Abstract
Research has demonstrated the toxic effects of methylmercury (MeHg), yet molecular mechanisms underlying its toxicity are not completely understood. Caenorhabditis elegans (C. elegans) offers a unique biological model to explore mechanisms of MeHg toxicity given many advantages associated with its ease of use and genetic power. Since our previous work indicated neurotoxic resistance of C. elegans to MeHg, the present study was designed to examine molecular mechanisms associated with this resistance. We hypothesized MeHg would induce expression of gst, hsp or mtl in vivo since glutathione (GSH), heat shock proteins (HSPs), and metallothioneins (MTs) have shown involvement in MeHg toxicity. Our studies demonstrated a modest, but significant increase in fluorescence in gst-4::GFP and mtl-1::GFP strains at an acute, low L1 MeHg exposure, whereas chronic L4 MeHg exposure induced expression of gst-4::GFP and hsp-4::GFP. Knockout gst-4 animals showed no alterations in lethality sensitivity compared to wildtype animals whereas mtl knockouts displayed increased sensitivity to MeHg exposure. GSH levels were increased by acute MeHg treatment and depleted with chronic exposure. We also demonstrate that MeHg induces hormesis, a phenotype whereby a sublethal exposure to MeHg rendered C. elegans resistant to subsequent exposure to the organometal. The involvement of gst-4, hsp-4, mtl-1, and mtl-2 in hormesis was examined. An increase in gst-4::GFP expression after a low-dose acute exposure to MeHg indicated that gst-4 may be involved in this response. Our results implicate GSH, HSPs, and MTs in protecting C. elegans from MeHg toxicity and show a potential role of gst-4 in MeHg-induced hormesis.
Keywords: Caenorhabditis elegans, methylmercury, hormesis
Introduction
Mercury (Hg) reaches aquatic environments as precipitation (Clarkson and Magos, 2006). Bacteria methylate Hg, forming MeHg, which moves up the food chain, bioconcentrating in predator fish. Humans are exposed to this toxicant when they consume seafood (Fitzgerald and Clarkson, 1991; Mason et al., 2005). Adult humans experience focal lesions to discreet areas of the brain including the loss of cerebellar granular cells and damage to the occipital lobe (Clarkson and Magos, 2006) while fetal and young individuals experience global brain lesions including microcephaly, distortion of the cortical layering system, cerebellar abnormalities, alterations in glial cells and disruptions of neurotransmitter systems upon exposure (Clarkson, 2002; Clarkson and Magos, 2006; Roh et al., 2006). Although MeHg has a high affinity for thiol (-SH) groups, specific cellular and molecular targets of MeHg are largely unknown (Kerper et al., 1992; Simmons-Willis et al., 2002). We designed experiments in Caenorhabditis elegans (C. elegans) to determine molecular mechanisms of proteins implicated in MeHg detoxification in an effort to better understand the adverse health effects of MeHg in humans.
Prior work by our group indicated no appreciable morphological alterations in GABAergic, dopaminergic, cholinergic, glutamatergic, or serotonergic neuronal subtypes in response to MeHg insult, showing that the C. elegans nervous system demonstrates resistance to the toxicant (Helmcke et al., 2009).
Many proteins are involved in the detoxification and excretion of MeHg; these include glutathione (GSH), heat shock proteins (HSPs), and metallothioneins (MTs). GSH is a tripeptide that can be oxidized to form GSSG in the presence of reactive oxygen species (ROS). GSSG can be converted back into GSH via glutathione reductase (GR) and the conversion of NADPH to NADP+ (Filomeni et al., 2005). Alternatively, glutathione s-transferases (GSTs) can catalyze the conversion of GSH to GS-, which binds to MeHg and facilitates its excretion from the body (Supplemental Figure 1) (Hirata and Takahashi, 1981). HSPs function as molecular chaperones under normal conditions. Upon stress, such as MeHg exposure (Sacco et al., 1997), HSPs promote refolding and repair of denatured proteins and facilitate protein synthesis (Hubbard and Sander, 1991). MTs are small, cysteine-rich metal binding proteins involved in metal detoxification, homeostasis and protection from oxidative stress (Maret, 2008). Due to their high cysteine content, MTs have a strong affinity for MeHg. Additionally, MeHg has been shown to induce MT expression (Rising et al., 1995; Tsui and Wang, 2005) and alterations in behavior of MT-null animals (Yoshida et al., 2008). Although GSH, HSP, and MT have been implicated in resistance to MeHg toxicity, researchers have yet to elucidate their precise roles in detoxification.
C. elegans is a free-living soil nematode often used in biological research due to the advantages it provides including its small size, quick generation time, self-fertilization, and simple genetic manipulation. Additionally, C. elegans has been well-studied, its genome has been sequenced, and nervous system mapped (Sulston and Horvitz, 1977; Sulston, 1983; Wood, 1988; 1998). C. elegans has been used as a model for studying various toxicants with a high predictive value for mammalian systems (Williams and Dusenbery, 1988; National Research Council, 2000; Cole et al., 2004; Leung et al., 2008). We chose C. elegans as a model system because of the resistance of its nervous system to MeHg (Helmcke et al., 2009). To determine whether GSTs, HSPs, or MTs play a role in MeHg detoxification, we examined their expression through the use of GFP reporter strains. We observed gst-4, hsp-4, and mtl-1 induction following exposure to MeHg.
Hormesis refers to a process whereby a sublethal stressor renders an organism resistant to subsequent stress. This effect has been demonstrated in many models, ranging from cell culture to humans under various stress conditions, including dietary restriction, exercise, radiation, and exposure to chemicals including metals, and heat (Damelin et al., 2000; Cypser et al., 2006; Mattson, 2008; Bourg, 2009). Although the mechanisms of hormesis are unknown, previous research has elucidated HSPs of the HSP70 family and MT proteins as contributors, both of which can be upregulated following exposure to heavy metals (Damelin et al., 2000). Additionally, hormesis has been implicated as an explanation of the time lag observed between MeHg exposure and the appearance of symptoms of toxicity (Weiss et al., 2002).
Following dietary restriction, exposure to sublethal heat stress (Cypser et al., 2006) or exposure to juglone (Przybysz et al., 2009), C. elegans display an increase in lifespan and a resistance to exposure to a subsequent stressor. We examined the ability of C. elegans to display a hormetic response following exposure to sublethal doses of MeHg and the role of GST-4, HSP-4, MTL-1, and MTL-2 in this response.
The overall goal of our studies was to address the mechanism of action of MeHg toxicity and gain a better understanding of the mechanism of resistance of the C. elegans nervous system to MeHg. We set out to explore several proteins previously shown to be involved in MeHg resistance with a secondary objective of linking them to the hormetic effect of MeHg.
Methods
C. elegans maintenance and strains
C. elegans were cultured on nematode growth medium (NGM) plates seeded with Escherichia coli strain OP50 as previously described (Brenner, 1974). In addition to the wildtype N2 Bristol strain, transgenic lines expressing GFP reporters used in this study were: CL2166 gst-4::GFP (Link and Johnson, 2002), SJ4005 hsp-4::GFP (Calfon et al., 2002) (obtained from the Caenorhabditis Genetic Center (CGC), Minneapolis, MN), mtl-1::GFP, and mtl-2::GFP (both gifts of the lab of Dr. Jonathan Freedman). The following knockout strains were also used: RB1823 gst-4 (ok2358), VC128 mtl-2 (gk125) (both obtained from the CGC, Minneapolis, MN), mtl-1 (tm1770), and double mtl-1/2 (zs1) knockouts (gifts from Hughes and Sturzenbaum) (Hughes and Sturzenbaum, 2007). With the exception of the hsp-4::GFP strain, which was kept at 15°C throughout experimentation due to induction of the heat shock proteins at higher temperatures, animals were kept at 20-23°C throughout experimentation, and hermaphroditic worms were used in all experiments.
MeHg treatments
Animals were treated as previously described (Helmcke et al., 2009). Briefly, animals were treated with an alkaline bleach solution to obtain a synchronous population (Stiernagle, 1999) and exposed to MeHg in one of two treatment regimes. In the first paradigm, animals were treated for 30 minutes at 18-24 hours after synchronization, at the L1 stage. In the second paradigm, synchronized animals were allowed to grow to the L4 stage then treated for 15 hours. All treatments involved combining larvae, (2500 L1s or 300 L4s), concentrated OP50, the appropriate volume of MeHgCl dissolved in water, and M9 buffer to a total volume of 500μL in 1.7 mL siliconized tubes. Following treatment, animals were washed twice with deionized water by centrifugation and placed on OP50-containing NGM plates. For hormesis experiments, animals were subjected to a combination of both treatments (Figure 6A). Animals were treated under control, 0.3, or 0.6 mM MeHg conditions at the L1 stage for 30 minutes, washed, allowed to recover and grow to the L4 stage on OP50-containing NGM plates, and then treated at the L4 stage for 15 hours, and washed again. Dose-response curves were generated for each treatment condition.
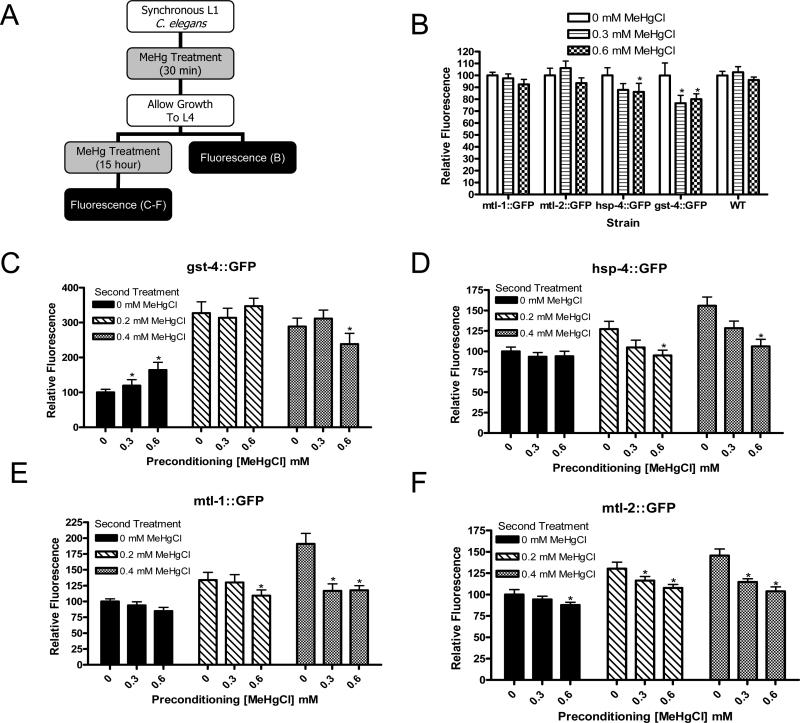
Figure 6.
Fluorescence of gst-4::GFP, hsp-4::GFP, mtl-1::GFP, and mtl-2::GFP strains following hormesis treatments. Treatment paradigm includes animals treated at the L1 stage for 30 minutes, allowed to grow to the L4 stage, assessed for fluorescence or treated again and assessed for fluorescence after second treatment (A). Decreases were noted in fluorescence in gst-4::GFP and hsp-4::GFP animals after a single treatment and recovery (B, n=4). After an initial treatment with MeHg and subsequent exposure to control treatment conditions, gst-4::GFP animals showed an increase in fluorescence (p<0.05). At higher subsequent MeHg levels and in all conditions of hsp-4::GFP, mtl-1::GFP, and mtl-2::GFP worms only decreases in fluorescence at increasing MeHg concentrations were noted (C-F, n=4).
Hg content
C. elegans larvae were treated with MeHg as described above. For L1 treatments, approximately 10,000 animals were pooled and assessed; for L4 treatments, approximately 900 animals were pooled and assessed. To separate live animals from dead animals, a sucrose floatation method was used. After treating and washing animals as described above, they were allowed to recover for 24 hours on OP50-containing NGM plates. They were then washed off the plate and into tubes with cold M9 buffer, centrifuged, and washed again with cold M9. After washing, a cold 30% sucrose solution was added to the worms, and they were centrifuged again. Worms floating on the top of the sucrose solution were live worms and were collected and washed an additional 3 times with M9. These samples were then sonicated and analyzed. Protein content was determined following manufacturer instructions for a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford IL). The remainder of the sample was used for inductively coupled plasma-mass spectrometry (ICP-MS) analysis of Hg content. Preparation of the sample for ICP-MS involved addition of nitric acid followed by heat digestion and dilution of the samples with water. The samples were digested in PP tubes (352059, BD) in a block heater after addition of 65% HNO3 (Merck, Suprapur). The samples were transferred to Teflon tubes and digested in an UltraClave (Milestone). After digestion the samples were diluted directly in the Teflon tubes with ultrapure water (PURLAB Ultra Analytic, Elga) to achieve a final acid concentration of 0.6 mol/L. High Resolution-Inductively Coupled Plasma-Mass Spectrometry (HR-ICP-MS) analysis was performed using a Thermo (Finnigan) model Element 2 instrument (Bremen, Germany). The RF power was 1400 W. The sample was introduced using an SC-2 with SC-FAST option auto sampler (ESI, NE, USA) with a peristaltic pump (pump speed 0.25 mL/min). The instrument was calibrated using 0.6 mol/L HNO3 solutions of multielement standards at appropriate concentrations. Internal standards were not used. To check for possible drift in the instrument, a standard solution with known elemental concentrations was analyzed for every 10 samples. In addition, blank samples (0.6 mol/L HNO3, Suprapur) were analyzed for approximately every 10 samples. The samples were analyzed in random order, and the analyst was not aware of the identity of the samples. Hg content was determined in the low resolution mode (M/Δm=300).
Lethality
Following treatment, wild type or knockout animals were placed on 60 mm NGM plates seeded with OP50 and allowed to grow for 24 hours. Animals were scored as dead or alive based on appearance and ability to move in response to being poked with a platinum wire (Bischof et al., 2006; Roh et al., 2007).
Measurement of fluorescence intensity
For each strain, control animals were imaged first. The imaging settings, including exposure time, were determined based on control animals. A Zeiss LSM 510 META upright confocal microscope was used for the imaging of hsp-4::GFP, mtl-1::GFP, and mtl-2::GFP strains. Autofluorescence was subtracted from each image, allowing for the analysis of GFP intensity using Metamorph software. For hormesis experiments and all experiments using the gst-4::GFP strain, C. elegans were imaged on a Nikon Eclipse 80i microscope. These images were analyzed using NIS-Element Basic Research Software to determine the fluorescence intensity of the animals.
Glutathione quantification
A scaled-up version of the treatment paradigms described above was conducted. C. elegans were treated in 50-mL conical tubes to a volume of 25 mL, using 125,000 animals for L1 treatments and 15,000 animals for L4 treatments. Following treatment, live and dead animals were washed 3x with dH2O, and duplicates of L4 treatments were pooled to yield 30,000 worms for glutathione analysis while L1 treatments were not pooled, yielding 125,000 animals for glutathione analysis. Immediately following washing, equal volumes of C. elegans and 10% perchloric acid/0.2 M boric acid/10 μM γ-glutamylglutamate were combined with approximately 500 mL of 1.0 mm zirconia beads in a 2 mL microtube. Samples were placed in a Mini-Beadbeater (Biospec Products, Bartlesville, OK) and beat for 20 seconds, then quickly placed in an ice water bath for 1 minute. This cycle was repeated 7 times before the samples were centrifuged for 30 minutes at 4°C. The supernatant was removed and frozen at -80°C until being subjected to further processing. For glutathione measurement, 300 μL sample was combined with 60 μL iodoacetic acid solution (14.8 mg/mL H2O). The pH was adjusted to 9.0±0.2 using KOH in saturated potassium tetraborate. After 20-minute incubation, 300 μL dansylchloride solution was added (20 mg/mL acetone), samples were mixed and allowed to incubate 24 hours in the dark. Following this incubation, 500 μL chloroform was added, samples were mixed and centrifuged, and aqueous layer was collected for injection to obtain HPLC results. HPLC separation was conducted as previously described (Reed et al., 1980), using an 80% methanol solution as solvent A, an acetate buffered methanol solution as solvent B, and a propylamine column (Custom LC, Huston, TX), with detection performed with a fluorescence detector with excitation maximum at 335 nm and emission at 515 nm. HPLC results were analyzed on a per-worm basis, assuming 125,000 animals per L1 treatment and 30,000 animals per L4 treatment.
Statistics
GraphPad Prism 4 was used to assess significance. For all experiments, ANOVA with Bonferroni's Multiple Comparison Test was applied to raw data. When p-values were less than 0.05, groups were considered significantly different, groups with p-values greater than 0.05 were not considered significantly different. For each experiment, ‘n’ indicates the number of independent experiments (not number of animals). For lethality tests, each experiment typically included three plates of approximately 200 animals for each concentration of MeHg tested. Fluorescence experiments included approximately 20 worms for each concentration in each experiment.
Results
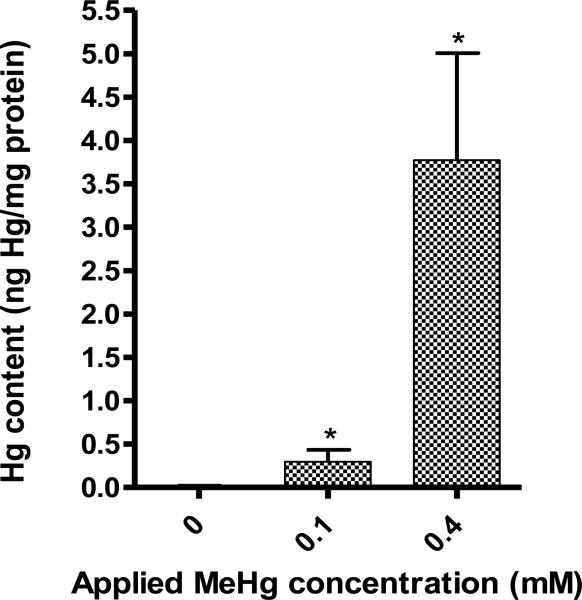
Hg accumulates in live animals following MeHg treatment
Hg content was measured in animals that survived a 15 hour exposure at the L4 stage at 0, 0.1, and 0.4 mM MeHg. Exposures tested were selected to represent a range of doses that corresponded to a low concentration (LC0), a medium concentration (LC20-LC80), and at a high concentration (LC100). As the MeHg concentration to which the worms were exposed increased, Hg content in the samples analyzed also increased, with Hg levels at 0.015±0.006 ng Hg/mg protein for samples treated with 0 mM MeHg, 0.297±0.136 ng Hg/mg protein for samples treated with 0.1 mM MeHg, and 3.775±1.231 ng Hg/mg protein (Figure 1). These results suggest that an increased accumulation of Hg in C. elegans is not responsible for the death of the animals that do not survive MeHg insult. However, measurements of Hg content of dead animals were not made, so there exists the unlikely possibility that these animals contain more MeHg than their surviving counterparts.
Figure 1.
Concentration of Hg in live animals following MeHg exposure.
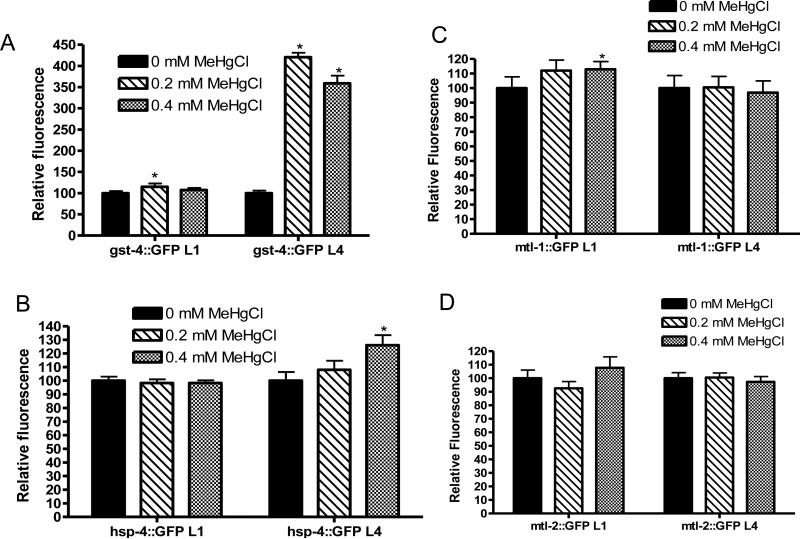
Increased expression of gst-4,hsp-4, mtl-1, and mtl-2 following MeHg exposure
Fluorescence intensities of gst-4::GFP, hsp-4::GFP, mtl-1::GFP and mtl-2::GFP strains were measured immediately following treatment of L1 C. elegans for 30 minutes and of L4 C. elegans for 15 hours with MeHg. hsp-4::GFP and mtl-2::GFP displayed no alteration in fluorescence after 30-minute treatment at the L1 stage at 0.2 or 0.4 mM MeHg. However, a significant increase was noted in fluorescence of gst-4::GFP following treatment of L1s at 0.2 mM MeHg (Figure 3A, p<0.01) and mtl-1::GFP following treatment at 0.4 mM MeHg (Figure 3C, p<0.05) (n=5). These data indicate that a short, low exposure to MeHg at the L1 stage can induce expression of gst-4 and mtl-1.
Figure 3.
MeHg induces increases in GFP fluorescence of gst-4::GFP (A), hsp-4::GFP (B), mtl-1::GFP (C) and mtl-2::GFP (D). After an acute treatment at the L1 stage, increases in fluorescence were observed in gst-4::GFP (A, p<0.01) and mtl-1::GFP (C, p<0.05) strains (n=5). Chronic treatment at the L4 stage induced in increases in fluorescence in gst-4::GFP (A, p<0.001) and hsp-4::GFP (B, p<0.05) strains (n=5).
Following a 15-hour treatment at the L4 stage (Figure 2), a significant increase in fluorescence was noted in gst-4::GFP [for which large (4 fold) increase in fluorescence was seen (Figure 3A, p<0.001)] and hsp-4::GFP (Figure 3B, p<0.05) while mtl-1::GFP and mtl-2::GFP worms displayed no changes in fluorescence intensity (Figure 3C-D) (n=5). The findings for the animals treated at the L4 stage were robust, indicating that this longer exposure or life stage may lead to increased induction of this protein.
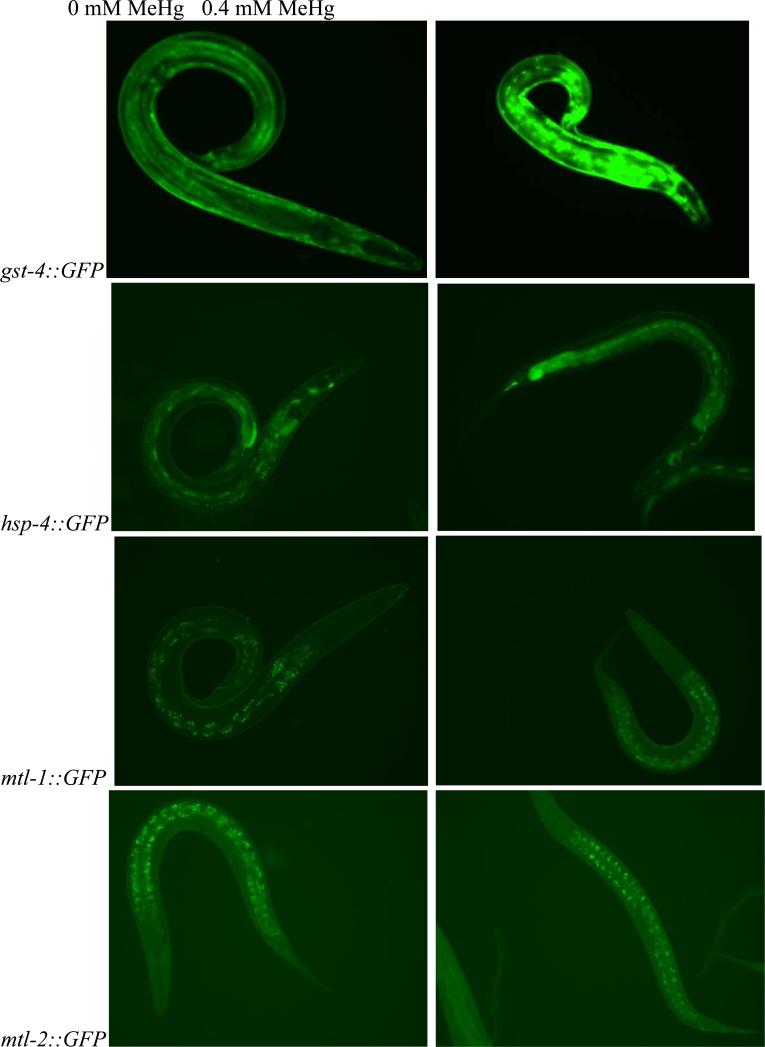
Figure 2.
Images of gst-4::GFP (top row), hsp-4::GFP (second row), mtl-1::GFP (third row), and mtl-2::GFP (bottom row) C. elegans taken after L4 animals were exposed for 15 hours to either control conditions (0 mM MeHg, left column) or 0.4 mM MeHg (right column). An increase in flourescense is observed upon exposure of gst-4::GFP and hsp-4::GFP to 0.4 mM MeHg compared to control while mtl-1::GFP and mtl-2::GFP remain unchanged.
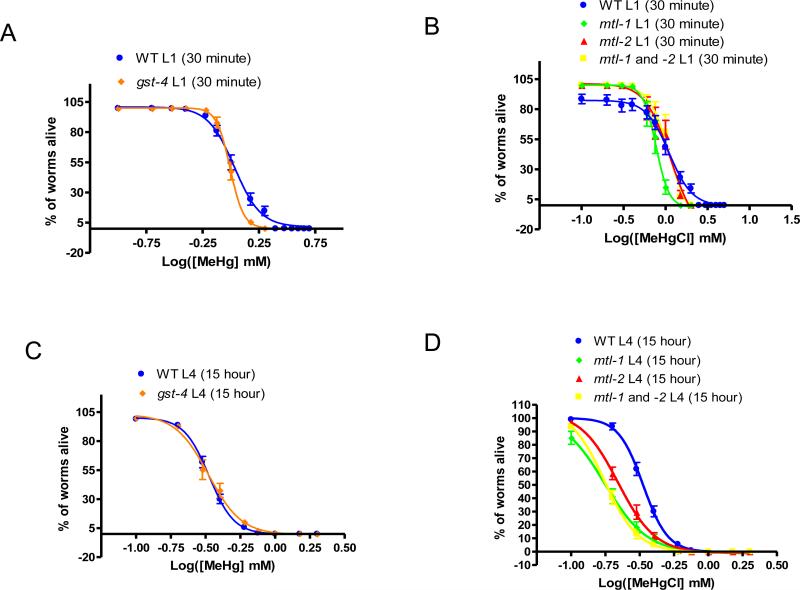
mtl but not gst-4 knockouts display increased sensitivity to MeHg
Lethality tests of L1 animals treated acutely and L4 animals treated chronically with MeHg were conducted on mtl-1, mtl-2, mtl-1/2, and gst-4 knockouts and compared to similarly treated wildtype animals. No significant shifts in dose-response curves were observed for animals treated acutely at the L1 stage (N2 LC50=1.08±0.02, mtl-1 LC50=0.78±0.02, mtl-2 LC50=1.15±0.1, mtl-1/2 LC50=1.12±0.05, gst-4 LC50=0.99±0.01, n=4) (Figure 4A-B) or in gst-4 knockout animals treated chronically at the L4 stage (N2 LC50=0.33±0.01, gst-4 LC50=0.33±0.02, n=4) (Figure 4C). However, all three mtl knockout strains (after treatment at the L4 stage) were significantly more sensitive to MeHg than wildtype (mtl-1 LC50=0.18±0.05, mtl-2 LC50=0.22±0.02 and mtl-1/2 LC50=0.17±0.02, n=6, p<0.05) (Figure 4D).
Figure 4.
Treatment of knockout animals reveals increased sensitivity in mtl null animals following chronic exposure to MeHg. Dose response curves following acute treatment at the L1 stage did not reveal shifts in gst-4 (LC50=0.99±0.01) (A), mtl-1 (LC50=0.78±0.02), mtl-2 (LC50=1.15±0.1), or mtl-1/2 (LC50=1.12±0.05) (B) strains when compared to wild type (LC50=1.08±0.02) (n=4). Chronic exposure of L4s did not induce a shift in gst-4 animals (LC50=0.33±0.02) (C) as compared to wild type (LC50=0.33±0.01) but did induce a significant shift in mtl-1 (LC50=0.18±0.05), mtl-2 (LC50=0.22±0.02), and mtl-1/2 (LC50=0.17±0.02) strains (D, p<0.05) (n=4).
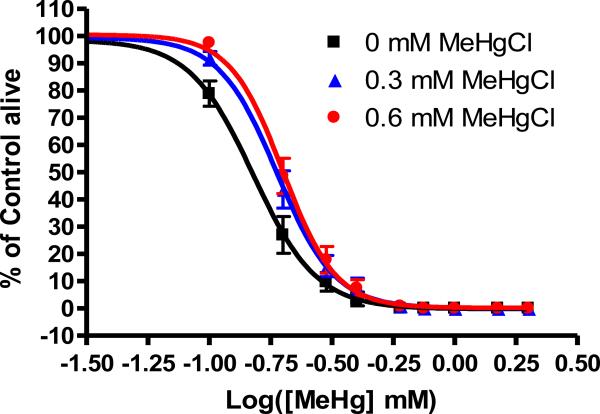
MeHg induces hormesis in wild-type C. elegans
We tested the ability of pre-treatment with MeHg to confer resistance to subsequent exposure. In these experiments, L1 animals were subjected to acute exposure to MeHg and then and allowed to recover until a second treatment at the L4 larval stage. L1 animals treated under control conditions (e.g., 0 mM MeHg) for the initial MeHg exposure (LC50=0.15±0.004 mM MeHg) were significantly (p<0.05) more sensitive to subsequent exposure to MeHg than those pre-treated with 0.3 mM MeHg (LC50=0.19±0.005 mM) or 0.6 mM MeHg (LC50=0.20±0.004 mM) when LC50 values were compared (Figure 5). The overall rightward shift in the dose-response curve is also indicative of the enhanced resistance of animals subjected to prior exposure to MeHg.
Figure 5.
Pretreatment with MeHg renders C. elegans more resistant to a subsequent exposure to the toxicant. Dose-response curves were significantly shifted rightward under 0.3 mM MeHg (LC50=0.19±0.005) or 0.6 mM MeHg (LC50=0.20±0.004) (p<0.05) from control (LC50=0.15±0.004 mM MeHg) pretreatment conditions.
Contribution of gst-4, hsp-4, mtl-1, and mtl-2 to hormesis
We conducted experiments to assess the involvement of gst-4, hsp-4, mtl-1, and mtl-2 in hormesis. Fluorescence of wild-type gst-4::GFP, hsp-4::GFP, mtl-1::GFP and mtl-2::GFP strains was measured following exposure to hormesis conditions. Animals were imaged immediately before and immediately following a second exposure (chronic L4) to MeHg. Images collected before the second MeHg treatment, (i.e., after an acute L1 treatment followed by washing and growth to the L4 stage in the absence of MeHg), revealed minimal changes in fluorescence intensity. It is important to note that these measurements were taken approximately 18 hours after removal of the worms from MeHg, in contrast to the experiments described above where animals were assessed immediately after removal from MeHg solution. No significant alterations in fluorescence intensity were noted in wild-type, mtl-1::GFP, or mtl-2::GFP animals. A significant decrease in fluorescence was observed in gst-4::GFP animals treated at both 0.3 and 0.6 mM MeHg and hsp-4::GFP animals treated at 0.6 mM MeHg (Figure 6B, p<0.05). These findings were surprising given the previous findings of increases in fluorescence when the hormesis model was not used.
After the second treatment of L4 larvae, alterations in fluorescence were similar to the trends observed following a single L4 chronic treatment. A slight decrease in baseline fluorescence of hsp-4::GFP (Figure 6D), mtl-1::GFP (Figure 6E), and mtl-2::GFP (Figure 6F) strains was noted as the initial, acute MeHg concentration increased, and this trend continued in animals treated at higher MeHg concentrations (p<0.05). In gst-4::GFP L4 animals, dramatic increases in fluorescence were observed following chronic treatments with increasing MeHg concentrations (Figure 6C, p<0.01). With the exception of hsp-4::GFP (Figure 5) animals treated at the 0.6 mM MeHg acute exposure level, significant increases in fluorescence occur in each of the treatment groups. In hsp-4::GFP and gst-4::GFP animals, these findings confirm the previous results described in this paper (Figure 6), whereby a chronic exposure to MeHg induces an increase in fluorescence in these animals. However, our previous data presented here did not reveal an increase in fluorescence in mtl-1::GFP (Figure 3C) or mtl-2::GFP (Figure 3D) animals. The results from these hormesis data, therefore, reveal that mtl-1 and mtl-2 can be induced upon a second exposure to MeHg, but not a single chronic exposure.
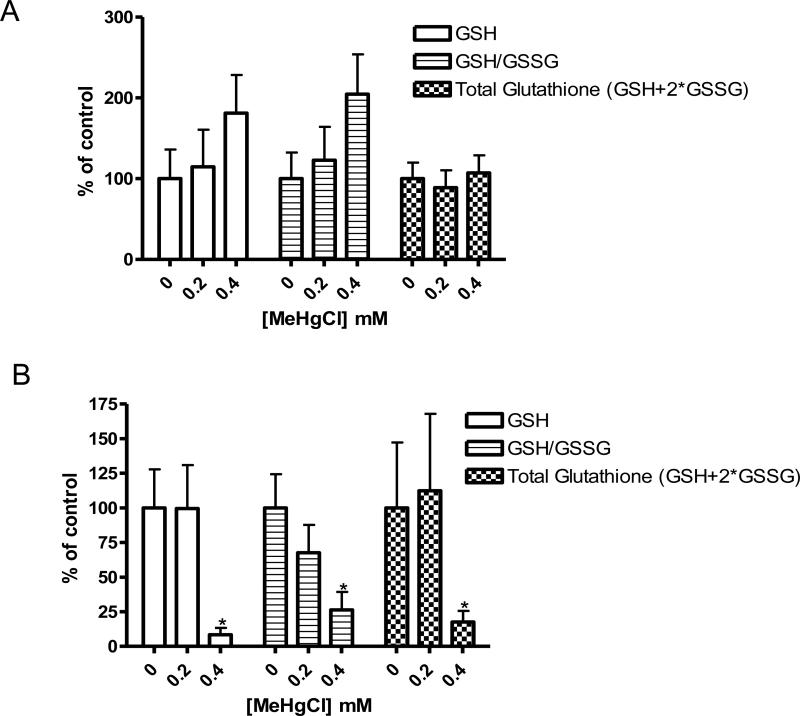
MeHg induces alterations in glutathione levels
Following the increase in fluorescence we observed in gst-4::GFP animals, we examined the role of the glutathione system in detoxification of MeHg in C. elegans, by measuring levels of GSH and GSSG following exposure to MeHg. Glutathione profiles were different between animals acutely and chronically exposed to MeHg. After an acute exposure to MeHg, a trend (not statistically significant) of increasing GSH and GSH/GSSG ratio were noted while no changes were observed in total glutathione levels (Figure 7A). Chronic exposure to MeHg induced statistically significant decreases in GSH, GSH/GSSG ratio, and total glutathione levels (Figure 7B). These data are consistent with the increase in gst-4::GFP expression we observed, as the increase in gst-4 indicates an increased conjugation of GSH to MeHg, facilitating its elimination from the system.
Figure 7.
Glutathione levels in C. elegans treated with MeHg. Levels of GSH and GSSG/GSH ratio increased after acute exposure (A) while these values along with total glutathione level decreased after chronic exposure (B).
Discussion
Previous data from our lab (Helmcke et al., 2009) showed that while lethality, pharyngeal pumping, growth (though an induction of the alternative dauer life stage was not observed), and development were affected in C. elegans that survived an exposure to MeHg, brood size, lifespan, thrashing rate, and nervous system morphology were surprisingly largely unaffected in intact animals that survived doses of MeHg lethal to some animals. This lack of effect, particularly in the nervous system, could be attributed to many factors including the difficulty of the toxicant to enter the animal through the cuticle or the absence of specific cells (such as the absence of traditional glial cells) or signaling pathways and enzymes in this model system. The studies reported here represent the first experiments to address the mechanism of action of MeHg toxicity in C. elegans and provide insights into the previously-observed unique relative resistance of the nematode's nervous system to this metal (Helmcke et al., 2009).
The current studies showed that MeHg exposure resulted in increasing levels of Hg accumulation in animals that survived exposure to the toxicant. This result indicates that the amount of accumulation of MeHg within the worm does not alter survival, but that viable animals respond differently to the toxicant than their non-surviving counterparts due to the similarities in the data described here and previously (Helmcke et al., 2009). Although concentrations of MeHg applied to C. elegans were high, our results from these and previous (Helmcke et al., 2009) studies indicate that the amount of Hg that accumulates in C. elegans is comparable to the levels observed in damaged mammalian brains following exposure to MeHg. In our study, Hg content in C. elegans ranged from 0-3.8 ng Hg/mg protein or parts per million (ppm). These values correlate with those levels observed in studies conducted in astrocyte cultures assessing toxicity (124 ng/mg protein) (Shapiro and Chan, 2008), in vivo rodent studies examining neurpathologic damage and neurobehavioral alterations at 4.5 and 0.5 ppm, respectively (Castoldi et al., 2008), and humans, where the threshold for observable clinical effects is 1 ppm (Burbacher et al., 1990) and the LOAEL is 0.5-1 ppm (Lewandowski et al., 2003). In our studies described here, the levels of Hg accumulation in worms that did not survive the MeHg insult were not measured. Since these values are unknown, the possibility remains that there is a slight increase in the Hg content of the worms that do not survive, though this is highly unlikely due to the comparison between these data and the data we previously described examining pooled samples and (Helmcke et al., 2009) along with the observation of a much higher concentration of Hg when animals were treated with higher concentrations of MeHg.
MeHg induced alterations in the expression of MT, GST, and HSP, all of which have been implicated in resistance to this organometal. These data show that expression of gst-4 and hsp-4 is induced by a long exposure of L4 animals to MeHg while expression of mtl-1 and gst-4 is induced by a short exposure of L1 animals to MeHg. Though no increase in MT expression was noted following a chronic exposure at the L4 stage, knockout of these proteins conferred increased lethality in C. elegans, indicating that while mtls are not induced by the toxicant, they are involved in protection and detoxification. These data indicate that proteins involved in toxicity function via different mechanisms and undergo various methods of transcriptional control. For example, the mtls may provide homeostatic protection from MeHg while gst-4 provides protection upon its dramatic upregulation.
Despite the induction of gst-4 upon exposure to MeHg, no shift in the lethality dose-response curve was observed of the gst-4 knockout strain, indicating that the absence of GST-4 does not increase the sensitivity to MeHg. One explanation for this result is that while gst-4 is involved in the response to the toxicant, other mechanisms are able to compensate in its absence. C. elegans express nearly 50 GSTs (van Rossum et al., 2001), approximately 10 HSPs of the HSP70 family (Heschl and Baillie, 1989), and 2 MTs (Freedman et al., 1993). Our results indicate that some of the same mechanisms are involved in detoxification in C. elegans as have been identified in other model systems (Sacco et al., 1997; Schlawicke Engstrom et al., 2008; Yoshida et al., 2008).
MeHg induces the generation of reactive oxygen species (ROS), mediators of MeHg toxicity in glial and neuronal cell culture (Sarafian and Verity, 1991; Yee and Choi, 1996). MTs are free radical scavengers and are induced in response to oxidative stress (Bauman et al., 1991; Maret, 2008) and also in response to MeHg exposure (Rising et al., 1995). The role of GSH in ROS elimination has been well-established and maintenance of GSH levels following MeHg exposure protects cells from oxidative injury (Kaur et al., 2006). Our results demonstrate increases in GST-4, GSH, HSP-4, and MTL-1, which implicate the induction of oxidative stress by MeHg, corroborating results in mammalian systems (Garg and Chang, 2006; Reardon, 2007).
Most parameters examined after the L1 acute treatment both in these and previous studies (Helmcke et al., 2009) demonstrated slight or no changes. The 30-minute exposure may be too short to induce alterations large enough to be quantified in our assays. Although the investigated proteins may contribute to the resistance of the C. elegans nervous system to MeHg, further investigation of these mechanisms can be used to further establish the mechanisms involved.
The hormesis phenomenon has been established in many systems upon exposure to various stressors. Given the effects of MeHg on gst-4, hsp-4, mtl-1, and mtl-2, we examined whether these proteins play a role in hormesis. We observed that animals with prior early exposure to MeHg showed a significant increase in resistance to a subsequent exposure to MeHg
Our results indicate that adaptation takes place in animals exposed to MeHg which renders them better-equipped to deal with a second exposure to the same stressor. There are many potential explanations and candidate proteins responsible for this phenomenon, including the upregulation of proteins involved in detoxification, upregulation of proteins involved in excretion or downregulation of proteins involved in uptake, only one of which we elucidated here. Here, we assessed whether MTs, a GST, or a HSP could contribute to the hormetic response of C. elegans. Since these proteins are not upregulated prior to the second insult of MeHg, these results cannot explain the hormetic phenotype observed in the lethality experiments. However, induction of these proteins upon the second exposure to MeHg may indicate that these proteins are more readily induced upon a second exposure, indicating that the initial exposure to MeHg may prime the system and facilitate their induction. However, expression of gst-4::GFP increased as the acute exposure concentration of MeHg increased under chronic control exposure conditions. Large increases in gst-4::GFP fluorescence are consistent with the proposal that GST levels are elevated in response to MeHg toxicity. However, the involvement of gst-4 in the hormetic response remains unclear. While increases in fluorescence were noted in groups with increasing initial exposure concentrations when the subsequent treatment was 0 mM MeHg, this same trend was not observed upon exposure to higher chronic concentrations of MeHg. This lack of a further increase in fluorescence could be attributed to a ceiling effect or an inability to differentiate very bright fluorescence. The increase in fluorescence, even at control conditions does provide some evidence that gst-4 could play a role in hormesis, since initial exposure to increasing MeHg concentrations induces an increase in fluorescence in subsequent stressful treatment conditions. We also observed increases in mtl-1 and mtl-2 expression when animals were preconditioned with MeHg.
We explored the contribution of GSH to the toxicity of MeHg. Exposure of L4 C. elegans to MeHg for 15 hours at 0.4 mM caused GSH, the GSH/GSSG ratio and the total glutathione levels to significantly decrease, indicating that GSH is converted to GSSG due to the presence of ROS and excreted in a complex with MeHg. These data suggest that the increase in gst-4 catalyzes the conjugation of MeHg to GSH, which is then excreted, causing GSH and total glutathione levels to decrease. These data corroborate what has been found in mammalian systems, with MeHg being excreted as a complex with glutathione, cysteine and glycine, or cysteine (Hirata and Takahashi, 1981). Additionally, alterations in the glutathione cycle can cause alterations in MeHg metabolism, such as a depletion of GSH leading to a decreased rate of conjugation to MeHg and a decrease in MeHg excretion (Schlawicke Engstrom et al., 2008). These findings indicate that independent lowering of GSH level would sensitize animals to MeHg while increasing GSH level may be protective. Taken together, our findings of alterations in GSH, gst-4, mtl-1 and mtl-2 confirm the involvement of ROS in MeHg toxicity and the ability of these proteins to confer resistance as shown by studies in other systems.
While previous researchers have shown the toxicity of MeHg and have identified some mechanisms involved in detoxification, we furthered their work by examining the role of GSH, HSP, and MTs in protection from MeHg, specifically by examining their role in hormesis. Our work begins to elucidate potential mechanisms of MeHg toxicity and neuroprotection in C. elegans; however, many other pathways are likely involved.
Supplementary Material
Acknowledgements
We thank the Caenorhabditis Genetics Center (CGC) at the University of Minnesota for providing worm strains. We also thank Lars Evje for running worm samples through ICP-MS procedures to determine Hg content and Jiyang Cai for his assistance with HPLC to determine GSH and GSSG levels. We also show our gratitude to David Miller for his experimental and manuscript advice and his lab for their assistance and technical expertise.
Funding
This work was supported by National Institute of Environmental Health Sciences, [grant numbers R01ES07731 and R01ES10563 to MA, and ES007028 to KJH].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Neither author has a conflict of interest.
References
- Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- Bauman JW, Liu J, Liu YP, Klaassen CD. Increase in metallothionein produced by chemicals that induce oxidative stress. Toxicol Appl Pharmacol. 1991;110:347–354. doi: 10.1016/s0041-008x(05)80017-1. [DOI] [PubMed] [Google Scholar]
- Bischof LJ, Huffman DL, Aroian RV. Assays for toxicity studies in C. elegans with Bt crystal proteins. Methods Mol Biol. 2006;351:139–154. doi: 10.1385/1-59745-151-7:139. [DOI] [PubMed] [Google Scholar]
- Bourg EL. Hormesis, aging and longevity. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbagen.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbacher TM, Rodier PM, Weiss B. Methylmercury developmental neurotoxicity: a comparison of effects in humans and animals. Neurotoxicol Teratol. 1990;12:191–202. doi: 10.1016/0892-0362(90)90091-p. [DOI] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Castoldi AF, Onishchenko N, Johansson C, Coccini T, Roda E, Vahter M, Ceccatelli S, Manzo L. Neurodevelopmental toxicity of methylmercury: Laboratory animal data and their contribution to human risk assessment. Regul Toxicol Pharmacol. 2008;51:215–229. doi: 10.1016/j.yrtph.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Clarkson TW. The three modern faces of mercury. Environ Health Perspect. 2002;110(Suppl 1):11–23. doi: 10.1289/ehp.02110s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Cole RD, Anderson GL, Williams PL. The nematode Caenorhabditis elegans as a model of organophosphate-induced mammalian neurotoxicity. Toxicol Appl Pharmacol. 2004;194:248–256. doi: 10.1016/j.taap.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Cypser JR, Tedesco P, Johnson TE. Hormesis and aging in Caenorhabditis elegans. Exp Gerontol. 2006;41:935–939. doi: 10.1016/j.exger.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damelin LH, Vokes S, Whitcutt JM, Damelin SB, Alexander JJ. Hormesis: a stress response in cells exposed to low levels of heavy metals. Hum Exp Toxicol. 2000;19:420–430. doi: 10.1191/096032700678816133. [DOI] [PubMed] [Google Scholar]
- Filomeni G, Rotilio G, Ciriolo MR. Disulfide relays and phosphorylative cascades: partners in redox-mediated signaling pathways. Cell Death Differ. 2005;12:1555–1563. doi: 10.1038/sj.cdd.4401754. [DOI] [PubMed] [Google Scholar]
- Fitzgerald WF, Clarkson TW. Mercury and monomethylmercury: present and future concerns. Environ Health Perspect. 1991;96:159–166. doi: 10.1289/ehp.9196159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman JH, Slice LW, Dixon D, Fire A, Rubin CS. The novel metallothionein genes of Caenorhabditis elegans. Structural organization and inducible, cell-specific expression. J Biol Chem. 1993;268:2554–2564. [PubMed] [Google Scholar]
- Garg TK, Chang JY. Methylmercury causes oxidative stress and cytotoxicity in microglia: attenuation by 15-deoxy-delta 12, 14-prostaglandin J2. J Neuroimmunol. 2006;171:17–28. doi: 10.1016/j.jneuroim.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Helmcke KJ, Syversen T, Miller DM, 3rd, Aschner M. Characterization of the effects of methylmercury on Caenorhabditis elegans. Toxicol Appl Pharmacol. 2009;240:265–272. doi: 10.1016/j.taap.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heschl MF, Baillie DL. Characterization of the hsp70 multigene family of Caenorhabditis elegans. DNA. 1989;8:233–243. doi: 10.1089/dna.1.1989.8.233. [DOI] [PubMed] [Google Scholar]
- Hirata E, Takahashi H. Degradation of methyl mercury glutathione by the pancreatic enzymes in bile. Toxicol Appl Pharmacol. 1981;58:483–491. doi: 10.1016/0041-008x(81)90101-0. [DOI] [PubMed] [Google Scholar]
- Hubbard TJ, Sander C. The role of heat-shock and chaperone proteins in protein folding: possible molecular mechanisms. Protein Eng. 1991;4:711–717. doi: 10.1093/protein/4.7.711. [DOI] [PubMed] [Google Scholar]
- Hughes S, Sturzenbaum SR. Single and double metallothionein knockout in the nematode C. elegans reveals cadmium dependent and independent toxic effects on life history traits. Environ Pollut. 2007;145:395–400. doi: 10.1016/j.envpol.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kaur P, Aschner M, Syversen T. Glutathione modulation influences methyl mercury induced neurotoxicity in primary cell cultures of neurons and astrocytes. Neurotoxicology. 2006;27:492–500. doi: 10.1016/j.neuro.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Kerper LE, Ballatori N, Clarkson TW. Methylmercury transport across the blood-brain barrier by an amino acid carrier. Am J Physiol. 1992;262:R761–765. doi: 10.1152/ajpregu.1992.262.5.R761. [DOI] [PubMed] [Google Scholar]
- Lapham LW, Cernichiari E, Cox C, Myers GJ, Baggs RB, Brewer R, Shamlaye CF, Davidson PW, Clarkson TW. An analysis of autopsy brain tissue from infants prenatally exposed to methymercury. Neurotoxicology. 1995;16:689–704. [PubMed] [Google Scholar]
- Leung MC, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci. 2008;106:5–28. doi: 10.1093/toxsci/kfn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski TA, Ponce RA, Charleston JS, Hong S, Faustman EM. Effect of methylmercury on midbrain cell proliferation during organogenesis: potential cross-species differences and implications for risk assessment. Toxicol Sci. 2003;75:124–133. doi: 10.1093/toxsci/kfg151. [DOI] [PubMed] [Google Scholar]
- Link CD, Johnson CJ. Reporter transgenes for study of oxidant stress in Caenorhabditis elegans. Methods Enzymol. 2002;353:497–505. doi: 10.1016/s0076-6879(02)53072-x. [DOI] [PubMed] [Google Scholar]
- Maret W. Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc. Exp Gerontol. 2008;43:363–369. doi: 10.1016/j.exger.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Mason RP, Abbott ML, Bodaly RA, Bullock OR, Jr., Driscoll CT, Evers D, Lindberg SE, Murray M, Swain EB. Monitoring the response to changing mercury deposition. Environ Sci Technol. 2005;39:14A–22A. doi: 10.1021/es053155l. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, U. S. Scientific Frontiers in Developmental Toxicology and Risk Assessment (NRC, US) The National Academies Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- Przybysz AJ, Choe KP, Roberts LJ, Strange K. Increased age reduces DAF-16 and SKN-1 signaling and the hormetic response of Caenorhabditis elegans to the xenobiotic juglone. Mech Ageing Dev. 2009;130:357–369. doi: 10.1016/j.mad.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon AM, Bhat HK. Methylmercury neurotoxicity: Role of oxidative stress. Toxicological and Environmental Chemistry. 2007;89:535–554. [Google Scholar]
- Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980;106:55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Rising L, Vitarella D, Kimelberg HK, Aschner M. Metallothionein induction in neonatal rat primary astrocyte cultures protects against methylmercury cytotoxicity. J Neurochem. 1995;65:1562–1568. doi: 10.1046/j.1471-4159.1995.65041562.x. [DOI] [PubMed] [Google Scholar]
- Roh JY, Jung IH, Lee JY, Choi J. Toxic effects of di(2-ethylhexyl)phthalate on mortality, growth, reproduction and stress-related gene expression in the soil nematode Caenorhabditis elegans. Toxicology. 2007;237:126–133. doi: 10.1016/j.tox.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Roh JY, Lee J, Choi J. Assessment of stress-related gene expression in the heavy metal-exposed nematode Caenorhabditis elegans: a potential biomarker for metal-induced toxicity monitoring and environmental risk assessment. Environ Toxicol Chem. 2006;25:2946–2956. doi: 10.1897/05-676r.1. [DOI] [PubMed] [Google Scholar]
- Sacco MG, Zecca L, Bagnasco L, Chiesa G, Parolini C, Bromley P, Cato EM, Roncucci R, Clerici LA, Vezzoni P. A transgenic mouse model for the detection of cellular stress induced by toxic inorganic compounds. Nat Biotechnol. 1997;15:1392–1397. doi: 10.1038/nbt1297-1392. [DOI] [PubMed] [Google Scholar]
- Sarafian T, Verity MA. Oxidative mechanisms underlying methyl mercury neurotoxicity. Int J Dev Neurosci. 1991;9:147–153. doi: 10.1016/0736-5748(91)90005-7. [DOI] [PubMed] [Google Scholar]
- Schlawicke Engstrom K, Stromberg U, Lundh T, Johansson I, Vessby B, Hallmans G, Skerfving S, Broberg K. Genetic variation in glutathione-related genes and body burden of methylmercury. Environmental health perspectives. 2008;116:734–739. doi: 10.1289/ehp.10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AM, Chan HM. Characterization of demethylation of methylmercury in cultured astrocytes. Chemosphere. 2008;74:112–118. doi: 10.1016/j.chemosphere.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Simmons-Willis TA, Koh AS, Clarkson TW, Ballatori N. Transport of a neurotoxicant by molecular mimicry: the methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Biochem J. 2002;367:239–246. doi: 10.1042/BJ20020841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. In: Hope IA, editor. C. elegans: A Practical Approach. Oxford University Press; New York: 1999. [Google Scholar]
- Sulston JE. Neuronal cell lineages in the nematode Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):443–452. doi: 10.1101/sqb.1983.048.01.049. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Tsui MT, Wang WX. Influences of maternal exposure on the tolerance and physiological performance of Daphnia magna under mercury stress. Environ Toxicol Chem. 2005;24:1228–1234. doi: 10.1897/04-190r.1. [DOI] [PubMed] [Google Scholar]
- van Rossum AJ, Brophy PM, Tait A, Barrett J, Jefferies JR. Proteomic identification of glutathione S-transferases from the model nematode Caenorhabditis elegans. Proteomics. 2001;1:1463–1468. doi: 10.1002/1615-9861(200111)1:11<1463::AID-PROT1463>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Weiss B, Clarkson TW, Simon W. Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environ Health Perspect. 2002;110(Suppl 5):851–854. doi: 10.1289/ehp.02110s5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PL, Dusenbery DB. Using the nematode Caenorhabditis elegans to predict mammalian acute lethality to metallic salts. Toxicol Ind Health. 1988;4:469–478. doi: 10.1177/074823378800400406. [DOI] [PubMed] [Google Scholar]
- Wood WB. Introduction to C. elegans Biology. In: W. B. Wood, Community of C. elegans Researchers, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, Cold Spring Harbor; New York: 1988. [Google Scholar]
- Yee S, Choi BH. Oxidative stress in neurotoxic effects of methylmercury poisoning. Neurotoxicology. 1996;17:17–26. [PubMed] [Google Scholar]
- Yoshida M, Shimizu N, Suzuki M, Watanabe C, Satoh M, Mori K, Yasutake A. Emergence of delayed methylmercury toxicity after perinatal exposure in metallothionein-null and wild-type C57BL mice. Environ Health Perspect. 2008;116:746–751. doi: 10.1289/ehp.10906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.