Abstract
Chondrocyte apoptosis is thought to be an important step in the calcification of cartilage in vivo; however there are conflicting reports as to whether or not this apoptosis is a necessary precursor to mineralization. The goal of this study was to determine whether or not apoptosis is necessary for mineralization in an in vitro murine micromass model of endochondral ossification. C3H10T1/2 murine mesenchymal stem cells were plated in micromass culture in the presence of 4mM inorganic phosphate with the addition of the apoptogens, camptothecin or staurosporine, to induce apoptosis. The rate and total accumulation of mineralization was measured with 45Ca uptake. In these studies, both apoptogens increased the rate of mineralization, with staurosporine increasing 45Ca accumulation by about 2.5 times that of controls and camptothecin increasing total amounts of mineralization about 1.5 times that of controls. Inhibiting cell apoptosis with the caspase inhibitor, ZVAD-fmk, to prevent apoptosis, caused slower rates of 45Ca uptake, however total amounts of 45Ca accumulation reached the same values by Day 30 of culture. FTIR data showed mineralization in all samples treated with 4mM inorganic phosphate, with the highest mineral to matrix ratios in the camptothecin treated samples.
Keywords: apoptosis, mineralization, micromass cultures
Introduction
Endochondral ossification occurs through a series of events that are not yet completely understood. There is evidence that terminally differentiated, hypertrophic chondrocyte apoptosis is one step in the process that leads to cartilage calcification and growth of long bones (Gibson et al., 1995, Roach, 1997, Roach et al., 1995, Adams and Shapiro, 2002, Gibson, 1998). There are, however, conflicting reports as to whether or not apoptosis is an essential step in the mineralization process (Felisbino and Carvalho, 2001, Magne et al., 2003, Mansfield et al., 2003, Kirsch et al., 2003, Gibson, 1998).
We previously reported that in primary chick limb bud micromass cultures, apoptosis is not necessary for mineralization to occur, and that the induction of apoptosis did not increase mineralization (Pourmand et al., 2007). These studies contrast with data from other groups using the murine cell line, ATDC5, which undergoes chondrogenic differentiation with the addition of insulin. Supplementation with exogenous inorganic phosphate triggers apoptotic cell death leading to mineralization in these cultures (Magne et al., 2003). These studies suggest that apoptosis mediated mineralization may be a species dependent phenomena, and that while there are similarities between avian and mammalian endochondral ossification, they may not be identical processes. These data also imply that the signals that the chondrocytes require to go through the process from differentiation to maturation and hypertrophy may be dependent on cell source.
Micromass spot cultures, or high density cultures, have previously been used as a model of endochondral ossification (Boskey et al., 1992a, Boskey et al., 1996a, Boskey et al., 1992b, Pourmand et al., 2007, Mello and Tuan, 1999, Mello and Tuan, 2006). The high density of cells and close cell-to-cell contacts create an environment that promotes and supports chondrocyte differentiation (DeLise et al., 2000, Haas and Tuan, 1999, Denker et al., 1999). These micromass cultures produce a mineralized matrix with the addition of exogenous inorganic phosphate, and provide a controlled environment in which the effects of apoptosis on calcification can be quantified (Boskey et al., 1992a, Boskey et al., 2008, Boskey et al., 1996a, Boskey et al., 1996b, Boskey et al., 2002, Boskey et al., 1997, Boskey et al., 1991, Boskey et al., 1992b).
The induction of apoptosis is an event that can be triggered by both internal and external cellular pathways (Mirkes, 2002). Activation of intracellular signaling involving caspase cascades has been implicated as the primary pathway of apoptosis (Yi and Yuan, 2009, Chowdhury et al., 2008, Cohen, 1997). Both staurosporine and camptothecin have been used as effective external apoptosis inducers (apoptogens) via activation of one or more of these caspase pathways. To inhibit apoptosis, Z-Val-Ala-Asp(O-Me)-fluoromethylketone (ZVAD-fmk), is an effective agent that prevents staurosporine and camptothecin induced apoptosis in chondrocytes and other cell types (Lee et al., 2000, Ceccatelli et al., 2004, Yue et al., 1998).
The objective of this study was to compare these apoptotic pathways with the in vitro micromass model of endochondral ossification and to clarify the role of apoptosis in mammalian vs. avian mineralization. It was hypothesized that apoptosis is a significant event in mineralization of the micromass cultures of the murine cell line C3H10T1/2, and that the induction of apoptosis will lead to increased mineralization in contrast to the previously reported data from avian cultures. Moreover, this study aimed to determine whether or there are species specific or reagent specific differences in the avian and murine systems.
Materials and Methods
Chick Cultures
Chick limb-buds were obtained at stage 21–24 (Hamburg and Hamilton, 1951) from white Leg Horn chick embryos. Mesenchymal cells released by digestion in trypsin-EDTA (GIBCO) were separated from debris by passage through a 20 μm Nitex membrane, counted with a hemocytometer, checked for viability by trypan blue dye exclusion, and pelleted in the cold at 1100 rpm. Cells, resuspended in DMEM with 100 units/mL of penicillin, 100μg/mL of streptomycin base, and 0.25mg/mL amphotericin B, (1% antibiotics/antimycotics) were plated using the micro-mass technique (Ahrens et al., 1977) at a density of 0.75×106 cells per 20 μl drop in 35 × 10 mm Falcon dishes, allowed to attach for 1 hr in a humidified atmosphere of 5% CO2 at 37C, and then flooded with DMEM (GIBCO 80-0303A; containing 1 g/l glucose and supplemented with 50 units/ml penicillin and 25 μg/ml streptomycin, and 10% fetal bovine serum (Atlanta Biologics). From day 2 onward, vitamin C (25 μg/ml) was added along with glutamine (0.3 mg/ml) and for all mineralizing cultures the total phosphate concentration (Pi) was adjusted to 4.0 mM with potassium acid phosphate. Control non-mineralizing cultures received no phosphate addition, and were ~1.0 mM in Pi. Cultures were incubated at 37° with 95% air, 5% CO2 with media changed three times per week. Cultures were treated with 11.5μM camptothecin (Camp) in DMSO beginning on day 7 and continuing through the entire culture period to induce apoptosis in some dishes as previously described. Control dishes were treated with DMSO only.
Murine Cells
C3H10T1/2 cells (ATCC, CCL-226) were expanded in DMEM (Invitrogen), 10% fetal bovine serum (FBS) (Invitrogen), and 1% antibiotics/antimycotics. Cells were passaged 2 times per week (0.05% trypsin, Invitrogen) at about 85% confluency. Passage 7–12 cells were used for experiments.
For micromass cultures cells were plated at a density of 100,000 cells per 10 μl spot in the center of 35mm tissue culture dishes and allowed to attach for two hours prior to flooding with media as detailed elsewhere (Roy et al.). Cultures were maintained in DMEM with 1% FBS, 6.25 μg/mL of recombinant insulin, 6.25 μg/mL human transferrin, and 6.25ng/mL selenious acid and 1% antibiotics/antimycotics in an incubator at 37°C with 5% CO2. From day 2 onward micromass cultures received 1.3mM CaCl2, 25ug/mL of ascorbic acid, and 1% L-glutamine. Also beginning on day 2 of culture, 4mM inorganic phosphate (4P) was added to media for mineralizing cultures while control cultures remained with 1mM inorganic phosphate (1P). On day 7 of micromass cultures, 40nM staurosporine (Stauro) or 11.5μM camptothecin (Camp) in DMSO was added to the media and for every media change thereafter to induce apoptosis in some dishes as previously described (Pourmand et al., 2007). Control dishes were treated with the carrier, DMSO. In experiments to inhibit apoptosis, the pan-caspase inhibitor, ZVAD-fmk, was given to cultures also from day 7 onward.
Flow Cytometry
Flow cytometry with FITC labeled annexin V and 7-AAD (BD Biosciences) was used to confirm apoptosis and cell death as previously described (Pourmand et al., 2007). In summary, the media from six samples were removed and combined with the 1mL of trypsin (0.25%, Invitrogen) that was added to cultures and incubated for 5 minutes at 37°C. The media and trypsin-cell mixture was centrifuged at 1100rpm for 8 minutes and the supernatant was discarded. The cell pellet was then mixed with 200μl of binding buffer (BD Biosciences). Cells were stained with annexinV-FITC to determine apoptosis and 7AAD to determine death for 15 minutes at room temperature and then placed on ice before flow cytometry. Samples were analyzed with FloJo software and were gated for cell death or apoptosis based on unstained and single stain controls.
Calcium Uptake
45Calcium was added to the media also starting on day seven and samples analyzed as previously described for mineral accretion (Pourmand et al., 2007). 45Calcium scintillation was normalized to Day 28 mineralizing DMSO cultures (4P) after subtracting control (1P) DMSO values. For each group there was a sample number of 5–7 from three independent experiments.
Fourier Transform Infrared Spectroscopy
For FTIR studies cultures were thoroughly washed with 100% ethanol and allowed to air dry overnight. Freshly dried (120°C, 24 hours) KBr (200mg) was mixed with the air dried samples in the tissue culture plates and pellets were made for spectroscopic analysis. For FTIR studies 4–6 samples were pooled for each spectrum and values were averaged from independent studies. Spectra were first baselined and the mineral to matrix ratio was determined as the area under the phosphate peak (900–1200cm-1) divided by the area under the amide I peak (1585–1720cm-1). Collagen maturity was defined as the ratio of reducible to non reducible crosslinks (1660/1690) (Paschalis et al., 2003) and hydroxyapatite crystallinity (Pleshko et al., 1991) was determined from the ratio of the peak intensity at 1030/1020.
Results
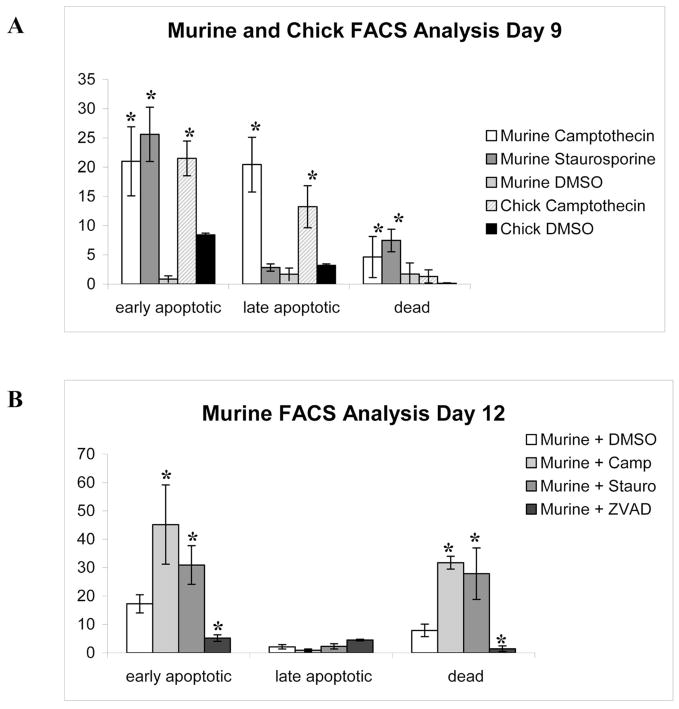
Flow cytometry was used to confirm the effectiveness of the apoptogens at selected time pointes. With camptothecin, in the C3H10T1/2 cultures, there was significantly more apoptosis (~21%) than with apoptogen-free (DMSO-treated) control cultures (~1%) at day 9 (two days after treatment with camptothecin). Similarly, in the chick cultures treated with camptothecin, there were significantly higher amounts of apoptosis (~21%) at day 9 than controls treated with DMSO (~8%) (Figure 1A). In chick cultures, camptothecin induced apoptosis earlier than in murine cultures (Figure 1A), and there were large amounts of late apoptotic cells (~13%). On day 9 staurosporine treated murine cultures had the highest percentage of early apoptotic cells (~25%) (Figure 1A). This is similar to rates of apoptosis previously reported for similar studies in chick micromass cultures (Pourmand et al., 2007). By day 12, in the murine cells, there were higher amounts of apoptosis in all cultures, including controls (Figure 1B). The murine cultures treated with camptothecin and staurosporine both had a large percentage of apoptotic cells (~30 and ~45% respectively) (Figure 1B). Control cultures at day 12 also had a percentage of cells undergoing apoptosis (~17%). By day 12 there were also higher numbers of dead murine cells in all conditions. ZAVD-fmk treated cultures showed significantly fewer cells undergoing apoptosis and cell death than all other conditions including control cultures (~5%) (Figure 1B).
Figure 1.
Figure 1A. FACS Analysis of Murine and Chick Cultures at Day 9. Both camptothecin and staurosporine induced apoptosis in murine cultures. There were more late apoptotic (double-labeled) cells with camptothecin treatment than staurosporine treatment. Chick cultures treated with camptothecin showed a similar profile for apoptosis. * p<0.05 vs. control cultures
Figure 1B. FACS Analysis of Murine Cultures at Day 12. At day 12 (5 days after start of apoptogen treatment) only murine cultures were analyzed. There were more cells undergoing apoptosis with staurosporine treatment and camptothecin treatment compared to controls. There were also more cells undergoing apoptosis in control cultures on day 12 than day 9, likely due to the start of mineralization. ZVAD-fmk treated cultures showed significantly less apoptosis than control cultures. There were significantly more dead cells with apoptogen treatment than control cells, and ZVAD treatment of the murine cultures resulted in a lower amount of dead cells than all other conditions, including control cultures. There were no differences in the amounts of late apoptotic cells by day 12. * p<0.05 vs. control cultures
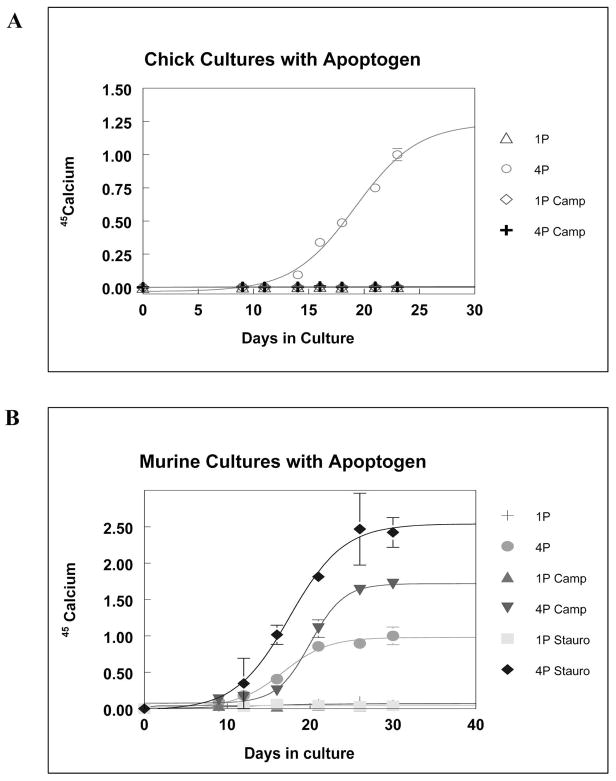
Based on earlier studies apoptosis in chick micromass cultures (Pourmand et al., 2007), spots were treated with an apoptogen beginning at day 7 and throughout the rest of the culture period. When the apoptogen, camptothecin, was given to 4P chick cultures at day 7, cultures failed to mineralize and 45Ca values remained at levels similar to the 1P controls. Chick cultures treated with camptothecin and 4P after the start of mineralization, at day 14, mineralized normally, with no differences compared to the 4P control (DMSO treated) cultures (Figure 2A).
Figure 2.
Figure 2A. 45Calcium Uptake in Chick Cultures Treated with Apoptogen. The treatment of chick cultures with camptothecin completely inhibited mineralization, and 45Ca uptake values were similar to non-mineralizing control (1P) cultures.
Figure 2B. 45Calcium Uptake in Murine Cultures Treated with Apoptogen. Both staurosporine and camptothecin caused an increase in the rate and extent of 45Ca uptake in murine cultures. Treatment with staurosporine resulted in the highest amount of mineral accretion, with values about 2.5 times that of control mineralizing (4P) cultures.
All mineralizing (4P) murine cultures showed increased 45Ca uptake, while 1mM cultures did not show significant mineralization. Both staurosporine and camptothecin treated mineralizing cultures showed higher amounts of 45Ca uptake compared to control mineralizing (4P DMSO) cultures, with staurosporine treated cultures reaching a plateau 2.5 times that of the controls (Figure 2B).
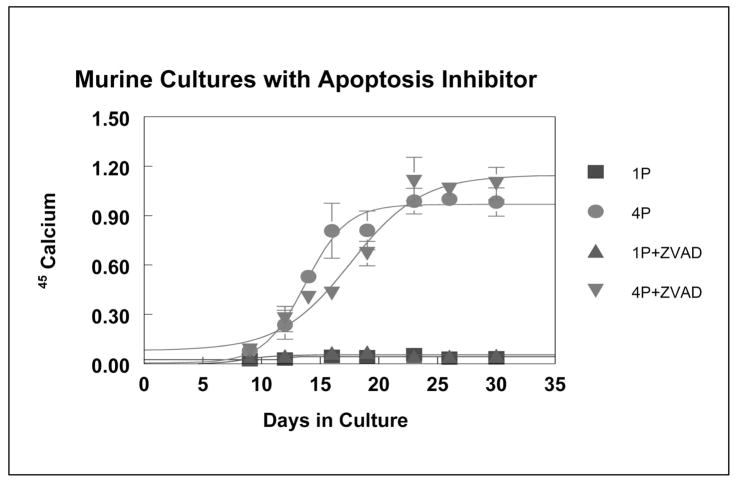
ZVAD-fmk is a pan-caspase inhibitor shown to block the pathways that lead to chondrocyte cell death by apoptosis (Garcia-Calvo et al., 1998). Mineralizing murine micromass cultures treated with ZVAD-fmk, had slower rates of 45Ca uptake compared to control. The total amount of mineralization, however, reached control levels (4P+DMSO) by day 23 and the plateau levels of 45Ca uptake were not significantly different (Figure 3).
Figure 3.
45Calcium Uptake in Murine Cultures Treated with an Apoptosis Inhibitor. Treatment of murine cultures with ZVAD-fmk resulted in a slower rate of mineralization than control mineralizing (4P) cultures, but the extent of mineralization was not significantly different than controls (4P).
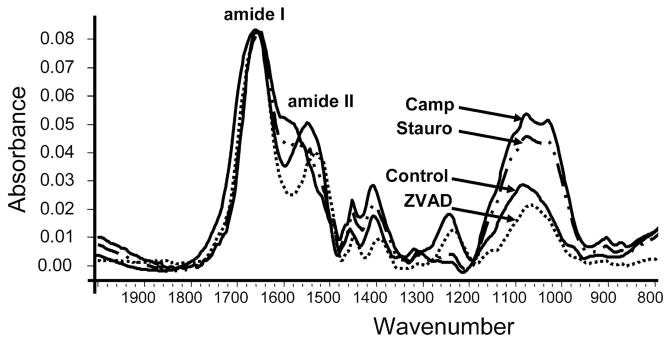
FTIR spectroscopy was performed on murine day 28 samples as apoptogen treated samples often detached, making analysis at later time points difficult. The FTIR spectroscopy shows some mineralization at day 28 in all groups given 4P (Figure 4). In the staurosporine and camptothecin treated samples, there were higher mineral to matrix ratios compared to control 4P cultures (camptothecin 1.91 ± .01, staurosporine 1.40 ± 0.16, control DMSO 0.51 ± 0.11, ZVAD-fmk 0.49 ± .03), supporting the 45Ca uptake data, and suggesting that increases in apoptosis parallel the extent of mineralization in the murine cultures. There were, however, changes in the amide II peak with the addition of both apoptogens, but not with the apoptosis inhibitor, possibly indicating a deficiency or defect in the matrix in these cultures.
Figure 4.
FTIR Spectroscopy of Murine Cultures. The FTIR spectroscopy showed more area under the mineral peaks in cultures treated with an apoptogen than control and ZVAD-fmk treated cultures. The shape of the amide ii peak in cultures treated with both apoptogens were different than control and ZVAD-fmk treated cultures, indicating differences in the collagen produced.
Discussion
The goal of this study was to determine whether or not apoptosis is a prerequisite to chondrocyte calcification in a mammalian system, or whether differences previously reported were due to the specific apoptogens used. In similar studies with primary chick limb-bud micromass cultures, apoptosis was not required for mineralization (Pourmand et al., 2007). In the chick, blocking apoptosis had no significant effect on measured mineralization parameters and inducing apoptosis with two different agents camptothecin, in the present study, and staurosporine, in a previous study, caused decreases in micromass matrix mineralization (Pourmand et al., 2007). This is contrary to some reports of what is hypothesized to occur in vertebrates in vivo (Bronckers et al., 1996, Zenmyo et al., 1996, Aizawa et al., 1997, Amling et al., 1997) and even studies of avian bone growth (Hatori et al., 1995, Ohyama et al., 1997, Gibson, 1998, Gibson et al., 1995). The present study employed a murine cell line to mimic endochondral ossification, in vitro, and to determine whether there are species specific roles of apoptosis in cartilage mineralization.
We found increases in mineralization of murine micromass cultures when treated with two different apoptogens. Based on previous studies and preliminary data, these agents were given prior to the start of mineralization (Pourmand et al., 2007), and were shown by fluorescence activated cell sorting (FACS) analysis to increase apoptosis. While staurosporine treated cultures showed the highest total amounts of calcium uptake, FTIR showed the highest mineral to matrix ratios for camptothecin treated cultures. This data along with the FACS analysis suggests that because the camptothecin treated cultures underwent apoptosis earlier they possibly may have synthesized less extracellular matrix. The staurosporine treated cultures may have had more time to deposit the extracellular matrix, resulting in the lower mineral to matrix values observed by FTIR. These data also confirm that the collagen or interactions of matrix proteins made by the cells with collagen is crucial to proper mineralization and crystallinity (Xu et al., 2008, Roschger et al., 2008, Kuznetsova et al., 2004).
The changes in the amide II region in the FTIR spectra of apoptogen-treated cultures may indicate changes in the matrix produced by apoptotic cells. It is possible that the early treatment with apoptogens does not give the cells time to make the correct post-translational modifications to the collagen. When given an apoptogen, there also may be little time for the chondrocytes to mature and hypertrophy causing deficiencies in matrix formation before cell death.
The differences in the increase in the total extent of mineralization, seen in the murine cultures, between the two apoptogens as measured by 45Ca could be due to the different caspase cascades involved. Staurosporine works through caspases 3 and 9 (Shiraishi et al., 2001, Yue et al., 1998, Ceccatelli et al., 2004), while camptothecin induces apoptosis through caspase 2 and 3 (Stefanis et al., 1999). In addition, camptothecin has been shown to interact with DNA topoisomerase I causing an intrinsic cascade of cell apoptotic death. It is possible that as shown by our flow cytometry data the different patterns of cell apoptosis and cell death led to slightly different responses for these apoptogens. This may be due to the differences in time of apoptosis in relation to the start of mineralization. It is important to note that camptothecin also induced apoptosis in the chick cultures in a similar manner to the chick cultures treated with staurosporine, and in both cases the chick cultures did not mineralize properly (Pourmand et al., 2007).
Both apoptogens also showed increases in the rate of mineralization as measured by 45Ca when given to murine cultures. This indicates that when increasing the amount and time course for apoptosis, mineralization occurs faster in the C3H10T1/2 cultures. This idea was also supported by the ZVAD-fmk studies. Inhibition of apoptosis through interference with the caspase cascade slowed the rate of mineralization in ZVAD-fmk treated murine micromass cultures. Control cultures also exhibited a certain amount of apoptosis and showed rates of mineralization in between the ZVAD-fmk treated and apoptogen treated cultures, indicating that apoptosis does play a role in mineralization in the murine system.
There were changes in the rate of mineralization with inhibition of apoptosis, but not in the total extent of mineralization as shown by the calcium uptake curves for ZVAD-fmk treated murine micromass cultures. These data indicate that while apoptosis may play a role in endochondral ossification, it is not a required step in mineralization. There are reports that vascular invasion of cartilage at the growth plate is linked to apoptosis, and furthermore that inhibition of angiogenesis results in an accumulation of hypertrophic chondrocytes in vivo (Gerber and Ferrara, 2000). Our in vitro results suggest that while induction of apoptosis may create an environment or one pathway to calcification, this is not the primary or only method of calcification of chondrocytic cells. Studies show that osteogenic differentiation, mineralization, and apoptosis and can be regulated by phosphate and calcium concentrations (Liu et al., 2009, Magne et al., 2003, Mansfield et al., 2001). It is possible that these are cell-type specific and that apoptosis is not the major factor in normal endochondral ossification. There are data that support this theory, as in fractures, the inhibition of apoptosis has been shown to increase healing (Garcia et al.).
When added to the chick cultures, camptothecin behaved like staurosporine and did not increase the extent of calcification, suggesting this was not a drug-specific effect. This is supported by data from the living mouse where treatment with camptothecin enhanced mineralization (Li et al., 2005). Based on the data in this study as well as others, it is likely that the role of apoptosis in mineralization is species specific.
Acknowledgments
This work was supported by NIH AR037661 and NIH AR046121.
References
- ADAMS CS, SHAPIRO IM. The fate of the terminally differentiated chondrocyte: evidence for microenvironmental regulation of chondrocyte apoptosis. Crit Rev Oral Biol Med. 2002;13:465–73. doi: 10.1177/154411130201300604. [DOI] [PubMed] [Google Scholar]
- AIZAWA T, KOKUBUN S, TANAKA Y. Apoptosis and proliferation of growth plate chondrocytes in rabbits. J Bone Joint Surg Br. 1997;79:483–6. doi: 10.1302/0301-620x.79b3.7221. [DOI] [PubMed] [Google Scholar]
- AMLING M, NEFF L, TANAKA S, INOUE D, KUIDA K, WEIR E, PHILBRICK WM, BROADUS AE, BARON R. Bcl-2 lies downstream of parathyroid hormone-related peptide in a signaling pathway that regulates chondrocyte maturation during skeletal development. J Cell Biol. 1997;136:205–13. doi: 10.1083/jcb.136.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSKEY AL, CAMACHO NP, MENDELSOHN R, DOTY SB, BINDERMAN I. FT-IR microscopic mappings of early mineralization in chick limb bud mesenchymal cell cultures. Calcif Tissue Int. 1992a;51:443–8. doi: 10.1007/BF00296678. [DOI] [PubMed] [Google Scholar]
- BOSKEY AL, DOTY SB, KUDRYASHOV V, MAYER-KUCKUK P, ROY R, BINDERMAN I. Modulation of extracellular matrix protein phosphorylation alters mineralization in differentiating chick limb-bud mesenchymal cell micromass cultures. Bone. 2008;42:1061–71. doi: 10.1016/j.bone.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSKEY AL, DOTY SB, STINER D, BINDERMAN I. Viable cells are a requirement for in vitro cartilage calcification. Calcif Tissue Int. 1996a;58:177–85. doi: 10.1007/BF02526884. [DOI] [PubMed] [Google Scholar]
- BOSKEY AL, GUIDON P, DOTY SB, STINER D, LEBOY P, BINDERMAN I. The mechanism of beta-glycerophosphate action in mineralizing chick limb-bud mesenchymal cell cultures. J Bone Miner Res. 1996b;11:1694–702. doi: 10.1002/jbmr.5650111113. [DOI] [PubMed] [Google Scholar]
- BOSKEY AL, PASCHALIS EP, BINDERMAN I, DOTY SB. BMP-6 accelerates both chondrogenesis and mineral maturation in differentiating chick limb-bud mesenchymal cell cultures. J Cell Biochem. 2002;84:509–19. [PubMed] [Google Scholar]
- BOSKEY AL, STINER D, BINDERMAN I, DOTY SB. Effects of proteoglycan modification on mineral formation in a differentiating chick limb-bud mesenchymal cell culture system. J Cell Biochem. 1997;64:632–43. doi: 10.1002/(sici)1097-4644(19970315)64:4<632::aid-jcb11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- BOSKEY AL, STINER D, DOTY SB, BINDERMAN I. Requirement of vitamin C for cartilage calcification in a differentiating chick limb-bud mesenchymal cell culture. Bone. 1991;12:277–82. doi: 10.1016/8756-3282(91)90076-u. [DOI] [PubMed] [Google Scholar]
- BOSKEY AL, STINER D, DOTY SB, BINDERMAN I, LEBOY P. Studies of mineralization in tissue culture: optimal conditions for cartilage calcification. Bone Miner. 1992b;16:11–36. doi: 10.1016/0169-6009(92)90819-y. [DOI] [PubMed] [Google Scholar]
- BRONCKERS AL, GOEI W, LUO G, KARSENTY G, D’SOUZA RN, LYARUU DM, BURGER EH. DNA fragmentation during bone formation in neonatal rodents assessed by transferase-mediated end labeling. J Bone Miner Res. 1996;11:1281–91. doi: 10.1002/jbmr.5650110913. [DOI] [PubMed] [Google Scholar]
- CECCATELLI S, TAMM C, SLEEPER E, ORRENIUS S. Neural stem cells and cell death. Toxicol Lett. 2004;149:59–66. doi: 10.1016/j.toxlet.2003.12.060. [DOI] [PubMed] [Google Scholar]
- CHOWDHURY I, THARAKAN B, BHAT GK. Caspases - an update. Comp Biochem Physiol B Biochem Mol Biol. 2008;151:10–27. doi: 10.1016/j.cbpb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- COHEN GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326 (Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELISE AM, STRINGA E, WOODWARD WA, MELLO MA, TUAN RS. Embryonic limb mesenchyme micromass culture as an in vitro model for chondrogenesis and cartilage maturation. Methods Mol Biol. 2000;137:359–75. doi: 10.1385/1-59259-066-7:359. [DOI] [PubMed] [Google Scholar]
- DENKER AE, HAAS AR, NICOLL SB, TUAN RS. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: I. Stimulation by bone morphogenetic protein-2 in high-density micromass cultures. Differentiation. 1999;64:67–76. doi: 10.1046/j.1432-0436.1999.6420067.x. [DOI] [PubMed] [Google Scholar]
- FELISBINO SL, CARVALHO HF. Growth cartilage calcification and formation of bone trabeculae are late and dissociated events in the endochondral ossification of Rana catesbeiana. Cell Tissue Res. 2001;306:319–23. doi: 10.1007/s004410100446. [DOI] [PubMed] [Google Scholar]
- GARCIA-CALVO M, PETERSON EP, LEITING B, RUEL R, NICHOLSON DW, THORNBERRY NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–13. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- GARCIA P, SCHWENZER S, SLOTTA JE, SCHEUER C, TAMI AE, HOLSTEIN JH, HISTING T, BURKHARDT M, POHLEMANN T, MENGER MD. Inhibition of angiotensin-converting enzyme stimulates fracture healing and periosteal callus formation - role of a local renin-angiotensin system. Br J Pharmacol. 159:1672–80. doi: 10.1111/j.1476-5381.2010.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERBER HP, FERRARA N. Angiogenesis and bone growth. Trends Cardiovasc Med. 2000;10:223–8. doi: 10.1016/s1050-1738(00)00074-8. [DOI] [PubMed] [Google Scholar]
- GIBSON G. Active role of chondrocyte apoptosis in endochondral ossification. Microsc Res Tech. 1998;43:191–204. doi: 10.1002/(SICI)1097-0029(19981015)43:2<191::AID-JEMT10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- GIBSON GJ, KOHLER WJ, SCHAFFLER MB. Chondrocyte apoptosis in endochondral ossification of chick sterna. Dev Dyn. 1995;203:468–76. doi: 10.1002/aja.1002030409. [DOI] [PubMed] [Google Scholar]
- HAAS AR, TUAN RS. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: II. Stimulation by bone morphogenetic protein-2 requires modulation of N-cadherin expression and function. Differentiation. 1999;64:77–89. doi: 10.1046/j.1432-0436.1999.6420077.x. [DOI] [PubMed] [Google Scholar]
- HATORI M, KLATTE KJ, TEIXEIRA CC, SHAPIRO IM. End labeling studies of fragmented DNA in the avian growth plate: evidence of apoptosis in terminally differentiated chondrocytes. J Bone Miner Res. 1995;10:1960–8. doi: 10.1002/jbmr.5650101216. [DOI] [PubMed] [Google Scholar]
- KIRSCH T, WANG W, PFANDER D. Functional differences between growth plate apoptotic bodies and matrix vesicles. J Bone Miner Res. 2003;18:1872–81. doi: 10.1359/jbmr.2003.18.10.1872. [DOI] [PubMed] [Google Scholar]
- KUZNETSOVA NV, FORLINO A, CABRAL WA, MARINI JC, LEIKIN S. Structure, stability and interactions of type I collagen with GLY349-CYS substitution in alpha 1(I) chain in a murine Osteogenesis Imperfecta model. Matrix Biol. 2004;23:101–12. doi: 10.1016/j.matbio.2004.03.002. [DOI] [PubMed] [Google Scholar]
- LEE D, LONG SA, ADAMS JL, CHAN G, VAIDYA KS, FRANCIS TA, KIKLY K, WINKLER JD, SUNG CM, DEBOUCK C, RICHARDSON S, LEVY MA, DEWOLF WE, JR, KELLER PM, TOMASZEK T, HEAD MS, RYAN MD, HALTIWANGER RC, LIANG PH, JANSON CA, MCDEVITT PJ, JOHANSON K, CONCHA NO, CHAN W, ABDEL-MEGUID SS, BADGER AM, LARK MW, NADEAU DP, SUVA LJ, GOWEN M, NUTTALL ME. Potent and selective nonpeptide inhibitors of caspases 3 and 7 inhibit apoptosis and maintain cell functionality. J Biol Chem. 2000;275:16007–14. doi: 10.1074/jbc.275.21.16007. [DOI] [PubMed] [Google Scholar]
- LI X, LIU P, LIU W, MAYE P, ZHANG J, ZHANG Y, HURLEY M, GUO C, BOSKEY A, SUN L, HARRIS SE, ROWE DW, KE HZ, WU D. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 2005;37:945–52. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- LIU YK, LU QZ, PEI R, JI HJ, ZHOU GS, ZHAO XL, TANG RK, ZHANG M. The effect of extracellular calcium and inorganic phosphate on the growth and osteogenic differentiation of mesenchymal stem cells in vitro: implication for bone tissue engineering. Biomed Mater. 2009;4:025004. doi: 10.1088/1748-6041/4/2/025004. [DOI] [PubMed] [Google Scholar]
- MAGNE D, BLUTEAU G, FAUCHEUX C, PALMER G, VIGNES-COLOMBEIX C, PILET P, ROUILLON T, CAVERZASIO J, WEISS P, DACULSI G, GUICHEUX J. Phosphate is a specific signal for ATDC5 chondrocyte maturation and apoptosis-associated mineralization: possible implication of apoptosis in the regulation of endochondral ossification. J Bone Miner Res. 2003;18:1430–42. doi: 10.1359/jbmr.2003.18.8.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSFIELD K, PUCCI B, ADAMS CS, SHAPIRO IM. Induction of apoptosis in skeletal tissues: phosphate-mediated chick chondrocyte apoptosis is calcium dependent. Calcif Tissue Int. 2003;73:161–72. doi: 10.1007/s00223-002-1056-z. [DOI] [PubMed] [Google Scholar]
- MANSFIELD K, TEIXEIRA CC, ADAMS CS, SHAPIRO IM. Phosphate ions mediate chondrocyte apoptosis through a plasma membrane transporter mechanism. Bone. 2001;28:1–8. doi: 10.1016/s8756-3282(00)00409-9. [DOI] [PubMed] [Google Scholar]
- MELLO MA, TUAN RS. High density micromass cultures of embryonic limb bud mesenchymal cells: an in vitro model of endochondral skeletal development. In Vitro Cell Dev Biol Anim. 1999;35:262–9. doi: 10.1007/s11626-999-0070-0. [DOI] [PubMed] [Google Scholar]
- MELLO MA, TUAN RS. Effects of TGF-beta1 and triiodothyronine on cartilage maturation: in vitro analysis using long-term high-density micromass cultures of chick embryonic limb mesenchymal cells. J Orthop Res. 2006;24:2095–105. doi: 10.1002/jor.20233. [DOI] [PubMed] [Google Scholar]
- MIRKES PE. 2001 Warkany lecture: to die or not to die, the role of apoptosis in normal and abnormal mammalian development. Teratology. 2002;65:228–39. doi: 10.1002/tera.10049. [DOI] [PubMed] [Google Scholar]
- OHYAMA K, FARQUHARSON C, WHITEHEAD CC, SHAPIRO IM. Further observations on programmed cell death in the epiphyseal growth plate: comparison of normal and dyschondroplastic epiphyses. J Bone Miner Res. 1997;12:1647–56. doi: 10.1359/jbmr.1997.12.10.1647. [DOI] [PubMed] [Google Scholar]
- PASCHALIS EP, RECKER R, DICARLO E, DOTY SB, ATTI E, BOSKEY AL. Distribution of collagen cross-links in normal human trabecular bone. J Bone Miner Res. 2003;18:1942–6. doi: 10.1359/jbmr.2003.18.11.1942. [DOI] [PubMed] [Google Scholar]
- PLESHKO N, BOSKEY A, MENDELSOHN R. Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophys J. 1991;60:786–93. doi: 10.1016/S0006-3495(91)82113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POURMAND EP, BINDERMAN I, DOTY SB, KUDRYASHOV V, BOSKEY AL. Chondrocyte apoptosis is not essential for cartilage calcification: evidence from an in vitro avian model. J Cell Biochem. 2007;100:43–57. doi: 10.1002/jcb.20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROACH HI. New aspects of endochondral ossification in the chick: chondrocyte apoptosis, bone formation by former chondrocytes, and acid phosphatase activity in the endochondral bone matrix. J Bone Miner Res. 1997;12:795–805. doi: 10.1359/jbmr.1997.12.5.795. [DOI] [PubMed] [Google Scholar]
- ROACH HI, ERENPREISA J, AIGNER T. Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J Cell Biol. 1995;131:483–94. doi: 10.1083/jcb.131.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSCHGER P, FRATZL-ZELMAN N, MISOF BM, GLORIEUX FH, KLAUSHOFER K, RAUCH F. Evidence that abnormal high bone mineralization in growing children with osteogenesis imperfecta is not associated with specific collagen mutations. Calcif Tissue Int. 2008;82:263–70. doi: 10.1007/s00223-008-9113-x. [DOI] [PubMed] [Google Scholar]
- ROY R, KUDRYASHOV V, DOTY SB, BINDERMAN I, BOSKEY AL. Differentiation and mineralization of murine mesenchymal C3H10T1/2 cells in micromass culture. Differentiation. 79:211–7. doi: 10.1016/j.diff.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIRAISHI J, TATSUMI T, KEIRA N, AKASHI K, MANO A, YAMANAKA S, MATOBA S, ASAYAMA J, YAOI T, FUSHIKI S, FLISS H, NAKAGAWA M. Important role of energy-dependent mitochondrial pathways in cultured rat cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2001;281:H1637–47. doi: 10.1152/ajpheart.2001.281.4.H1637. [DOI] [PubMed] [Google Scholar]
- STEFANIS L, PARK DS, FRIEDMAN WJ, GREENE LA. Caspase-dependent and -independent death of camptothecin-treated embryonic cortical neurons. J Neurosci. 1999;19:6235–47. doi: 10.1523/JNEUROSCI.19-15-06235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU P, HUANG J, CEBE P, KAPLAN DL. Osteogenesis imperfecta collagen-like peptides: self-assembly and mineralization on surfaces. Biomacromolecules. 2008;9:1551–7. doi: 10.1021/bm701365x. [DOI] [PubMed] [Google Scholar]
- YI CH, YUAN J. The Jekyll and Hyde functions of caspases. Dev Cell. 2009;16:21–34. doi: 10.1016/j.devcel.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUE TL, WANG C, ROMANIC AM, KIKLY K, KELLER P, DEWOLF WE, JR, HART TK, THOMAS HC, STORER B, GU JL, WANG X, FEUERSTEIN GZ. Staurosporine-induced apoptosis in cardiomyocytes: A potential role of caspase-3. J Mol Cell Cardiol. 1998;30:495–507. doi: 10.1006/jmcc.1997.0614. [DOI] [PubMed] [Google Scholar]
- ZENMYO M, KOMIYA S, KAWABATA R, SASAGURI Y, INOUE A, MORIMATSU M. Morphological and biochemical evidence for apoptosis in the terminal hypertrophic chondrocytes of the growth plate. J Pathol. 1996;180:430–3. doi: 10.1002/(SICI)1096-9896(199612)180:4<430::AID-PATH691>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]