Abstract
Stomata are microscopic pores formed by pairs of guard cells in the epidermis of terrestrial plants; they are essential for gas exchange with the environment and controlling water loss. Accordingly, plants regulate stomatal aperture in response to environmental conditions, such as relative humidity, CO2 concentration, and light intensity. Stomatal openings are also a major route of pathogen entry into the plant and plants have evolved mechanisms to regulate stomatal aperture as an immune response against bacterial invasion. In this review, we highlight studies that begin to elucidate signaling events involved in bacterium-triggered stomatal closure and discuss how pathogens may have exploited environmental conditions or, in some cases, have evolved virulence factors to actively counter stomatal closure to facilitate invasion.
Introduction
The phyllosphere (i.e., aerial parts of terrestrial plants) provides one of the most important niches for microbial inhabitation [1]. Numerous bacteria, including plant and human pathogens, can survive and even proliferate on the plant surface as epiphytes. To initiate pathogenesis, plant pathogenic bacteria must first enter plant tissues. Unlike fungal pathogens, bacteria lack the ability to directly penetrate the plant epidermis; therefore they rely entirely on natural openings or accidental wounds to enter internal tissues.
The stomate is one such natural opening in the plant epidermis and has long been recognized as a major point of entry for plant pathogenic bacteria [2]. However, until recently stomata have generally been considered to be passive portals of entry for plant pathogenic bacteria. Melotto and colleagues [3] noted that plant stomata close in response to a plant pathogen, Pseudomonas syringae pv. tomato (Pst) DC3000, and a human pathogen, Escherichia coli O157:H7. Interestingly, this response can also be triggered by well-characterized pathogen/microbe-associated molecular patterns (PAMPs or MAMPs; see below), such as flg22 (a peptide derived from bacterial flagellin) and lipopolysaccharide (LPS). This observation suggests that bacterium-triggered stomatal closure is an output of PAMP-triggered immunity [3]. In the past few years, further studies have been published on this topic; we will discuss these in this review.
Role of pattern recognition receptors (PRRs) in stomatal closure
MAMPs are molecules that are generally conserved among pathogenic and non-pathogenic microbes [4]. Well-defined MAMPs include flg22, elf18 (a peptide derived from elongation factor EF-Tu), LPS, peptidoglycan (PGN) and A×21 (activator of XA21-mediated immunity) from bacteria; xylanase, chitin, chitosan (a deacylated derivative of chitin) and ergosterol from fungi; and glucan, pep13, and elicitin from oomycetes [5,6]. Some of these MAMPs were shown to induce stomatal closure in tomato [3], Commelina communis [7], grape [8], Pisum sativum [9], and Arabidopsis [3,10•; W. Zeng and S.Y. He, unpublished]. Interestingly, in addition to triggering stomatal closure, flg22 also prevents stomatal opening in response to light [11•].
MAMPs are recognized by PRRs located in the plant plasma membrane [5]. For example, flg22 is perceived by its cognate PRR FLS2, which is required for flg22 to trigger stomatal closure [3]. Stomata from fls2 mutant plants, however, still respond to purified LPS, illustrating both specificity in MAMP recognition by stomatal guard cells and the capacity of guard cells to recognize multiple MAMPs [3]. Because each pathogen can potentially release multiple MAMPs, it is of interest to determine the relative importance of different PRRs in controlling stomatal closure during actual infection. A recent study addressed this question and found that in the Arabidopsis-Pst DC3000 interaction, FLS2 plays a decisive role [10•]. This finding suggests that not all potential MAMPs from a given pathogen are necessarily presented simultaneously to the stomatal guard cell at all stages of an infection. Alternatively, Pst DC3000 releases multiple MAMPs during infection, but most Pst DC3000-derived MAMPs may not be as potent as flagellin in eliciting stomatal defense in Arabidopsis. This possibility is supported by observations that, at the same concentration (e.g., 1 μM), flg22 is more potent than elf18 to induce the oxidative burst in Arabidopsis leaves [12] and stomatal closure [10•], and elf26 peptides from Agrobacterium tumefaciens and Erwinia amylovora are 50 times more potent to induce medium alkalinization of Arabidopsis cell culture than elf26 from Pst DC3000 [13]. An important conclusion from these studies is that different PAMP/PRR combinations may play different roles in different plant-bacteria interactions.
Signaling cascade involved in pathogen- or MAMP-induced stomatal closure
Studies using purified MAMPs have shown that stomatal closure in response to biotic signals requires the phytohormone abscisic acid (ABA), the guard cell-specific OPEN-STOMATA 1 (OST1) kinase, the production of reactive oxygen species (ROS) and nitric oxide (NO), the heterotrimeric G protein, and the regulation of K+ channels—all of which are hallmarks of abiotic signal-induced stomatal closure [3,11•, 14; Figure 1]. These findings suggest that the guard cell signal transductions in response to biotic and abiotic signals share some common steps. Besides shared signaling components, however, bacterium- and/or MAMP-triggered stomatal closure also requires mitogen-activated protein kinase 3 (MPK3) [15•], the plant defense hormone salicylic acid (SA) [3], and the SA signaling component NPR1 [10•]. Epistasis analysis suggests that PAMP signaling precedes SA signaling, which in turn precedes ABA signaling [10•]. How the three signaling cascades connect to each other at the molecular level remains unclear.
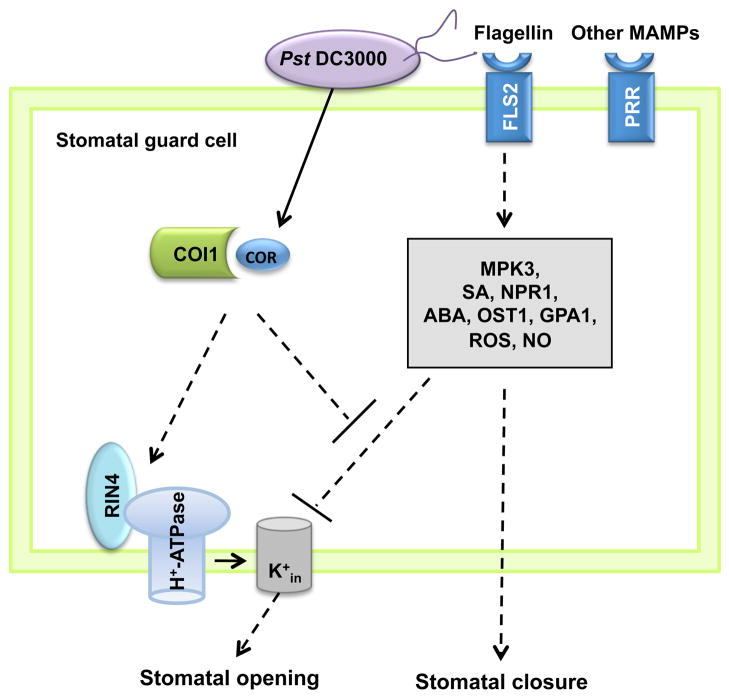
Figure 1.
A model showing some of the signaling components involved in MAMP/bacterium-triggered stomatal closure. Pst DC3000 is illustrated as an example. In guard cells, MAMPs (e.g., flagellin) are perceived by their cognate PRRs (pattern recognition receptors), such as the flagellin receptor FLS2. Perception of MAMPs triggers stomatal closure, which requires phytohormones SA and ABA, as well as some SA and ABA signaling components. MAMP signaling prevents stomatal opening by inhibiting inwardly rectifying K+ channels (K+in) through some ABA signaling components (GPA1 and likely others). COR inhibits MAMP-triggered stomatal closure; this process requires COI1 (a receptor of COR) and RIN4. RIN4 binds and activates the proton pump (H+-ATPase), causing membrane hyperpolarization, activation of K+ influx (K+in), and stomatal opening. Dashed lines indicate uncertain connections.
Recently, an important role of the plasma membrane-localized H+-ATPases (AHA1 and AHA2) in regulating bacterium- and PAMP-triggered stomatal regulation has been reported [16••]. When activated, these ATPases generate a transmembrane electrochemical gradient, which drives the uptake of charged solutes and then water, causing swelling of the guard cells and opening of stomata. Accordingly, the guard cells with constitutive activation of AHA1 in the ost2 mutant plant are insensitive to exogenous application of ABA [17,18]. Liu and colleagues also showed that stomata of ost2 plants do not close in response to bacteria, LPS, or flg22 and ost2 plants are more susceptible to Pst DC3000 bacterial infection when surface-inoculated [16••]. AHA1 and AHA2 were found to interact with RIN4 (Rpm1-interacting protein 4), which is a well-known regulator of multiple plant immune responses [16••]. Overexpression and knockout of RIN4 are correlated with higher and lower H+-ATPase activities, suggesting that RIN4 is a positive regulator of AHA1/2 in promoting stomatal opening [16••; Figure 1].
Pathogen virulence factors involved in counter stomatal closure
Stomatal closure results in reduced pathogen entry into the plant, thereby having a negative impact on pathogenesis. It is not known how different pathogens overcome this host immune response to cause massive infection. As already mentioned, stomata are also regulated by environmental conditions, such as humidity; it is therefore possible that some pathogens may have developed strategies to survive as epiphytes on the plant surface until environmental conditions, such as high humidity, favor stomatal opening. Indeed, many bacterial disease outbreaks occur after heavy rain and/or a prolonged period of high humidity.
In addition to possibly exploiting environmental regulation of stomatal movements, some pathogens apparently have evolved virulence factors to actively counter stomatal closure. For example, coronatine (COR), a phytotoxin produced by Pst DC3000 and several other pathovars of P. syringae, can reverse stomatal closure induced by bacteria or MAMPs [3]. As a result, wild-type COR-producing Pst DC3000 promotes stomatal opening as a virulence strategy [3,16••, 19•]. In contrast, COR-deficient mutants of Pst DC3000 cannot open stomata efficiently and are greatly reduced in their ability to cause infection in Arabidopsis when inoculated on the leaf surface [3,10•]. However, the virulence of COR-deficient mutants can be restored in Arabidopsis mutants that are defective in stomatal closure [3,10•]. How COR promotes stomatal opening remains to be elucidated. Of note, the stomata of rin4 mutant plants cannot be re-opened by virulent Pst DC3000 [16••], implicating a potential role of RIN4 in regulating COR-mediated stomatal response. COR has also been shown to interfere with the inhibition of flg22-induced K+in currents, consistent with its ability to interfere with flg22-inhibition of stomatal opening [3,11•].
Besides COR, the stomatal closure-suppressing activity has also been detected in the bacterial pathogen Xanthomonas campestris pv. campestris (Xcc) [15•]. In this case, a soluble factor of smaller than 2 kDa is responsible for inhibiting stomatal closure, but the molecular identity of this factor remains unknown. Intriguingly, the supernatants of Xcc cultures containing this factor can restore the ability of the COR-deficient PstDC3118 to infect Arabidopsis via surface-inoculation, suggesting that COR and this factor have analogous functions [15•]. Production of the Xcc anti-stomate factor is under the control of the diffusible signal factor (DSF), which is involved in bacterial cell-to-cell signaling [15•].
In addition to bacterial factors, stomatal regulation by fungal factors has also been observed. Fusicoccin is a toxin produced by Fusicoccum amygdali, the fungal pathogen of almond and peach canker [20]. When applied to leaves, fusicoccin promotes stomate opening in tobacco, sorghum, cucumber, lucerne (Medicago sativa), and pokeweed (Phytolacca Americana) [20]. Fusicoccin promotes stomatal opening under light or dark conditions, and inhibit dark-induced stomatal closure in Commelina communis [21]. Fusicoccin has been shown to activate a plasma membrane H+-ATPase in guard cells [21,22]. Very recently, fusicoccin was also shown to inhibit dark-induced stomatal closure in broad bean, probably by removing NO, similar to the action of c-PTIO, a NO scavenger, and L-NAME, an inhibitor of nitric oxide synthase (NOS) [23]. In addition, actin depolymerization seems to be involved in fusicoccin-induced stomate opening in Commelina communis [24].
Oxalate is another fungal virulence factor implicated in stomatal regulation. This virulence factor is produced by the fungal pathogen Sclerotinia sclerotiorum [25,26]. In Vicia faba leaves, oxalate is shown to induce stomatal opening, and S. sclerotiorum seems to exploit open stomata for exiting the plant tissue at late stages of infection [26]. This oxalate-induced stomatal opening is likely due to an increase in osmotically active solutes [26]. In addition, oxalate could suppress the defense-related oxidative burst in soybean and tobacco cells [27].
Role of stomate closure in other branches of plant immunity
Recent studies have shown a molecular “arms race” between the plant immune system and pathogen virulence factors. As mentioned above, plants mount PAMP-triggered immunity by recognizing MAMPs/PAMPs. However, PAMP-triggered immunity is often suppressed by virulence effectors produced by pathogens. Plants in turn evolve disease resistance proteins to recognize some of these virulence effectors and activate another branch of immune response known as effector-triggered immunity [28,29]. Stomatal closure appears to be part of effector-triggered immunity as well. For example, effector-triggered immunity following recognition of the effector AvrRpt2 by the cognate disease resistance protein RPS2 promotes stomatal closure in Arabidopsis [3,19•].
Stomatal regulation in plant interaction with human pathogens
In addition to phytopathogenic bacteria, human pathogens are also capable of occupying the phyllosphere, an aspect of the biology of plant-microbe interactions that has major implications for the safety of fresh fruits and vegetables. It is estimated that 76 million cases of food-borne diseases occur yearly in the US (CDC, www.cdc.gov) and there were over 35 major outbreaks in the past decade [30]. The number of serious cases leading to death has been increasing and outbreaks associated with fresh produce have emerged as an important public health concern. In particular, enterohemorrhagic Escherichia coli and Salmonella enterica appear to be some of the most common causal agents of food poisoning associated with the consumption of fresh leafy vegetables [30].
Escherichia coli O157:H7 apparently cannot overcome stomatal closure in Arabidopsis leaves, resulting in constitutive activation of the stomatal immune response [3]. However, a recent study documented a remarkable ability of Salmonella enterica serovar Typhimurium (STM) to migrate toward stomata and enter plant tissues without triggering stomatal immune response [31•]. After incubating lettuce leaves with GFP-labeled STM bacteria, many bacteria were found to attach to lettuce leaves near and within stomata, and in the leaf apoplast space, especially underneath stomata [31•]. However, when tested in the dark, under which condition stomata are closed, bacteria were detected only on the leaf surface [31•]. Interestingly, STM does not trigger stomatal closure, in contrast to Pst DC3000, which induced stomatal closure in lettuce leaves, as in Arabidopsis [31•]. It is not known whether the inability of STM to trigger stomatal closure is because PAMPs produced by STM are not recognizable by stomatal guard cells or because, like some plant pathogens, STM produces a factor that actively suppresses PAMP-induced stomatal closure. Either mechanism would be interesting.
Conclusions
Invasion of plants by microbial pathogens is a critical step in causing plant disease and human pathogen contamination, yet our knowledge in this area remains incomplete. As discussed in this review, stomata represent a major route of pathogen invasion and recent studies have begun to shed light on the signal transduction cascades underlying bacterial regulation of stomatal closure and opening. Current results suggest that stomatal closure is a functional output of both PAMP-triggered and effector-triggered immunity. Because stomata respond to both abiotic and biotic signals, pathogens may exploit abiotic environmental conditions, such as high humidity, and/or produce virulence factors to actively suppress stomatal closure as part of their infection strategy. In the future, it would be important to learn about the detailed molecular and biochemical mechanisms by which PAMP, SA, and ABA signaling cascades intersect each other at the molecular level and to elucidate how different pathogen virulence factors inhibit stomatal closure. Knowledge derived from such studies could lead to novel methods of managing plant disease and human pathogen contamination of plant tissues.
Acknowledgments
We thank Karen Bird for editing and members of the S. Y. H. lab for their comments on the manuscript. We also thank National Institutes of Health (5R01AI068718) and the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy (DE-FG02-91ER20021) for funding support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Lindow SE, Brandl MT. Microbiology of the phyllosphere. Appl environ Microbiol. 2003;69:1875–1883. doi: 10.1128/AEM.69.4.1875-1883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang J. Ultrastructure of bacterial penetration in plants. Annu Rev Phytopathol. 1986;24:141–157. [Google Scholar]
- 3.Melotto M, Underwood W, Koczan J, Nomura K, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–980. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 4.Mackey D, McFall AJ. MAMPs and MIMPs: proposed classifications for inducers of innate immunity. Mol Microbiol. 2006;61:1365–1371. doi: 10.1111/j.1365-2958.2006.05311.x. [DOI] [PubMed] [Google Scholar]
- 5.Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 6.Lee SW, Han SW, Sririvanum M, Park CJ, Seo YS, Ronald PC. A type I-secreted, sulfated peptide triggers XA21-mediated innate immunity. Science. 2009;326:850–853. doi: 10.1126/science.1173438. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Choi H, Suh S, Doo I-S, Oh K-Y, Choi EJ, Taylor ATS, Low PS, Lee Y. Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelinacommunis. Plant Physiol. 1999;121:147–152. doi: 10.1104/pp.121.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allègre M, Héloir M-C, Trouvelot S, Daire X, Pugin A, Wendehenne D, Adrian M. Are grapevine stomata involved in the elicitor-induced protection against downy mildew? Mol Plant-Microbe Interact. 2009;22:977–986. doi: 10.1094/MPMI-22-8-0977. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava N, Gonugunta VK, Puli MR, Raghavendra AS. Nitric oxide production occurs downstream of reactive oxygen species in guard cells during stomatal closure induced by chitosan in abaxial epidermis of Pisum sativum. Planta. 2009;229:757–765. doi: 10.1007/s00425-008-0855-5. [DOI] [PubMed] [Google Scholar]
- 10•.Zeng W, He SY. A prominent role of the flagellin receptor FLS2 in mediating stomatal response to Pseudomonas syringae pv. tomato DC3000 in Arabidopsis. Plant Physiol. 2010 doi: 10.1104/pp.110.157016. The study shows that flagellin perception by the FLS2 receptor is essential for stomatal response in the Arabidopsis-Pst DC3000 pathosystem. Epistasis analyses suggest that PAMP signaling in the guard cell precedes SA signaling, which precedes ABA signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Zhang W, He SY, Assmann SM. The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J. 2008;56:984–996. doi: 10.1111/j.1365-313X.2008.03657.x. This paper reports PAMP inhibition of light-induced stomatal opening and inward K+ channels of guard cells that mediate K+ uptake during stomatal opening. Coronatine was shown to reverse the inhibitory effects of flg22 on inward K+ currents and stomatal opening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G. Perception of the bacterial PAMPEF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell. 2004;16:3496–3507. doi: 10.1105/tpc.104.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I. Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- 15•.Gudesblat GE, Torres PS, Vojnov AA. Xanthomonas campestris overcomes Arabidopsis innate immunity through a DSF Cell-to-cell signal-regulated virulence factor. Plant Physiol. 2009;149:1017–1027. doi: 10.1104/pp.108.126870. This paper reports that Xcc can modulate stomatal movement. A soluble factor of < 2 kDa was found in Xcc cultures. This factor can suppress bacterium-induced stomatal closure and restores the ability of COR-deficient Pst DC3118 bacteria to infect Arabidopsis. The authors also show an important role of MPK3 in modulating bacterium-induced stomatal closure in Arabidopsis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Liu J, Elmore JM, Fuglsang AT, Palmgren MG, Staskawicz BJ, Coaker G. RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack. PLoS Biol. 2009;7:e1000139. doi: 10.1371/journal.pbio.1000139. The authors show that RIN4, a negative regulator of plant defense against bacteria, interacts with plasma membrane-localized H+-ATPases (AHA1 and AHA2) and positively regulates their activity to promote stomatal opening. Accordingly, rin4 mutant plants have reduced AHA1 activity and are impaired in Pst DC3000-induced stomatal opening. Conversely, stomata in plants expressing constitutively active AHA1 do not close in response to Pst DC3000, LPS, or flg22, and are hypersusceptible to Pst DC3000 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merlot S, Mustilli A-C, Genty B, North H, Lefebvre V, Sotta B, Vavasseur A, Giraudat J. Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 2002;30:601–609. doi: 10.1046/j.1365-313x.2002.01322.x. [DOI] [PubMed] [Google Scholar]
- 18.Merlot S, Leonhardt N, Fenzi F, Valon C, Costa M, Piette L, Vavasseur A, Genty B, Boivin K, Müller A, Giraudat J, Leung J. Constitutive activation of a plasma membrane H+-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J. 2007;26:3216–3226. doi: 10.1038/sj.emboj.7601750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Freeman BC, Beattie GA. Bacterial growth restriction during host resistance to Pseudomonas syringae is associated with leaf water loss and localized cessation of vascular activity in Arabidopsis thaliana. Mol Plant-Microbe Interact. 2009;22:857–867. doi: 10.1094/MPMI-22-7-0857. The real-time recording results of stomatal conductance reported in this paper significantly extend the observations on MAMP/bacterium-triggered stomatal closure observed in previous studies. [DOI] [PubMed] [Google Scholar]
- 20.Turner NC, Graniti A. Fusicoccin: a fungal toxin that opens stomata. Nature. 1969;223:1070–1071. [Google Scholar]
- 21.Assmann SM, Schwartz A. Synergistic effect of light and fusicoccin on stomatal opening. Plant Physiol. 1992;98:1349–1355. doi: 10.1104/pp.98.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao R, Dielen V, Kinet J-M, Boutry M. Cosuppression of a plasma membrane H+-ATPaseisoform impairs sucrose translocation, stomatal opening, plant growth, and male fertility. Plant Cell. 2000;12:535–546. doi: 10.1105/tpc.12.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.She X-P, Li J, Huang A-X, Han X-Z. Fusicoccin inhibits dark-induced stomatal closure reducing nitric oxide in the guard cells of broad bean. Aust J Bot. 2010;58:81–88. [Google Scholar]
- 24.Eun S-O, Lee Y. Stomatal opening by fusicoccin is accompanied by depolymerization of actin filaments in guard cells. Planta. 2000;210:1014–1017. doi: 10.1007/s004250050711. [DOI] [PubMed] [Google Scholar]
- 25.Godoy G, Steadman JR, Dickman MB, Dam R. Use of mutants to demonstrate the role of oxalic acid in pathogenicity of Sclerotinia sclerotiorum on Phaseolus vulgaris. Physiol Mol Plant Pathol. 1990;37:179–191. [Google Scholar]
- 26.Guimarães RL, Stotz HU. Oxalate production by Sclerotinia sclerotiorum deregulates guard cells during infection. Plant Physiol. 2004;136:3703–3711. doi: 10.1104/pp.104.049650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cessna SG, Sears VE, Dickman MB, Low PS. Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell. 2000;12:2191–2199. doi: 10.1105/tpc.12.11.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 29.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Doyle MP, Erickson MC. Summer meeting 2007 – the problems with fresh produce: an overview. J Appl Microbiol. 2008;105:317–330. doi: 10.1111/j.1365-2672.2008.03746.x. [DOI] [PubMed] [Google Scholar]
- 31•.Kroupitski Y, Golberg D, Belusov E, Pinto R, Swartzberg D, Granot D, Sela S. Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Appl Environ Microb. 2009;75:6076–6086. doi: 10.1128/AEM.01084-09. Using GFP-labeled Salmonella enterica bacteria and 3-D reconstruction of confocal microscopic pictures, the authors show that Salmonella enter lettuce leaves without triggering stomatal closure. Interestingly, Salmonella enterica enter through open stomata only under light and this process requires bacterial motility and chemotaxis. [DOI] [PMC free article] [PubMed] [Google Scholar]