed tumor growth delay for both the radiation therapy alone and combination with TW-37 (Figure 5A). Despite the termination of treatment at day 10, we observed a separation of the curves for combination therapy and radiotherapy alone starting around day 30 and continuing through the completion of the experiment (Figure 5A). Analysis of time to treatment failure (defined as a 4-fold increase in tumor volume, as compared to baseline) showed that combination treatment of TW-37 and ionizing radiation produced a significantly greater effect than single drug therapies or vehicle controls (p<0.05) (Figure 5B). In light of the inhibitory action of TW-37 on non-tumor related angiogenesis in vivo (11) we examined the effects of single and combination treatments, collecting samples immediately after cessation of treatment. These tumors displayed a significant reduction in blood vessel density with TW-37 treatment compared to vehicle control (p=0.01). Blood vessel density in TW-37 plus RT treated tumors nearly reached a significant reduction compared to control (p=0.051). There was no significant difference in blood vessel density of tumors treated with RT alone (p=0.07) (Figure 5C).
Figure 5. Effect of TW-37 and radiation treatment on time to tumor failure and tumor angiogenesis.
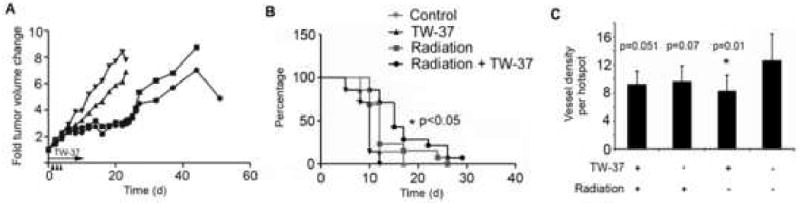
HDMEC and OSCC3 bearing scaffolds were implanted in immunodefficient mice. (A) Mice were submitted to radiotherapy (0.8 Gy × 3 days) with or without TW-37 (15 mg/kg/day) administered daily for 10 days, as indicated by arrows in the graph. Graph depicts the fold tumor volume change as compared to pre-treatment volume. (B) Kaplan-Meier plot depicts time to treatment failure, defined as a 4-fold increase in tumor volume as compared to pre-treatment values. Data are representative of three independent experiments of n=14 tumors per treatment. (C) Microvessel density was assessed in 3 hotspot fields (at 400×) per tissue section. N=8 tumors per treatment group.
Assessment of tumor pathological phenotype
Tumors from the radiation alone and vehicle control groups shared similarities between themselves (Figure 6), but were clearly distinct from the experimental conditions that involved TW-37 treatment. Collectively, the TW-37 and combination treatment groups were represented by moderately differentiated tumors, characterized by large islands of tumor cells surrounded by inflammatory cells (Figure 6). Generally cohesive fronts were displayed by these tumors. The specimens from the TW-37 group showed a pattern of growth characterized by the presence of cohesive islands. Numerous atypical mitoses as well as atypical squamous cells with enlarged and angulated nuclei were seen. Increased nuclear-to-cytoplasmic diameter ratio, hyperchromasia, inflammatory cells including neutrophils, eosinophils, and lymphocytes were also present. Tumors from the combination treatment group also showed large pushing fronts of dozens of cohesive squamous cells. They formed large islands surrounded by mixed inflammatory components, numerous typical and atypical mitosis, as well as nuclear and cytoplasmic pleomorphism.
Figure 6. TW-37-treated tumors present less aggressive phenotype.
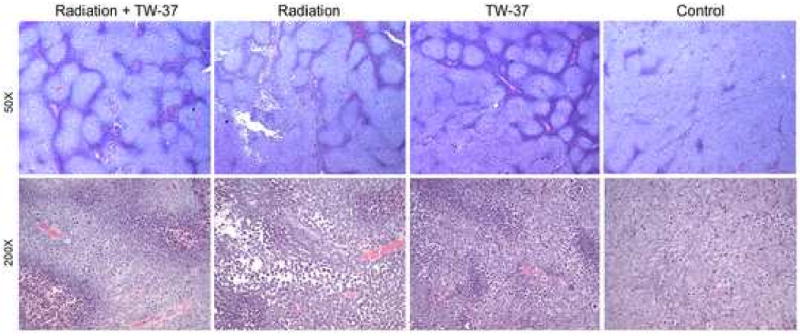
Tumors treated with TW-37 alone, TW-37 + radiation, radiation alone, or vehicle were retrieved 24 hours after last treatment administration. Histological sections were stained with hematoxylin/eosin and assessed microscopically by a trained pathologist blinded for experimental condition. Representative images were captured under bright-field microscopy at 50× and 200× magnification (n=8 tumors per treatment group).
In contrast, tumors from radiation alone and vehicle groups were classified as poorly differentiated although also showing moderate differentiation in some areas (Figure 6). These two groups were characterized by the presence of cells forming large anastomizing areas and pronounced cytonuclear atypia. Diffuse infiltrating patterns represented by a non-cohesive front and diffuse single cell spread were only seen in tumors from radiation and vehicle groups. Areas of the radiation alone group were also represented by intercellular bridges that radiated from the cytoplasm and connected adjacent cells. Intratumoral areas mimicking a non-cohesive front were also seen. Other features presented in this group were mixed inflammatory components, typical and atypical mitosis, as well as nuclear and cytoplasmic pleomorphism. Samples from the vehicle treated group also showed tumors with large cohesive islands which contrasted with areas of dense masses or diffusely spread, loosely associated single cells. As seen with radiation alone, non-cohesive fronts, mixed inflammatory components, typical and atypical mitosis, as well as nuclear and cytoplasmic pleomorphism were present.
Discussion
Double-stranded DNA breakage signaling cell death through the p53 pathway is probably the best-described pathway for radiation-induced apoptosis. BAX may also be induced by radiation via the death receptor signaling pathway and subsequent ceramide induced protein kinase activation (30,31). Pharmacological inhibition of Bcl-2 has shown efficacy in a variety of co-therapeutic regimens for cancer using both in vitro and in vivo tumor models (13). The effect of Bcl-2 inhibition and radiation on the endothelial fraction of tumors is less well understood. Endothelial apoptosis, senescence and proliferation are mediated by ionizing radiation under varying conditions and through clearly different mechanisms (31,32). In the present study, we demonstrate the cooperative anti-tumor effects of a novel Bcl-2 inhibitor and radiotherapy on a tumor model with humanized tumor endothelium.
There is limited literature regarding the effects of ionizing radiation on endothelial cells alone and the data that has been presented is occasionally contradictory. Here, we observed little effect on proliferation when microvascular cells were exposed to ionizing radiation up to 10 Gy. It was recently demonstrated that 4-8 Gy ionizing radiation induced a senescent phenotype in proliferating bovine aortic endothelial cells and human umbilical vein endothelial cells, but not in confluent monolayers (33). Interestingly, whilst they observed significant reduction in DNA replication, cell proliferation and invasion capabilities, they found no significant effects on their ability to form sprout-like tubes on Matrigel. Here, a significant reduction was observed in the ability of HDMEC to form sprouting structures in 3-D collagen matrices. The collagen matrix sprouting assay involves both migration and invasion over an extended period (2-8 days), unlike the Matrigel assay which is a rapid (6-24 hours) primarily migration based assay. The inclusion of an invasive element may account for the observed differences between the two studies. Indeed, a recent study showed some significant inhibition of HUVEC sprouting on a Matrigel bed (34). The combination of TW-37 (0.5 μM) and radiation (1 Gy) significantly inhibited endothelial cell sprouting compared to either treatment alone. This suggested the potential for both treatments to cooperate via an anti-angiogenic mechanism. The combination effects of radiation and TW-37 on cell cycle are more difficult to understand. Radiation alone produces an expected G2 block and TW-37 has little noticeable effect until the concentration reaches 0.5 μM, still below a concentration for which TW-37 induces apoptosis or inhibits proliferation of endothelial cells. From 0.5 μM to 5 μM, the G1 and G2 phase fractions of cells fall while the proportion of cells in S-phase rises. It is unclear how this effect alters the fate of cells as, consistently, 5-10% of these cells will undergo apoptosis and the greater majority will cease proliferating (11).
Similarly to previous studies of TW-37 and angiogenic function (11) the concentrations of TW-37 that do not induce apoptosis still had an anti-proliferative effect that was sufficient to sensitize endothelial cells to radiation mediated growth inhibition in a clonogenic assay. This was in contrast to the SRB-based proliferation assay, which demonstrated no such sensitizing effect. Cell proliferation or cytotoxicity assays generally measure the whole population of cells in a well, plate or tube. The clonogenic assay is rarely used in endothelial cell analysis, however it serves a useful purpose here in determining individual endothelial cell survival, as shown (35,36). That a sensitizing effect was observed in assay of individual cells and not in assay of the population as a whole suggested that there is a sub-population of endothelial cells that are selectively responsive to the radio-sensitizing effects of TW-37. We have recently demonstrated that 22% of endothelial cells are required to undergo apoptosis to cause a significant reduction in blood vessel density (37). This suggests that even a small sub-population of endothelial cells sensitized to radiation may potentially have a significant effect on the tumor vasculature.
Sub-maximal doses of TW-37 and radiation were used intentionally for in vivo studies to allow for observation of combination effects on the head and neck tumor xenografts. Radiation doses as low as 0.8 Gy administered for 5 consecutive days abolished tumor growth. Interestingly, the SCID-mouse xenograft model used in this study was consistently more sensitive to radiation in every experiment performed than might be expected for other immunodeficient murine models. The radiation exposures were carefully determined and performed by an experienced radiologist. Ultimately, 0.8 Gy administered for 3 days was used in our studies. It allowed a transient, but significant, decrease in tumor growth rate. Likewise, 60 mg/kg in combination with a chemotherapeutic regimen was necessary to cause growth inhibition on a lymphoma xenograft model (38). Here, the tumor inhibitory effect persisted in the combination group with metronomic TW-37 over two weeks after cessation of treatment. These data supports the use of a metronomic administration of the Bcl-2 inhibitor as an effective dosing regimen that shows low toxic effects.
Pathological assessment of tumor status immediately after final treatment reflected the observed long-term tumor growth patterns. The cohesive pattern of tumor growth presented by the TW-37 and TW-37 plus ionizing radiation groups has a more favorable prognosis than the diffuse growth presented by specimens from radiation alone and vehicle control. It is widely described that tumors invading with pushing borders are less aggressive than tumors showing a non-cohesive front and diffuse spread with tiny strands or single cells (27). Additionally, invasive fronts are typically correlated with metastases (27). These data strengthen the argument for usage of low dose TW-37 as a positive tumor modifier.
The present study demonstrates the ability of TW-37 to sensitize endothelial cells to the effects of low dose radiation. The results further suggest that this effect may derive from a sensitive sub-population of endothelial cells that respond to TW-37 treatment and might contribute to the significant reduction of tumor blood vessel density seen in vivo. The anti-angiogenic effects of radiation demonstrated here compliment the known anti-tumor cell effects of both TW-37 and radiation in vitro and may account for an enhanced anti-tumor effect for the combination therapy in vivo. These results suggest that head and neck patients might benefit from the combination of a metronomic regimen of TW-37, or related drugs, with traditional radiotherapy.
Acknowledgments
The authors thank Chris Strayhorn for help with histology and Kari Wilders-Roman for help with irradiation studies. Supported by grant P50-CA97248 (University of Michigan Head & Neck SPORE) (SW, JEN), U19-CA113317 (SW), R01-DE14601, R01-DE15948, R01-DE16586, and R21-DE19279 from the NIH (JEN).
Footnotes
Conflict of Interest Notification: Shaomeng Wang: Commercial research grant, ownership interest, and consultant of Ascenta Therapeutics.
The remaining authors of this manuscript have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Argiris A, Karamouzis MV, Raben D, et al. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- 3.Le Tourneau C, Siu LL. Molecular-targeted therapies in the treatment of squamous cell carcinomas of the head and neck. Curr Opin Oncol. 2008;20:256–263. doi: 10.1097/CCO.0b013e3282f9b575. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Moran MS, Haffty BG. Bcl-2 expression predicts local relapse for early-stage breast cancer receiving conserving surgery and radiotherapy. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-0068-4. [DOI] [PubMed] [Google Scholar]
- 5.Chen MK, Lai JC, Chang CC, et al. Prognostic impact of bcl-2 expression on advanced nasopharyngeal carcinoma. Head Neck. 2008 doi: 10.1002/hed.20839. [DOI] [PubMed] [Google Scholar]
- 6.Lee KH, Im SA, Oh DY, et al. Prognostic significance of bcl-2 expression in stage III breast cancer patients who had received doxorubicin and cyclophosphamide followed by paclitaxel as adjuvant chemotherapy. BMC Cancer. 2007;7:63. doi: 10.1186/1471-2407-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khor LY, Moughan J, Al-Saleem T, et al. Bcl-2 and Bax expression predict prostate cancer outcome in men treated with androgen deprivation and radiotherapy on radiation therapy oncology group protocol 92-02. Clin Cancer Res. 2007;13:3585–3590. doi: 10.1158/1078-0432.CCR-06-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contu PC, Contu SS, Moreira LF. Bcl-2 expression in rectal cancer. Arq Gastroenterol. 2006;43:284–287. doi: 10.1590/s0004-28032006000400008. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko T, Zhang Z, Mantellini MG, et al. Bcl-2 orchestrates a cross-talk between endothelial and tumor cells that promotes tumor growth. Cancer Res. 2007;67:9685–9693. doi: 10.1158/0008-5472.CAN-07-1497. [DOI] [PubMed] [Google Scholar]
- 10.Ashimori N, Zeitlin BD, Zhang Z, et al. TW-37, a small-molecule inhibitor of Bcl-2, mediates S-phase cell cycle arrest and suppresses head and neck tumor angiogenesis. Mol Cancer Ther. 2009;8:893–903. doi: 10.1158/1535-7163.MCT-08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeitlin BD, Joo E, Dong Z, et al. Antiangiogenic Effect of TW37, a Small-Molecule Inhibitor of Bcl-2. Cancer Res. 2006;66:8698–8706. doi: 10.1158/0008-5472.CAN-05-3691. [DOI] [PubMed] [Google Scholar]
- 12.Spalding AC, Lawrence TS. New and emerging radiosensitizers and radioprotectors. Cancer Invest. 2006;24:444–456. doi: 10.1080/07357900600705706. [DOI] [PubMed] [Google Scholar]
- 13.Zeitlin BD, Zeitlin IJ, Nor JE. Expanding circle of inhibition: small-molecule inhibitors of Bcl-2 as anticancer cell and antiangiogenic agents. J Clin Oncol. 2008;26:4180–4188. doi: 10.1200/JCO.2007.15.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Yang D, Wang S, et al. (-)-Gossypol enhances response to radiation therapy and results in tumor regression of human prostate cancer. Mol Cancer Ther. 2005;4:197–205. [PubMed] [Google Scholar]
- 16.An J, Chervin AS, Nie A, et al. Overcoming the radioresistance of prostate cancer cells with a novel Bcl-2 inhibitor. Oncogene. 2007;26:652–661. doi: 10.1038/sj.onc.1209830. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, Nikolovska-Coleska Z, Yang CY, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–6142. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 18.Verhaegen M, Bauer JA, Martin de la Vega C, et al. A novel BH3 mimetic reveals a mitogen-activated protein kinase-dependent mechanism of melanoma cell death controlled by p53 and reactive oxygen species. Cancer Res. 2006;66:11348–11359. doi: 10.1158/0008-5472.CAN-06-1748. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Song W, Aboukameel A, et al. TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth and invasion in pancreatic cancer. Int J Cancer. 2008;123:958–966. doi: 10.1002/ijc.23610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Stempak D, Seely D, Baruchel S. Metronomic dosing of chemotherapy: applications in pediatric oncology. Cancer Invest. 2006;24:432–443. doi: 10.1080/07357900600705599. [DOI] [PubMed] [Google Scholar]
- 21.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 22.Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 23.Spalding AC, Zeitlin BD, Wilder-Romans K, et al. Enzastaurin, an inhibitor of PKCbeta, Enhances Antiangiogenic Effects and Cytotoxicity of Radiation against Endothelial Cells. Transl Oncol. 2008;1:195–201. doi: 10.1593/tlo.08151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 25.Nör JE, Hu Y, Song W, et al. Ablation of microvessels in vivo upon dimerization of iCaspase-9. Gene Ther. 2002;9:444–451. doi: 10.1038/sj.gt.3301671. [DOI] [PubMed] [Google Scholar]
- 26.Nör JE, Christensen J, Mooney DJ, et al. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shingaki S, Suzuki I, Nakajima T, et al. Evaluation of histopathologic parameters in predicting cervical lymph node metastasis of oral and oropharyngeal carcinomas. Oral Surg Oral Med Oral Pathol. 1988;66:683–688. doi: 10.1016/0030-4220(88)90318-0. [DOI] [PubMed] [Google Scholar]
- 28.Vermeulen PB, Gasparini G, Fox SB, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38:1564–1579. doi: 10.1016/s0959-8049(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 29.Nör JE, Christensen J, Liu J, et al. Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 2001;61:2183–2188. [PubMed] [Google Scholar]
- 30.Rodemann HP, Blaese MA. Responses of normal cells to ionizing radiation. Semin Radiat Oncol. 2007;17:81–88. doi: 10.1016/j.semradonc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- 32.Oh CW, Bump EA, Kim JS, et al. Induction of a senescence-like phenotype in bovine aortic endothelial cells by ionizing radiation. Radiat Res. 2001;156:232–240. doi: 10.1667/0033-7587(2001)156[0232:ioaslp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Igarashi K, Sakimoto I, Kataoka K, et al. Radiation-induced senescence-like phenotype in proliferating and plateau-phase vascular endothelial cells. Exp Cell Res. 2007;313:3326–3336. doi: 10.1016/j.yexcr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Tu T, Thotala D, Geng L, et al. Bone marrow X kinase-mediated signal transduction in irradiated vascular endothelium. Cancer Res. 2008;68:2861–2869. doi: 10.1158/0008-5472.CAN-07-5743. [DOI] [PubMed] [Google Scholar]
- 35.Franken NA, Rodermond HM, Stap J, et al. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 36.Kataoka Y, Murley JS, Baker KL, et al. Relationship between phosphorylated histone H2AX formation and cell survival in human microvascular endothelial cells (HMEC) as a function of ionizing radiation exposure in the presence or absence of thiol-containing drugs. Radiat Res. 2007;168:106–114. doi: 10.1667/RR0975.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong Z, Zeitlin BD, Song W, et al. Level of endothelial cell apoptosis required for a significant decrease in microvessel density. Exp Cell Res. 2007;313:3645–3657. doi: 10.1016/j.yexcr.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohammad RM, Goustin AS, Aboukameel A, et al. Preclinical studies of TW-37, a new nonpeptidic small-molecule inhibitor of Bcl-2, in diffuse large cell lymphoma xenograft model reveal drug action on both Bcl-2 and Mcl-1. Clin Cancer Res. 2007;13:2226–2235. doi: 10.1158/1078-0432.CCR-06-1574. [DOI] [PubMed] [Google Scholar]


