Abstract
The interaction of cytoplasmic proteins with intracellular domains of membrane receptors can occur at several opportunities, including: during biosynthesis, while in membrane residency and during internalization and recycling following ligand binding. Since the initial discovery that it interacts with the FSH receptor (FSHR) together with additional members of a potential signaling complex, APPL1 has been shown to interact with a variety of membrane receptors. Recent subcellular localizations of APPL1 place it in dynamic and varied venues in the cell, including at the cell membrane, the nucleus and the early endosomes. Another adapter protein family the 14-3-3 proteins, are largely recognized as binding to phosphorylation sites but recent work demonstrated that in the case of FSHR, the 14-3-3 site overlaps with the canonical G-protein binding site. G-proteins appear to sample the environment and exchange between the membrane and intracellular locales and this binding could be mediated by or modulated by receptor interactions at the 14-3-3 binding site. Observations that multiple proteins can interact with cytoplasmic domains of GPCRs leads to the inescapable conclusion that either the interactions occur via orderly replacement or exchange, or that receptors are simultaneously occupied by a variety of adapters and effectors or even that oligomers of dimeric GPCRs provide for platforms that can simultaneously interact with effectors and adaptors.
FSH Receptor (FSHR) Organization
Follicle stimulating hormone (FSH) is essential for ovarian folliculogenesis in females and spermatogenesis in males. The follicle stimulating hormone receptor (FSHR) protein is a member of the G-protein coupled receptor (GPCR) family and is comprised of a large extracellular domain and a transmembrane domain. The seven α-helices of the transmembrane domain are interconnected by three extracellular (ecL1, ecL2 and ecL3) and three intracellular loops (icL1, icL2 and icL3) (1). It is an open question as to how many of the FSHR subunits form a functional receptor. Biochemical studies demonstrated that the minimal unit of FSHR that is stable to detergent extraction appears to be a dimer (2). It is likely that higher order arrays exist (3).
A model of the FSH receptor transmembrane domain has been proposed which has proven useful in understanding the role of cysteine residues as attachment sites for lipid anchors (4). The formation of palmitoylation sites on the FSH receptor C-terminal tail potentially provides for the formation of an additional loop icL4 (4). The recent crystal structures of opsin and beta 2 adrenergic receptors have provided further opportunities to visualize the three dimensional structure of the cytoplasmic face of a G-protein coupled receptor (5;6). Whereas activation of GPCRs appears to require only the monomeric form of receptor, it is anticipated that regulation and activation will be more complex and dependent on cellular localization (7). Also, such data suggest the idea that the relationships of the monomeric receptors to each other may be more important for targeting than how they form functional units for signaling (8;9). The FSH receptor forms oligomers in the endoplasmic reticulum and clipping of the C-terminal tail is required for insertion into the plasma membrane (2). Dimerization of receptor in the endoplasmic reticulum will have consequences for genetic diseases of heterozygosity, where mutant forms of receptor which do not traffick to the cell surface may impair normal processing of receptors from the wild type allele by heterodimerization followed by retrograde proteosomal degradation of the heterodimeric complex (10). Conversely, it could be anticipated that FSHR heterozygosity may yield fertility even in the presence of inactivating mutations, by virtue of the wild type alleles giving rise to functional homodimers as well as functional heterodimers.
Juxtaposition of the receptor monomers while not necessarily positioning the intracellular loops’ three dimensional binding sites for various effectors such as G-proteins, can certainly affect the extent to which one or more adapter proteins might associate with the receptor. In its simplest embodiment, G-protein interaction is thought to be facilitated by a movement of helix VI and a shift in icL3, allowing for docking of the G-protein (6). It is now suggested that G-protein interaction with receptors may involve random collisions and not stable complexes (11). In this paradigm, ligand binding could lead to amplification of the signal by the expected dissociation of the G-protein subunits, one of which activates adenylyl cyclase leading to production of cAMP, which in turn activates protein kinase A (PKA) (12). It is not known if more than one protein can bind to a receptor dimer at a time, if an adapter-occupied receptor can accommodate a G-protein or what the ratio’s are of differentially occupied receptors.
FSH Receptor Signaling
In recent years the complexity of FSH signaling has become appreciated. This complexity is likely governed by cytoplasmic FSH receptor-interacting proteins which determine the residency time of signaling complexes in different cellular compartments. Biological efficacy has been typically evaluated by the kinetics of ligand receptor interaction. The binding event is clearly important from a standpoint of ensuring interaction of ligand and receptor, despite low physiological levels of hormone in the blood. However, the biological responsiveness of cells can be transient or long lived depending on the readout chosen. The best example of transient response is the production of cAMP. Rat granulosa cells in culture secrete most of the cAMP produced in response to FSH (data not shown). The low levels of cAMP detected in the cells is probably further reduced in specific compartments where phosphodiesterases are targeted (13). A recent example of sustained activation of shows that persistent phosphorylation of extracellular regulated kinase (ERK) can lead to aromatase activation in granulosa cells (14). Less clear is the fate of internalized occupied receptors, and their interactions with signaling effectors and terminators within the cell.
FSH activates p38 mitogen activated protein kinase (MAPK) in rat granulosa cells (15) and the ERK pathway (16). FSH also stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-induced kinase (SGK) in rat granulosa cells (17). Stimulation of the downstream kinases such as ERK1/2 occur via activation of the small G-protein Ras or via β-arrestins (18) whereas the activation of Akt appears to involve the Src family of kinases (19). Following FSH stimulation elevation in mRNA for SGK, Activin A, P450scc and CYP19 (aromatase) is observed while caveolin message is dramatically decreased (20). Interestingly, constitutively active PKA raises P450 scc similarly as FSH but CYP19 message is dramatically elevated by FSH compared to constitutively active PKA (20). Additionally, dominant negative PKB abolishes CYP19 expression (21). These data demonstrate that additional pathways work in concert with PKA for full FSH induction of estrogen production. One potential pathway which has been largely ignored and deserves further study is the IP3/Ca+2/calmodulin/protein phosphatase pathway which may be related to the PKB pathway. This pathway controls phosphorylation status of GATA family members and in particular, the control of ovarian and testicular gene transcription by the GATA family members is of interest. The role of calcium signaling in granulosa cells has been suggested to affect variants of FSH receptor which may signal as growth factor-like receptors (22). Extracellular calcium is not required for cAMP production in response to FSH but does play a role in expression of P450 scc in porcine granulosa cells cultured in defined media without serum or growth factors (23). FSH also causes an increase in intracellular calcium in a cAMP-independent manner (24).
While the steroidogenesis pathways provide for monitoring of differentiation, less is known about pathways that influence apoptosis, proliferation or metabolic support to the oocyte. Treatment of granulosa cells with estradiol lessens the effect of Fas ligand-induced apoptosis (25;26). Although the field has used steroid production as an indicator of differentiated function of granulosa cells, these readouts may not be optimal for elucidation of intracellular signaling pathways that could govern metabolism and proliferation, or even recruitment of adjacent cells into a synchronized community (27). Indeed, these parameters might best be studied not by stimulation with FSH alone. Rather, it seems appropriate that future studies examine the interactions of FSH stimulation with adiponectin, EGF and epiregulin stimulation, to name a few. Moreover, educated guesses about which pathways to interrogate may not be as fruitful as interrogation of the transcriptome using mutants of the FSHR so that timing is not an issue.
Expanding roles of FSH Receptor Interacting Protein APPL1
Clearly, the idea that movement of FSHR within the cell is governed by interaction with cytoplasmic proteins which then influence the quality and quantity of signaling pathway output, seems a reasonable paradigm. Previous studies, using a yeast interaction trap, identified several proteins expressed by an ovarian cDNA library as interacting partners of human FSHR (hFSHR) icL1, icL2 or icL3 (28–30). One of the proteins that interacts with hFSHR icL1was identified as an adaptor protein named APPL1 (Figure 1) which was originally identified as an Akt2 (Akt;Protein Kinase B; PKB) interacting protein (31). Initial consideration of the physiological role of FSHR-APPL1 interaction in the ovary was as an effector of FSH mediating PI3K signaling involved in the promotion and regulation of cellular metabolism, proliferation and apoptosis (30). The idea was that if Akt is bound by APPL1 then the interaction of an FSHR-FOXO1a complex with an FSHR-APPL1-Akt complex could lead to phosphorylation of FOXO1a. Indeed FSH stimulation results in phosphorylation of FOXO1a and its exclusion from the nucleus (32;33) thereby regulating cell cycle progression by regulating p27 Kip in granulosa cells (34;35). Activation of the PI3K/Akt pathway in granulosa cells by FSH is concurrent with the induction of expression of several differentiation markers, mediated through transcriptional activation by Hif 1 (36).
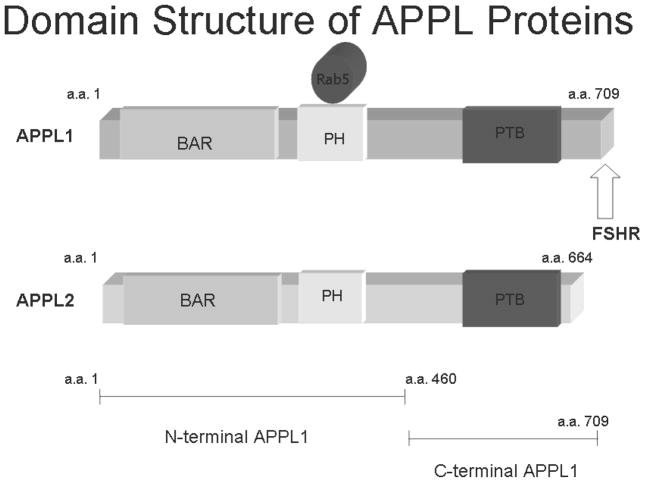
Figure 1.
Schematic of the domain architecture of APPL proteins. The BAR domain is the dimerization domain (42). The pleckstrin homology domain (PH) is the site of Rab5 interaction. The FSH receptor binding site is in the C-terminal domain, which also contains the phosphotyrosine binding domain (PTB).
Some support for the concept of a large FSHR signaling complex was engendered when it was found that in addition to APPL1, FSHR interacts with FOXO1a which is a downstream effector of the PI3K pathway (37). FOXO1a does not co-immunoprecipitate with APPL1 and at this time, the mechanism of FOXO1a association with FSH receptor is not known. However, an intriguing possibility is that Akt-APPL1-FSHR holds Akt inactive, and that FSHR-FOXO1a keeps FOXO1a tethered so that upon activation of Akt, FOXO1a can be phosphorylated. However, it is not clear if activation of Akt would rely solely on PDK1. Recently it has been shown that protein kinase CK2 can phosphorylate Akt (38). Protein kinase CK2 is also associated with the FSHR (39) providing an interesting possibility that protein kinase CK2 is the missing link for Akt phosphorylation in the absence of PDK1 activation at the FSHR signaling complex. It has recently been shown that APPL1 is selectively required for cell survival and that Akt and GSK-3β but not TSC2 dynamically associate with APPL1 endosomes upon growth factor stimulation (40). Interestingly, the early endosomes which form with APPL1 do not contain PI(3,4,5)P3 which the pleckstrin homology domains of Akt would bind to and so a mechanism needs to be in place for Akt to be tethered to this endosome. Thus it has been proposed that Akt may be retained at, or recruited to, the endosomal surface after PI(3,4,5)P3 dephosphorylation by its interaction with APPL1 (41).
A homolog of APPL1 is APPL2 which lacks the C-terminal domain that interacts with FSHR (Figure 1) (37). At the time, it was reasoned based on what was known about Bin-Amphiphysin-Rvs (BAR) domain proteins, that APPL2 would co-immunoprecipitate with FSHR because it associated with APPL1 via their N-termini as would be expected for a BAR domain protein. Since APPL2 lacks the C-terminal residues that likely interacted with FSHR it would not be anticipated to interact directly with FSHR (42). Elegant cell biology studies have shown that as expected, APPL1 and APPL2 co-localize in DLD-1 cells(43). However, the subcellular co-localization of APPL1 and APPL2 may be predicted by the BAR dimerization domains. Thus the majority of APPL1 minimal BAR domains localize to large clusters of round vesicles and ring-like structures while APPL2 minimal BAR domains associate primarily with curved networks of elongated membrane structures(43). The significance of these differences is not yet appreciated.
The recent crystal structures of APPL1 dimers has provided a structural basis for the supposition of BAR domain interaction through the amine terminus (44;45). The significance of this observation is that, in theory, the dimers of APPL1 and APPL2 or heterodimers of each, can bridge interactions between two proteins, in addition to their role as a Rab5 activator. The formation of heterodimers could also have consequences. For example, Akt2 associates with APPL1 as reported but does not associate with APPL2 (37). This was the first documented difference in function between APPL1 and APPL2. Likewise, interactions between FSHR and APPL2 and between FSHR and FOXO1a evidently are distinct since FOXO1a does not associate with either APPL1 or with APPL2.
Since the discovery that APPL1 interacts with Akt and FSHR, it has become clear that APPL1 is a major player in a variety of membrane protein functions (Figure 2). Interestingly full length APPL1 and APPL2 do not co-localize with subcellular compartment markers such as cis or trans Golgi, caveosomes, endoplasmic reticulum or actin, but APPL1 does recruit Rab5 to APPL1-associated cytosolic membrane structures (43) and likely also recruits others such as GTP-bound Rab21 (45). Moreover, the association of APPL proteins with phosphoinositides may impart them with regulatory properties distinct from those of the Rab5 effector EEA1 (early endosome marker) that only binds PtdIns(3)P (43). APPL also plays a role in phoshphoinositide biosynthesis. Ocrl1 (occulocerebralrenal syndrome of Lowe) is an inositol polyphosphate 5–phosphatase that can be found on early endosomes. Its substrates are PI(4,5)P2 and PI(3,4,5)P3. This protein is recruited to endosomes and all mutations of this uncommon disease prevent its interaction with APPL1 containing endosomes (46;47). APPL1 endosomes are precursors of PI(3,4,5)P3 positive endosomes and depletion of PI(3,4,5)P3 causes a reversion to the precursor (41). Thus it has been proposed that the 5-dephophorylation of PI(4,5)P2 and PI(3,4,5)P3 provide a switch for the maturation of APPL1 endosomes (41).
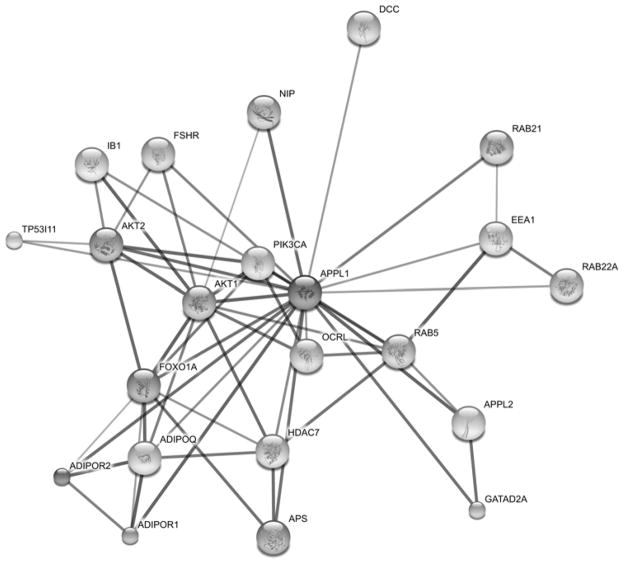
Figure 2.
APPL1 interactome MAP. FSHR interacts with APPL1 potentially leading to additional associations. APPL1 forms heterodimers with APPL2. APPL1 associates with the adiponectin receptor (ADPOR1) as well as the neurotropin receptor TrkA (48). It interacts with protein kinase B (Akt) and phosphoinositide-3-kinase (PI3K) (31) holding them in an inactive complex. Rab5 an effector of APPL1 is bound at the Cterminus (45). APPL1 is present in early endosomes associated with the marker EEA1(81). Following EGF stimulation it is found associated with Histone Deacetylase (HDZC) (50). It has been shown to associate with OCRL (47) and netrin receptor protein referred to as Deleted in Colorectal Carcinoma (DCC) (82).
A mutation of FSHR, which prevents interaction with APPL1 does not affect the canonical FSH-mediated granulosa cell readouts of cAMP production and steroidogenesis (Thomas, R. et al, 2009, submitted). This has prompted consideration of equally important but less studied pathways that enable granulosa cell proliferation, differentiation and metabolism. In this regard, the interplay between growth factors and FSH action seems most interesting. The APPL1 endosome plays host to growth factor receptors, and this interaction suggests that APPL1 could be involved in crosstalk between FSHR and epidermal growth factor receptor (EGR).
It has been shown that APPL1 interacts with the neurotropin receptor (TrkA) via two modes; directly through its phosphotyrosine binding site, and indirectly through its interaction with GIPC1, a protein that is constitutively bound to TrkA and is found associated with clathrin coated pits. Reduction of either GIPC1 or APPL1 decreased signaling by the TrkA receptor to MEK, ERK and Akt. (48). Although GIPC interacts with the cytoplasmic tail of the LH receptor, we do not know at this time if APPL1 interacts with the LHR (49). FSH activation of Ras requires Src family kinases and epidermal growth factor (EGF) receptor tyrosine kinase activities (19). This is of considerable interest because APPL1 translocates from membranes to the nucleus in response to EGF (50) where it interacts with the nucleosome remodeling and histone deacetylase multiprotein complex. Importantly, APPL1 has been linked to the signaling activity of EGFR via the Akt and MAPK pathways (40;50) and it has been demonstrated that prolonged presence of EGFR in the APPL compartment increases not only Akt-dependent signaling but also MAPK signaling (41). Relevant questions with regard to FSH signaling are whether FSHR signal strength is affected by residency time in the APPL endosome, if EGFR and FSHR co-localize in APPL endosomes and further, whether EGFR-FSHR crosstalk is regulated by co-residency in APPL endosomes.
Another potentially fascinating connection of FSH signaling with APPL stems from the interaction of APPL with the adiponectin receptor (51). Adiponectin and its receptors are present in rat ovaries (52) and testis (53) and decreased adiponectin levels in blood are associated with polycystic ovary syndrome (PCOS) (54). Energy regulation in the cell is mediated by adenosine monophosphate kinase (AMPK) which is activated by AMP. Adiponectin can activate AMPK through APPL1-mediated cytosolic localization of the upstream kinase LKB1 or to a lesser extent by activation of PLC. This leads to release of intracellular calcium and activation of calmodulin dependent protein kinase-kinase (CaMKK) (55). Here again, it is not clear if FSHR and adiponectin receptors share the same APPL1 endosomes and if their ligands cross-regulate their actions. However, results suggest that FSH inhibits AMPK activation through an Akt-dependent pathway and promotes granulosa cell proliferation by increasing cyclin D2 mRNA expression and by reducing p27 kip expression (56). In particular it would be important to determine if FSH inhibits the actions of adiponectin with regards to signaling leading to lipid oxidation, glucose uptake and membrane translocation of the glucose transport 4 protein (GLUT4). Expanding roles of APPL1 have pointed to potential areas of crosstalk that may occur upon FSH stimulation. A potential area of interest for future research is the effect of FSH on energy balance and proliferation in the granulosa cell, likely mediated via calcium signaling.
Overlap of the 14-3-3 interaction site on FSHR icL2 with a G-protein binding site
The 14-3-3 family of proteins has been known for some time to be involved in regulation of signaling pathways, specifically via interaction and sequestration of phosphorylated signaling molecules and recently specific interactions have been identified by transgenic mouse proteomics (57). Of interest to FSH signaling, proteins found to associate with 14-3-3 included calcium/calmodulin dependent protein kinase (CaMK), regulatory subunit of protein kinase A, protein phosphatase 2 and 3, phosphodiesterases, G-protein receptor kinase interactor 1 and inositol 1,4,5 triphosphate receptor (IP3R) (57). Interaction of FSHR icL2 with 14-3-3τ protein has been shown by a yeast two-hybrid screen (29). Over-expression of 14-3-3τ in HEK293 cells stably transfected with FSHR decreased cAMP levels by two-fold at the highest dose of FSH tested, suggesting a role for 14-3-3τ in signal transduction. In concert with this finding, FSHR icL2 has also been shown to be involved in the coupling of the receptor to the Gs protein and maintaining the receptor molecule in an inactive state (58).
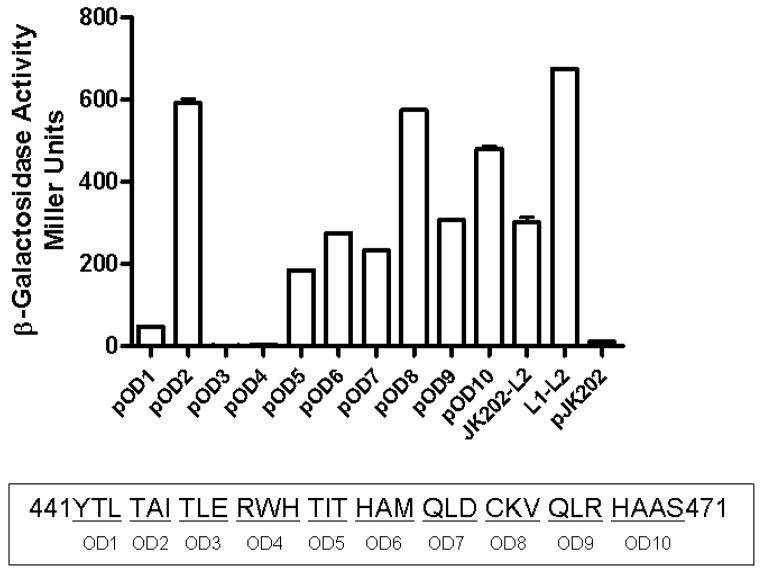
To precisely determine the site of interaction between 14-3-3τ and FSHR icL2, a series of alanine scanning mutants was constructed in icL2. As seen in Figure 3, mutation of amino acids (a.a.) 447TLE449 and 450RWH452 to alanine abrogates the association between 14-3-3τ and FSHR icL2 in a β-galactosidase assay in a yeast two hybrid assay. This assay was performed with the original clone of 14-3-3τ identified in a yeast interaction trap (29) which had a deletion of the first 42 a.a. The deletion encompasses α-helices 1 and 2 (59)(60) and much of the dimerization domain of 14-3-3τ but not the protein interaction domains (61). No interaction was observed between full-length 14-3-3τ and wild type (Wt) FSHR icL2 or with the alanine scanning mutants (data not shown). Monomers of the 14-3-3 proteins have been shown to be active (62) and they exist in both monomeric and dimeric states, possibly using the dimerization process to control their cellular activities (61). Since the primary sequence of icL2: 447TLE RWH452 encompasses the ERW motif important in G-protein interaction and receptor activation (63).
Figure 3.
Scanning alanine mutant of the FSHR icL2 bait protein tested for interaction with 14-3-3τ as previously described (29). Each underlined set of residues was changed to alanine (YTL/AAA). Region 447–452 (OD3 and OD4) of FSHR-icL2 appeared to be involved in the interaction with 14-3-3τ and so single mutations were made in full-length FSHR and tested for function.
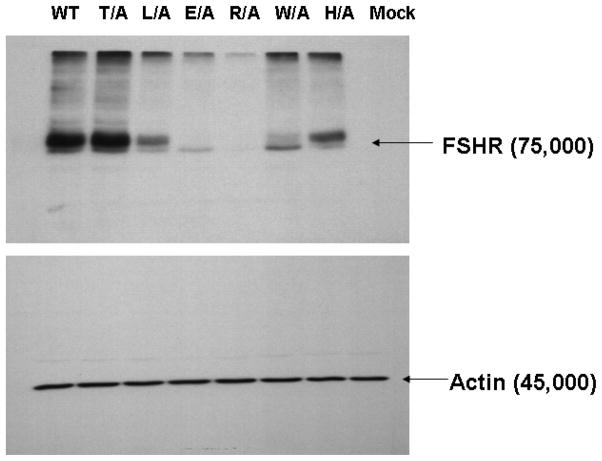
A set of single mutations was generated in which alanine was substituted for each amino acid, so that functionality could be tested. The mutants T447A, L448A and H452A demonstrated high levels of surface binding to radiolabeled hFSH (Figure 4A), with lower levels of binding for R450A and W451A and barely detectable binding for E449A. The ability of the mutants to bind hFSH is consistent with the levels of protein expression when the mutants are overexpressed in HEK293 cells (Figure 4B). The mutants T447A, L448A and H452A have high levels of mature fully glycosylated FSHR and also appear to bind FSH, whereas E449A, R450A and W451A have little or no mature FSHR and consequently, little FSH binding. It has recently become appreciated that 14-3-3 proteins play a role in regulation of endoplasmic reticulum localization of membrane proteins but these are largely considered to involve phosphorylation dependent interactions (64). Whether the defective trafficking of these mutants is due to abrogation of 14-3-3 interaction will require further study. The observation that these residues constrain the helices raises a concern that mutation of these residues could have induced an untoward and deleterious conformational change. The two charged residues in the E(D)RY motif are known to constrain TMVI in the helix bundle constituting the so called “ionic lock”. For example in rhodopsin, Arg135 (TMIII) interacts with Glu247 (TMVI) and Glu134 (TMIII) interacts with Thr251 (TMVI). However in the active structure of opsin there is an interhelical association of Lys231 and Glu247 (TMVI) whereas Arg135 (TMIII) interacts with Tyr223 (TMVI) (6;65;65). Moreover the interaction between TMIII and TMVI enabled by the E(D)RY motif are less so in the β2-adrenergic receptor (66;67). Taken together these data suggest that mutagenesis of these residues would not necessarily result in a conformational instability which would result in retrograde degradation and decreased membrane residency of GPCRs. Consistent with all structures is the orientation of the E(D) residues towards the inner helix bundle and the Y(x) residues towards solvent. Importantly, since the carboxyl terminal peptide of Gsα interacts with R135 (of the ERY motif) in the active structure of opsin (6), it is clear that orientation of these residues towards the inner helix bundle does not preclude their interactions with cytoplasmic adapter proteins.
Figure 4.
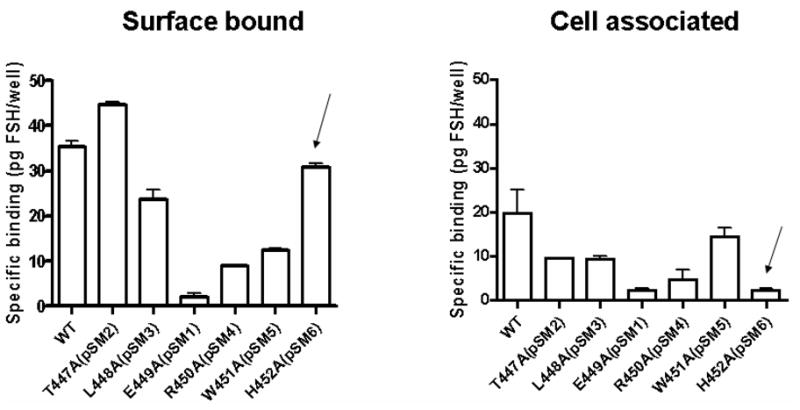
Figure 4A. Binding of 125I-hFSH to HEK293 cells transiently transfected with plasmids encoding single amino acid substitution mutants of hFSHR as previously described (2). Decreased or absent binding was consistent with the level of protein expression (Figure 4B) and indicated that the mutations did not adversely affect conformation of the receptor. Arrows indicate the H452A mutant which has good binding but did not internalize 125I-hFSH.
Figure 4B. Immunoblot of cellular lysates following transfection with single amino acid substitution mutants of hFSHR was done as previously described (2). The region of hFSHR icL2 represented as single mutations is underlined: 441YTLTAITLERWHTITHAMQLDCKVQLRHAAS471. Loading controls were immunostained with actin antibody.
Interestingly, although H452A FSHR mutant bound hFSH at Wt levels, very little FSH appeared to be internalized (Figure 4B). The mutants T447A, L448A and W451A internalized hFSH although at reduced levels. The mutants that internalized hFSH also produced cAMP when stimulated with hFSH. T447A, L448A and W451A, which show considerable levels of internalization, produce high levels of cAMP when exposed to hFSH (Figure 5). As expected, H452A has very low levels of internalization and cAMP production was not detectable.
Figure 5.
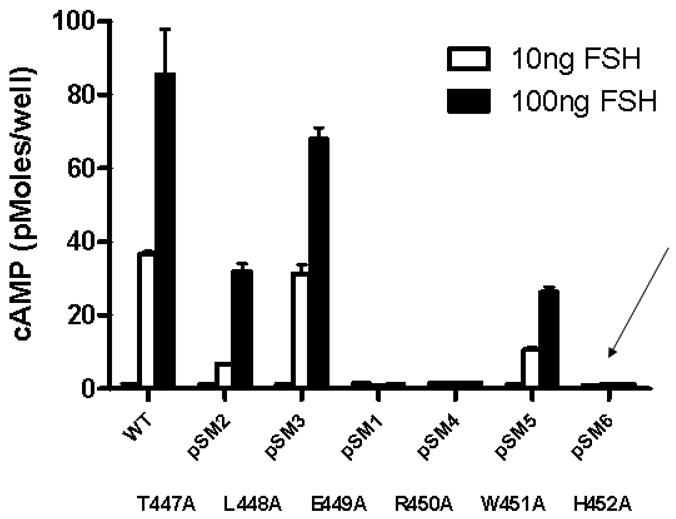
Induction of cAMP production following binding of hFSH to HEK293 cells transiently transfected with plasmids encoding single amino acid substitution mutants of hFSHR as previously described (2). Decreased or absent cAMP production was consistent with the level of protein expression (Figure 4B) and binding of radiolabeled hFSH (Figure 4A) with the exception of the H452A mutation (arrow). This mutant of hFSHR exhibited good binding, but failed to internalize and did not induce cAMP production.
These data show that internalization is linked to signaling. Thus H452A FSHR mutant exhibited little conformational perturbation, as evidence by its ability to bind FSH with high affinity but it was not internalized and could not signal. It is tempting to speculate that signaling is a concerted process requiring endosome formation, and that this is linked to activation of G-protein. It is of course entirely possible that H452A failed to load G-protein, since it has been shown using FRET measurements that heterotrimeric G-proteins precouple with GPCRs (68). In any case, this is the first demonstration that an amino acid contiguous with the canonical ERW motif has been implicated in G-protein signaling. In fact, recent structural studies of G-protein coupled receptors have indicated that the ERW motif may be more relevant to conformational stability of the transmembrane helices, than to direct interaction with G-proteins. In this regard, it is interesting to note that in the model of the FSHR intracellular domains (4), the histidine 452 residue is at the interval between helix III and the random coil of icL2 (Figure 6). Mutations of residues that progress into helix IV were of no consequence to interaction of icL2 with the 14-3-3 bait in the yeast two hybrid assay. Notably, the side chains of histidine and tryptophan (ERWH) project into the same space and are solvent accessible. The juxtaposition of these two residues could play an important role in the process of G-protein loading and activation and/or internalization. The most resolved information available so far on the receptor domains participating in the intermonomer interfaces in GPCR homo-dimers concerns a very limited number of systems, namely rhodopsin (8;69) opsin (65), squid rhodopsin(70), the β-2-adrenergic receptor (67) and the D2-dopamine receptor (71–73). Recently, a disulphide trapping approach was used to study 5HT2c serotonin receptor dimerization (9). Collectively, in vitro evidence seems to highlight different portions even for the same receptor as being involved in intermonomer contacts. The most recurrent interface would involve either H1-H1 (H stands for helix) or H4-H4 or H4-H5 contacts. These arrangements of the dimeric receptor would seem to provide for molecular access to H452 and W451 residues.
Figure 6.
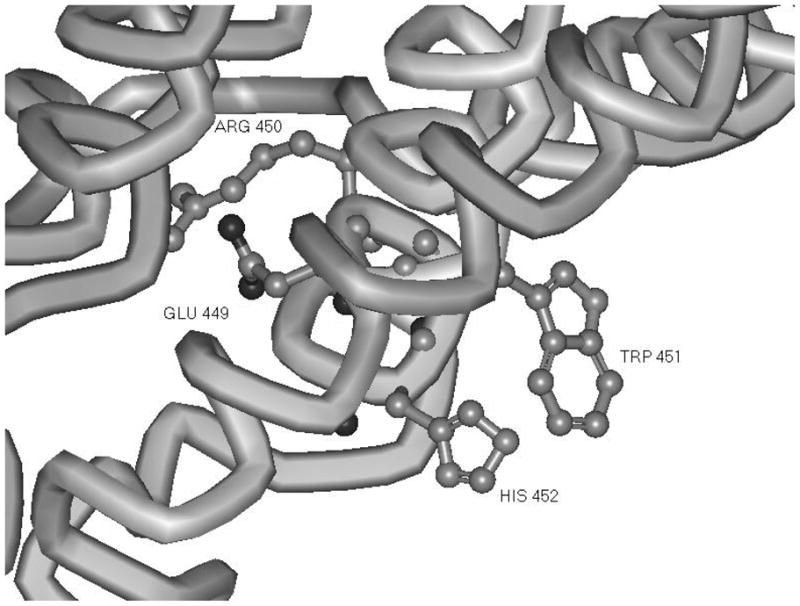
Orientation of a G-protein interaction motif (ERW)H in a model of FSHR cytoplasmic domain structure (4). Substitution of alanine at position His 452 affects neither trafficking to the membrane nor FSHR binding. This mutation results in a block to cAMP production and failure of the hormone receptor to internalize. The Glu 449 and Arg 450 are oriented into the center of the transmembrane domain helices whereas side chains of both His 452 and Trp 451 are oriented towards solvent.
It is difficult to ignore the significance of the ERWH sequence as a canonical G-protein interaction site and how mutations of this sequence abrogate interaction in the yeast two hybrid assay with the 14-3-3 bait. The (E/D)R(W/Y) motif of the majority of GPCRs belonging to the rhodopsin/β-adrenergic receptor subfamily is present as ERW in the glycoprotein hormone receptors and has been implicated in G protein interaction and receptor activation (63). Therefore a future hypothesis to be tested is whether this motif may play a role in FSH mediated signaling and that 14-3-3 interaction with this motif may modulate such signaling.
Recently it has been demonstrated that G proteins do not only localize at the plasma membrane, but rather shuttle between the plasma membrane and intracellular membranes such as the endoplasmic reticulum and the Golgi (74;75). Indeed assembly of signaling complexes prior to insertion at the plasma membrane is a current paradigm(76;77). Since it seems that receptors interact freely with G-proteins (11) it seems reasonable to propose that premature loading of G-protein coupled receptors may be sterically blocked by the association of adapter proteins with the G-protein interaction site. Further research on this topic with regard to the FSH receptor will require imaging of fluorescently labeled FSHR interacting proteins and similarly labeled FSHR.
Supplementary Material
Acknowledgments
Supported by NIH grant HD18407; O.D. supported by an undergraduate summer research fellowship from the Endocrine Society. SDM supported by an Overseas Associateship from the Department of Biotechnology, Government of India. A.U.-A. is recipient of a Career Development Award from the Fundación IMSS, Government of Mexico.
Abbreviations
- FSH
follicle stimulating hormone
- FSHR
FSH receptor
Footnotes
The Authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Dias JA, Cohen BD, Lindau-Shepard B, Nechamen CA, Peterson AJ, Schmidt A. Molecular, Structural, and Cellular Biology of Follitropin and Follitropin Receptor. In: Litwack G, editor. Vitamins and Hormones. 1. New York, NY: Academic Press; 2002. pp. 249–322. [DOI] [PubMed] [Google Scholar]
- 2.Thomas RM, Nechamen CA, Mazurkiewicz JE, Muda M, Palmer S, Dias JA. Follice-stimulating hormone receptor forms oligomers and shows evidence of carboxyl-terminal proteolytic processing. Endocrinology. 2007;148:1987–1995. doi: 10.1210/en.2006-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X, Depasquale JA, Griswold MD, Dias JA. Accessibility of rat and human follitropin receptor primary sequence (R265–S296) in situ. Endocrinology. 1994;135:682–691. doi: 10.1210/endo.135.2.8033817. [DOI] [PubMed] [Google Scholar]
- 4.Uribe A, Zarinan T, Perez-Solis MA, Gutierrez-Sagal R, Jardon-Valadez E, Pineiro A, Dias JA, Ulloa-Aguirre A. Functional and structural roles of conserved cysteine residues in the carboxyl-terminal domain of the follicle-stimulating hormone receptor in human embryonic kidney 293 cells. Biol Reprod. 2008;78:869–882. doi: 10.1095/biolreprod.107.063925. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 6.Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 7.Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, Sunahara RK. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci U S A. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodowski DT, Salom D, Le TI, Teller DC, Ballesteros JA, Palczewski K, Stenkamp RE. Crystal packing analysis of Rhodopsin crystals. J Struct Biol. 2007;158:455–462. doi: 10.1016/j.jsb.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancia F, Assur Z, Herman AG, Siegel R, Hendrickson WA. Ligand sensitivity in dimeric associations of the serotonin 5HT2c receptor. EMBO Rep. 2008;9:363–369. doi: 10.1038/embor.2008.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulloa-Aguirre A, Zariñán T, Pérez-Solís MA, Conn PM. Dominant negative effects of human follicle-stimulating hormone receptor (hFSHR) mutants on wild type receptor expression. (Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azpiazu I, Gautam N. A fluorescence resonance energy transfer-based sensor indicates that receptor access to a G protein is unrestricted in a living mammalian cell. J Biol Chem. 2004;279:27709–27718. doi: 10.1074/jbc.M403712200. [DOI] [PubMed] [Google Scholar]
- 12.Richards JS. New signaling pathways for hormones and cyclic adenosine 3′, 5′-monophosphate action in endocrine cells. [Review] [119 refs] Molecular Endocrinology. 2001;15:209–218. doi: 10.1210/mend.15.2.0606. [DOI] [PubMed] [Google Scholar]
- 13.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 14.Donadeu FX, Ascoli M. The differential effects of the gonadotropin receptors on aromatase expression in primary cultures of immature rat granulosa cells are highly dependent on the density of receptors expressed and the activation of the inositol phosphate cascade. Endocrinology. 2005;146:3907–3916. doi: 10.1210/en.2005-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maizels ET. Follicle stimulating hormone (FSH) activates the p38 mitogen- activated protein kinase pathway, inducing small heat shock protein phosphorylation and cell rounding in immature rat ovarian granulosa cells. Endocrinology. 1998;139:3353–6. doi: 10.1210/endo.139.7.6188. [DOI] [PubMed] [Google Scholar]
- 16.Cottom J, Salvador LM, Maizels ET, Reierstad S, Park Y, Carr DW, Davare MA, Hell JW, Palmer SS, Dent P, Kawakatsu H, Ogata M, Hunzicker-Dunn M. Follicle-stimulating hormone activates extracellular signal-regulated kinase but not extracellular signal-regulated kinase kinase through a 100-kDa phosphotyrosine phosphatase. Journal of Biological Chemistry. 2003;278:7167–7179. doi: 10.1074/jbc.M203901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS. Follicle-Stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid- lnduced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol. 2000;14:1283–1300. doi: 10.1210/mend.14.8.0500. [DOI] [PubMed] [Google Scholar]
- 18.Kara E, Crepieux P, Gauthier C, Martinat N, Piketty V, Guillou F, Reiter E. A phosphorylation cluster of five serine and threonine residues in the C-terminus of the follicle-stimulating hormone receptor is important for desensitization but not for beta-arrestin-mediated ERK activation. Molecular Endocrinology. 2006;20:3014–3026. doi: 10.1210/me.2006-0098. [DOI] [PubMed] [Google Scholar]
- 19.Wayne CM, Fan HY, Cheng XD, Richards JS. Follicle-stimulating hormone induces multiple signaling cascades: Evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Molecular Endocrinology. 2007;21:1940–1957. doi: 10.1210/me.2007-0020. [DOI] [PubMed] [Google Scholar]
- 20.Escamilla-Hernandez R, Little-Ihrig L, Orwig KE, Yue J, Chandran U, Zeleznik AJ. Constitutively active protein kinase A qualitatively mimics the effects of follicle-stimulating hormone on granulosa cell differentiation. Mol Endocrinol. 2008;22:1842–1852. doi: 10.1210/me.2008-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeleznik AJ, Saxena D, Little-Ihrig L. Protein kinase B is obligatory for follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology. 2003;144:3985–3994. doi: 10.1210/en.2003-0293. [DOI] [PubMed] [Google Scholar]
- 22.Touyz RM, Jiang LG, Sairam MR. Follicle-stimulating hormone mediated calcium signaling by the alternatively spliced growth factor type I receptor. Biol Reprod. 2000;62:1067–1074. doi: 10.1095/biolreprod62.4.1067. [DOI] [PubMed] [Google Scholar]
- 23.Jayes FC, Day RN, Garmey JC, Urban RJ, Zhang G, Veldhuis JD. Calcium ions positively modulate follicle-stimulating hormone- and exogenous cyclic 3′, 5′-adenosine monophosphate-driven transcription of the P450(scc) gene in porcine granulosa cells. Endocrinology. 2000;141:2377–2384. doi: 10.1210/endo.141.7.7558. [DOI] [PubMed] [Google Scholar]
- 24.Flores JA, Veldhuis JD, Leong DA. Follicle stimulating hormone evokes an increase in intracellular free calcium ion concentrations in single ovarian (granulosa) cells. 1990;127:3172–3179. doi: 10.1210/endo-127-6-3172. [DOI] [PubMed] [Google Scholar]
- 25.Quirk SM, Cowan RG, Harman RM. Estradiol inhibits apoptosis of granulosa cells induced by Fas ligand. Biol Reprod. 2001;64:291. doi: 10.1095/biolreprod64.2.518. [DOI] [PubMed] [Google Scholar]
- 26.Quirk SM, Cowan RG, Harman RM. The susceptibility of granulosa cells to apoptosis is influenced by oestradiol and the cell cycle. J Endocrinol. 2006;189:441–453. doi: 10.1677/joe.1.06549. [DOI] [PubMed] [Google Scholar]
- 27.Sharma OP, Flores JA, Leong DA, Veldhuis JD. Cellular basis for follicle-stimulating hormone-stimulated calcium signaling in single rat Sertoli cells: possible dissociation from effects of adenosine 3′, 5′-monophosphate. Endocrinology. 1994;134:1915–1923. doi: 10.1210/endo.134.4.8137759. [DOI] [PubMed] [Google Scholar]
- 28.Cohen BD, Bariteau JT, Magenis LM, Dias JA. Regulation of follitropin receptor cell surface residency by the ubiquitin-proteasome pathway. Endocrinology. 2003;144:4393–4402. doi: 10.1210/en.2002-0063. [DOI] [PubMed] [Google Scholar]
- 29.Cohen BD, Nechamen CA, Dias JA. Human follitropin receptor (FSHR) interacts with the adapter protein 14-3-3 tau. Molec Cell Endocrinol. 2004;220:1–7. doi: 10.1016/j.mce.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Nechamen CA, Thomas RM, Cohen BD, Acevedo G, Poulikakos PI, Testa JR, Dias JA. Human follicle-stimulating hormone (FSH) receptor interacts with the adaptor protein APPL1 in HEK 293 cells: Potential involvement of the PI3K pathway in FSH signaling. Biol Reprod. 2004;71:629–636. doi: 10.1095/biolreprod.103.025833. [DOI] [PubMed] [Google Scholar]
- 31.Mitsuuchi Y, Johnson SW, Sonoda G, Tanno S, Golemis EA, Testa JR. Identification of a chromosome 3p14.3-21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene. 1999;18:4891–4898. doi: 10.1038/sj.onc.1203080. [DOI] [PubMed] [Google Scholar]
- 32.Nechamen CA, Dias JA. Human follicle-stimulating hormone (hFSH) receptor N-terminus as an assembled hFSH binding site. Biol Reprod. 1999;60:245. [Google Scholar]
- 33.Cunningham MA, Zhu Q, Unterman TG, Hammond JM. Follicle-stimulating hormone promotes nuclear exclusion of the forkhead transcription factor FoxO1a via phosphatidylinositol 3-kinase in porcine granulosa cells. Endocrinology. 2003;144:5585–5594. doi: 10.1210/en.2003-0678. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham MA, Zhu Q, Hammond JM. FoxO1a can alter cell cycle progression by regulating the nuclear localization of p27(kip) in granulosa cells. Molecular Endocrinology. 2004;18:1756–1767. doi: 10.1210/me.2004-0071. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham MA, Zhu Q, Hammond JM. FoxO1a increases p27(kip) and restricts cell cycle progression by negatively regulating the ubiquitin ligase Skp2. Biol Reprod. 2004:163–164. [Google Scholar]
- 36.Alam H, Weck J, Maizels E, Park Y, Lee EJ, Ashcroft M, Hunzicker-Dunn M. Role of the PI3-Kinase and ERK Pathways in the Induction of HIF-1 Activity and the HIF-1 Target VEGF in Ovarian Granulosa Cells in response to Follicle Stimulating Hormone. Endocrinology. 2008 doi: 10.1210/en.2008-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nechamen CA, Thomas RM, Dias JA. APPL1, APPL2, Akt2 and FOXO1a interact with FSHR in a potential signaling complex. Molec Cell Endocrinol. 2007;260:93–99. doi: 10.1016/j.mce.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di MG, Salvi M, Arrigoni G, Marin O, Sarno S, Brustolon F, Pinna LA, Ruzzene M. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005;12:668–677. doi: 10.1038/sj.cdd.4401604. [DOI] [PubMed] [Google Scholar]
- 39.Dias JA, Nechamen CA, Atari R. Identifying protein interactors in gonadotropin action. Endocrine. 2005;26:241–247. doi: 10.1385/ENDO:26:3:241. [DOI] [PubMed] [Google Scholar]
- 40.Schenck A, Goto-Silva L, Collinet C, Rhinn M, Giner A, Habermann B, Brand M, Zerial M. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 41.Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, De CP. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell. 2009;136:1110–1121. doi: 10.1016/j.cell.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Habermann B. The BAR-domain family of proteins: a case of bending and binding? The membrane bending and GTPase-binding functions of proteins from the BAR-domain family. Embo Reports. 2004;5:250–255. doi: 10.1038/sj.embor.7400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chial HJ, Wu R, Ustach CV, McPhail LC, Mobley WC, Chen YQ. Membrane targeting by APPL1 and APPL2: dynamic scaffolds that oligomerize and bind phosphoinositides. Traffic. 2008;9:215–229. doi: 10.1111/j.1600-0854.2007.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Mao X, Dong LQ, Liu F, Tong L. Crystal structures of the BAR-PH and PTB domains of human APPL1. Structure. 2007;15:525–533. doi: 10.1016/j.str.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Zhu G, Chen J, Liu J, Brunzelle JS, Huang B, Wakeham N, Terzyan S, Li X, Rao Z, Li G, Zhang XC. Structure of the APPL1 BAR-PH domain and characterization of its interaction with Rab5. EMBO J. 2007 doi: 10.1038/sj.emboj.7601771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erdmann KS, Mao Y, McCrea HJ, Zoncu R, Lee S, Paradise S, Modregger J, Biemesderfer D, Toomre D, De CP. A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev Cell. 2007;13:377–390. doi: 10.1016/j.devcel.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCrea HJ, Paradise S, Tomasini L, Addis M, Melis MA, De Matteis MA, De CP. All known patient mutations in the ASH-RhoGAP domains of OCRL affect targeting and APPL1 binding. Biochem Biophys Res Commun. 2008;369:493–499. doi: 10.1016/j.bbrc.2008.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin DC, Quevedo C, Brewer NE, Bell A, Testa JR, Grimes ML, Miller FD, Kaplan DR. APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction. Mol Cell Biol. 2006;26:8928–8941. doi: 10.1128/MCB.00228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirakawa T, Galet C, Kishi M, Ascoli M. GIPC binds to the human lutropin receptor (hLHR) through an unusual PDZ domain binding motif, and it regulates the sorting of the internalized human choriogonadotropin and the density of cell surface hLHR. Journal of Biological Chemistry. 2003;278:49348–49357. doi: 10.1074/jbc.M306557200. [DOI] [PubMed] [Google Scholar]
- 50.Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 51.Deepa SS, Dong LQ. APPL1: role in adiponectin signaling and beyond. Am J Physiol Endocrinol Metab. 2009;296:E22–E36. doi: 10.1152/ajpendo.90731.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chabrolle C, Tosca L, Dupont J. Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotrophin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis. Reproduction. 2007;133:719–731. doi: 10.1530/REP-06-0244. [DOI] [PubMed] [Google Scholar]
- 53.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 54.Ardawi MSM, Rouzi AA. Plasma adiponectin and insulin resistance in women with polycystic ovary syndrome. Fertility and Sterility. 2005;83:1708–1716. doi: 10.1016/j.fertnstert.2004.11.077. [DOI] [PubMed] [Google Scholar]
- 55.Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X, Fang Q, Liu DD, Torres JM, Jia W, Lechleiter JD, Liu F, Dong LQ. Adiponectin Activates AMP-activated Protein Kinase in Muscle Cells via APPL1/LKB1-dependent and Phospholipase C/Ca2+/Ca2+/Calmodulin-dependent Protein Kinase Kinase-dependent Pathways. J Biol Chem. 2009;284:22426–22435. doi: 10.1074/jbc.M109.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kayampilly PP, Menon KM. Follicle-stimulating hormone inhibits adenosine 5′-monophosphate-activated protein kinase activation and promotes cell proliferation of primary granulosa cells in culture through an Akt-dependent pathway. Endocrinology. 2009;150:929–935. doi: 10.1210/en.2008-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Angrand PO, Segura I, Volkel P, Ghidelli S, Terry R, Brajenovic M, Vintersten K, Klein R, Superti-Furga G, Drewes G, Kuster B, Bouwmeester T, cker-Palmer A. Transgenic mouse proteomics identifies new 14-3-3-associated proteins involved in cytoskeletal rearrangements and cell signaling. Mol Cell Proteomics. 2006;5:2211–2227. doi: 10.1074/mcp.M600147-MCP200. [DOI] [PubMed] [Google Scholar]
- 58.Timossi C, Maldonado D, Vizcaino A, Lindau-Shepard B, Conn PM, Ulloa-Aguirre A. Structural determinants in the second intracellular loop of the human follicle-stimulating hormone receptor are involved in G(s) protein activation. Mol Cell Endocrinol. 2002;189:157–168. doi: 10.1016/s0303-7207(01)00720-1. [DOI] [PubMed] [Google Scholar]
- 59.Xiao B, Smerdon SJ, Jones DH, Dodson GG, Soneji Y, Aitken A, Gamblin SJ. Structure of a 14-3-3 protein and implications for coordination of multiple signalling pathways. Nature. 1995;376:188–191. doi: 10.1038/376188a0. [DOI] [PubMed] [Google Scholar]
- 60.Liu D, Bienkowska J, Petosa C, Collier RJ, Fu H, Liddington R. Crystal-Structure of the Zeta-Isoform of the 14-3-3 Protein. Nature. 1995;376:191–194. doi: 10.1038/376191a0. [DOI] [PubMed] [Google Scholar]
- 61.Yang X, Lee WH, Sobott F, Papagrigoriou E, Robinson CV, Grossmann JG, Sundstrom M, Doyle DA, Elkins JM. Structural basis for protein-protein interactions in the 14-3-3 protein family. Proc Natl Acad Sci U S A. 2006;103:17237–17242. doi: 10.1073/pnas.0605779103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Y, Schopperle WM, Murrey H, Jaramillo A, Dagan D, Griffith LC, Levitan IB. A dynamically regulated 14-3-3, Slob, and Slowpoke potassium channel complex in Drosophila presynaptic nerve terminals. Neuron. 1999;22:809–818. doi: 10.1016/s0896-6273(00)80739-4. [DOI] [PubMed] [Google Scholar]
- 63.Gershengorn MC, Osman R. Minireview: Insights into G protein-coupled receptor function using molecular models. [Review] [69 refs] Endocrinology. 2001;142:2–10. doi: 10.1210/endo.142.1.7919. [DOI] [PubMed] [Google Scholar]
- 64.Shikano S, Coblitz B, Wu M, Li M. 14-3-3 proteins: regulation of endoplasmic reticulum localization and surface expression of membrane proteins. Trends Cell Biol. 2006;16:370–375. doi: 10.1016/j.tcb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- 66.Kobilka B, Schertler GF. New G-protein-coupled receptor crystal structures: insights and limitations. Trends Pharmacol Sci. 2008;29:79–83. doi: 10.1016/j.tips.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 67.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nobles M, Benians A, Tinker A. Heterotrimeric G proteins precouple with G protein-coupled receptors in living cells. Proc Natl Acad Sci U S A. 2005;102:18706–18711. doi: 10.1073/pnas.0504778102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang Y, Fotiadis D, Filipek S, Saperstein DA, Palczewski K, Engel A. Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J Biol Chem. 2003;278:21655–21662. doi: 10.1074/jbc.M302536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murakami M, Kouyama T. Crystal structure of squid rhodopsin. Nature. 2008;453:363–367. doi: 10.1038/nature06925. [DOI] [PubMed] [Google Scholar]
- 71.Guo W, Shi L, Javitch JA. The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. Journal of Biological Chemistry. 2003;278:4385–4388. doi: 10.1074/jbc.C200679200. [DOI] [PubMed] [Google Scholar]
- 72.Guo W, Shi L, Filizola M, Weinstein H, Javitch JA. Crosstalk in G protein-coupled receptors: changes at the transmembrane homodimer interface determine activation. Proc Natl Acad Sci U S A. 2005;102:17495–17500. doi: 10.1073/pnas.0508950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo W, Urizar E, Kralikova M, Mobarec JC, Shi L, Filizola M, Javitch JA. Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 2008;27:2293–2304. doi: 10.1038/emboj.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saini DK, Kalyanaraman V, Chisari M, Gautam N. A family of G protein betagamma subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J Biol Chem. 2007;282:24099–24108. doi: 10.1074/jbc.M701191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saini DK, Chisari M, Gautam N. Shuttling and translocation of heterotrimeric G proteins and Ras. Trends Pharmacol Sci. 2009;30:278–286. doi: 10.1016/j.tips.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dupre DJ, Robitaille M, Rebois RV, Hebert TE. The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol. 2009;49:31–56. doi: 10.1146/annurev-pharmtox-061008-103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dupre DJ, Robitaille M, Richer M, Ethier N, Mamarbachi AM, Hebert TE. Dopamine receptor-interacting protein 78 acts as a molecular chaperone for Ggamma subunits before assembly with Gbeta. J Biol Chem. 2007;282:13703–13715. doi: 10.1074/jbc.M608846200. [DOI] [PubMed] [Google Scholar]
- 78.Cohen BD, Sertil O, Abramova NE, Davies KJ, Lowry CV. Induction and repression of DAN1 and the family of anaerobic mannoprotein genes in Saccharomyces cerevisiae occurs through a complex array of regulatory sites. Nucleic Acids Res. 2001;29:799–808. doi: 10.1093/nar/29.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nechamen CA, Dias JA. Point mutations in follitropin receptor result in ER retention. Mol Cell Endocrinol. 2003;201:123–131. doi: 10.1016/s0303-7207(02)00424-0. [DOI] [PubMed] [Google Scholar]
- 80.Nechamen CA, Dias JA. Human follicle stimulating hormone receptor trafficking and hormone binding sites in the amino terminus. Mol Cell Endocrinol. 2000;166:101–110. doi: 10.1016/s0303-7207(00)00281-1. [DOI] [PubMed] [Google Scholar]
- 81.Varsano T, Dong MQ, Niesman I, Gacula H, Lou X, Ma T, Testa JR, Yates JR, Farquhar MG. GIPC is recruited by APPL to peripheral TrkA endosomes and regulates TrkA trafficking and signaling. Mol Cell Biol. 2006;26:8942–8952. doi: 10.1128/MCB.00305-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J, Yao F, Wu R, Morgan M, Thorburn A, Finley RL, Jr, Chen YQ. Mediation of the DCC apoptotic signal by DIP13 alpha. J Biol Chem. 2002;277:26281–26285. doi: 10.1074/jbc.M204679200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.