Abstract
Background
Associations between diagnosed unipolar depression, depressive symptoms, and cerebrovascular disease are well known. Yet, minimal research has investigated whether sex may modify such associations, despite known sex differences in depression and depressive symptoms. The present study examined whether depressive symptoms were disproportionately related to subclinical cerebrovascular disease in women versus men.
Methods
One hundred one older adults (58% male; mean age=67 years), free of major co-morbidities, completed the Beck Depression Inventory and underwent magnetic resonance imaging (MRI). MRI scans were neuroradiologist-rated for markers of subclinical cerebrovascular disease (SCD; periventricular and deep white matter hyperintensities, number of silent infarcts) and brain atrophy (ventricular enlargement, sulcal widening). We then created two rank-sum outcome variables (SCD, brain atrophy).
Results
On average, depressive symptoms were relatively low in magnitude (M=3.8, SD=3.6, range= 0–17). Multiple regression analyses, adjusted for age, sex, education, systolic blood pressure, fasting glucose, maximal oxygen consumption, body mass index, average weekly alcohol consumption, and Mini-Mental State Examination performance, revealed sex to be a significant effect modifier of depressive symptoms in the prediction of SCD. Sex-stratified regression analyses indicated depressive symptoms and SCD were strongly related among women, but not men. Depressive symptoms were not related to brain atrophy, regardless of inclusion of sex as an effect modifier.
Conclusions
Depressive symptoms, even in a subclinical range, are significantly associated with an MRI-derived index of SCD among women, but not men, in the present sample of relatively healthy older adults.
Keywords: depression, depressive symptoms, subclinical cerebrovascular disease, white matter hyperintensities, magnetic resonance imaging
Associations between unipolar depression and cerebrovascular disease among older adults are well recognized (1). These relations span the domains of both clinical (2) and subclinical (3) cerebrovascular disease (SCD). Select findings have been extended to the range of depressive symptoms, rather than clinical depression per se (4, 5), In contrast, the bulk of prior research does not support an association between depression and measures of generalized cerebral atrophy, including ventricular enlargement and sulcal widening (6), although regional volumetric differences have been identified (7).
Evidence suggests that vascular disease contributes etiologically to the onset of late-life depression, consistent with the “vascular depression hypothesis” (8, 9). Importantly, the relation is also bidirectional, such that depression may facilitate the development of cerebrovascular disease via numerous pathways, including hypercortisolemia, immune activation, increased platelet aggregation, endothelial dysfunction, abnormal folate metabolism, or behavioral factors such as treatment nonadherence (1, 10). In addition, depression and cerebrovascular disease may have shared genetic, biomedical (e.g., atherosclerosis), or behavioral etiologies. Causal inferences aside, both depression and cerebrovascular disease are common and compromise the health of our aging population (11–13).
Epidemiologic studies consistently replicate a higher prevalence of depression in adult women compared to adult men (14). Various hypotheses regarding the reasons for this sex difference exist, including variable reporting tendency and pathophysiologic differences of a biological, psychological, and/or social nature (15). Sex differences in the epidemiology of later life depression have been less well characterized, but at a minimum, the higher prevalence rates among women appear to persist (16). Sex differences in SCD and brain atrophy have also been identified, including higher prevalence of white matter lesions among elderly women in the population-based Rotterdam Scan Study (13) and increased brain atrophy with aging among men (17, 18). Moderation of depression by sex has typically been examined only in patients with clinical depression, clinical cerebrovascular disease, or both. For instance, women may be twice as likely to develop poststroke depression than men (19), but increased white matter hyperintensities among elderly, clinically depressed men have also been reported (20). In spite of this existing literature, little to no research has examined effect modification by sex in associations between depressive symptoms and SCD among healthy older adults.
The present study aimed to identify whether depressive symptoms were differentially associated with an index of SCD, comprised of periventricular white matter hyperintensities (WMH), deep WMH, and silent infarcts, in men versus women among a sample of relatively healthy, non-demented, community-dwelling older adults. We also sought to identify any depressive symptom-related sex difference in an index of global brain atrophy, comprised of ventricular enlargement and sulcal widening. We hypothesized that depressive symptoms and SCD would be more strongly associated among women than men; no association between depressive symptoms and brain atrophy was expected in either group.
Methods
Participants
Participants were 101 healthy, community-dwelling older adults (ages 54 to 81; 58% male, 89% white), with available data to date, who had participated in an ongoing parent study of relations of cardiovascular risk factors to structural and functional neuroimaging and cognitive performance. Participants were recruited by newspaper and other local advertisement, from the Geriatric Research Education and Clinical Center at the Baltimore Veterans Affairs Medical Center (B-VAMC) and by general advertisement at the B-VAMC. Inclusion criteria were age≥55 years1, absence of contraindication to magnetic resonance imaging, systolic blood pressure (SBP)<180 mm Hg, and diastolic blood pressure (DBP)106 mm Hg on at least two study visits. Exclusionary criteria were history or clinical evidence of cardiovascular disease (other than mild-to-moderate hypertension, defined as systolic blood pressure 140–179 mm Hg and/or diastolic blood pressure 90–105 mm Hg, diabetes mellitus, other major medical disease (e.g., renal, hepatic, pulmonary), neurologic disease, stroke, known or suspected dementia [Mini-Mental State Examination (MMSE) score <22], self-reported psychiatric disorder, heavy alcohol use (>14 drinks per week), severe head injury (loss of consciousness >30 minutes), or medications affecting central nervous system function. Power analysis was conducted using the statistical software G*Power, Version 3 (21). The available sample size of 101 participants was powered to detect a small to medium Cohen’s f2 estimate of .079 at conventional levels of power (.80) and alpha (.05), according to conventional guidelines (22). Participants provided written informed consent according to the guidelines of the University of Maryland, Baltimore and University of Maryland, Baltimore County’s Institutional Review Boards.
Depressive Symptoms
The Beck Depression Inventory, a well-validated measure among both community and clinical samples, assessed current level of depressive symptoms (23). This 21-item measure asks participants to rate a variety of symptoms of depression on a scale of 0 to 3. Scores range from 0 to 63, with higher values signifying higher levels of depressive symptoms.
Magnetic Resonance Imaging
Magnetic resonance imaging was performed utilizing a Phillips 1.5 Tesla scanner. The imaging protocol consisted of sagittal T1 (TR/TE/thickness/matrix/FOV/averages = 465/14/6 mm/192X256/24/1) axial T1 (600/14/5 mm/192X256/23/2), dual contrast proton density/T2 (3500/16,96/5 mm/192x256/23/2), and fluid attenuated inversion recovery (FLAIR) (TR/TE/TI/thickness/ matrix/FOV/averages = 8000/120/2200/5 mm/192x256/21/2) sequences. Images were rated blindly for periventricular white matter hyperintensities (WMH), deep WMH, number of silent infarctions, sulcal widening, and ventricular enlargement by a board-certified neuroradiologist (D.M.L.). Periventricular and deep white matter hyperintensities were rated using the method of Fazekas (24). Specifically, periventricular white matter hyperintensities were rated with the following coding scale: 0=absent; 1=cap; 2=band; and 3=irregular hyperintensity extending into the deep white matter. Deep white matter hyperintensities were rated as 0=absent, 1=punctuate, limited, 2=beginning confluent, and 3=confluent. Silent brain infarction was coded using modified Cardiovascular Health Study criteria (25); infarcts were defined as =3 millimeters in size. Brain atrophy was rated according to the apparent size of the ventricles (a measure of subcortical atrophy) and sulcal widening (an indication of cortical atrophy) using the following coding scale: 0=absent; 1=mild; 2=moderate; 3=severe.
Covariates
Participants underwent an extensive medical evaluation that included history, physical examination, blood chemistries, a graded exercise treadmill test, and an oral glucose tolerance test. Age and education were assessed in years. Total cholesterol and fasting glucose levels were determined enzymatically. Clinical assessment of blood pressure was performed on 2 to 3 occasions with patients in a seated position using an automated vital signs monitor (Dinamap Model #1846SX, Critikon, Tampa, FL) and appropriately sized occluding cuff. The readings were averaged to yield an estimate of participants’ resting SBP and DBP. Body mass index (BMI) was computed as measured weight in kilograms divided by height in meters squared. Maximal oxygen consumption (VO2max), an indicator of physical fitness, was obtained during exercise treadmill test. Alcohol consumption was assessed as the average number of drinks consumed per week over the past month, with one drink defined as 12 oz. beer, 4 oz. wine, or 1 oz. hard liquor. The Mini-Mental State Examination measured global cognitive status (26).
Data Reduction and Analysis
All statistical analyses were performed using SAS version 9.1 (Cary, NC). Principal components analysis of the five brain measures yielded two components with eigenvalues >1. Based on component loadings, the first component best represented periventricular WMH, deep WMH, and silent infarcts (hereafter labeled SCD), whereas the second component represented ventricular enlargement and sulcal widening (hereafter labeled brain atrophy). The linear combinations of the SCD variables and brain atrophy variables, respectively, were exceptionally skewed due to the ordinal categorical nature of the neuroradiologist ratings. To address this issue, we ranked each of the brain measures and used a normal scores transformation on the sum of the ranks of the three SCD variables and the two brain atrophy variables. These procedures yielded normalized composite indices of SCD and brain atrophy, to be used as the two outcome variables of all subsequent analyses.
T-tests were used to identify any group differences between the men and women in our sample. We then conducted ordinary least squares (OLS) general linear model (GLM) multiple regression analyses to examine relations of depressive symptoms with the rank-sum indices of SCD and brain atrophy. Due to positive skewness of the BDI score distribution, GLM analyses were also conducted with logarithmically (base 10) transformed BDI values. Covariates were selected based upon prior literature, to account for potential confounding by variables highly related to either depressive symptoms and/or brain outcomes (1, 27). Sex was treated categorically (0=women, 1=men), and all covariates (age, education, SBP, glucose, VO2max, BMI, alcohol use, and MMSE) were analyzed continuously. Separate multiple regression equations were constructed to examine effect modification of depressive symptoms by sex on SCD and brain atrophy individually. Significant interactive effects were followed up with sex-stratified regression analyses.
Results
Sample characteristics are displayed in Table 1. On average, participants reported relatively low levels of depressive symptoms. An independent samples t-test revealed that levels of depressive symptoms reported by men and women did not significantly differ (t(99)=1.06, p>.05). Additional t-tests revealed that men had higher SBP (t(99)=−3.44, p<.01), higher VO2max (t(99)=−3.86, p<.01), higher fasting glucose (t(99)=−2.55, p<.01), greater ventricular enlargement (t(99)=−4.80, p<.01), greater sulcal widening (t(99)=−2.08, p<.05), and greater brain atrophy rank sum scores (t(99)=−4.06, p<.01). Men and women were not statistically different on any other covariate or term of interest.
Table 1.
Characteristics of Study Sample
| Total sample (n=101) | Men (n=59) | Women (n=42) | ||||
|---|---|---|---|---|---|---|
| Variable | Mean (SD) or Percent |
Range | Mean (SD) or Percent |
Range | Mean (SD) or Percent |
Range |
| Age (years) | 66.7 (6.8) | 54–81 | 67.5 (6.55) | 54–81 | 65.4 (7.06) | 55–80 |
| Sex (% male) | 58.4 | -- | -- | -- | -- | -- |
| Race (% white) | 89.1 | -- | 93.2 | -- | 83.3 | -- |
| Education (years) | 16.4 (2.8) | 9–23 | 16.5 (2.85) | 9–22 | 16.4 (2.67) | 12–23 |
| SBP (mm Hg)* | 129 (17.7) | 95–169 | 133 (16.8) | 95–178 | 122 (16.8) | 99–169 |
| VO2max (ml/kg/min)* | 24.9 (7.05) | 11.7–48.7 | 27.1 (6.78) | 15.3–48.7 | 21.9 (6.34) | 11.7–44.8 |
| BMI (kg/m2) | 27.1 (4.96) | 18.0–42.5 | 27.6 (4.44) | 18.0–38.0 | 26.4 (5.59) | 18.6–42.5 |
| Fasting glucose (mg/dL)* | 93.0 (9.20) | 54–116 | 94.9 (8.06) | 79–116 | 90.3 (10.1) | 54–112 |
| Alcohol use† | 2.61 (3.26) | 0–14 | 2.93 (3.67) | 0–14 | 2.14 (2.56) | 0–10.5 |
| MMSE (total correct) | 29.2 (1.01) | 26–30 | 29.2 (1.03) | 26–30 | 29.2 (1.00) | 26–30 |
| BDI (total score) | 3.81 (3.59) | 0–17 | 3.49 (3.64) | 0–17 | 4.26 (3.51) | 0–14 |
| Periventricular WMH (rating) | 0.96 (0.65) | 0–3 | 0.88 (0.62) | 0–3 | 1.07 (0.68) | 0–3 |
| Deep WMH (rating) | 0.91 (0.66) | 0–3 | 0.88 (0.65) | 0–3 | 0.95 (0.70) | 0–3 |
| Total silent infarcts | 4.59 (8.62) | 0–40 | 3.80 (7.39) | 0–31 | 5.71 (10.09) | 0–40 |
| Ventricular enlargement (rating)* | 0.91 (0.80) | 0–2 | 1.20 (0.76) | 0–2 | 0.50 (0.67) | 0–2 |
| Sulcal widening (rating)* | 1.09 (0.57) | 0–2 | 1.19 (0.57) | 0–2 | 0.95 (0.54) | 0–2 |
| SCD (rank sum score) | 225 (90.4) | 63–445 | 214 (89.9) | 63–440 | 239 (90.1) | 63–445 |
| Brain atrophy (rank sum score)* | 150 (68.3) | 36–266 | 172 (66.8) | 36–266 | 120 (58.6) | 36–266 |
Means differ between men and women, p<.05;
average # of drinks per week over past month;
SD=standard deviation; SBP=systolic blood pressure; VO2max=maximal oxygen consumption; BMI=body mass index; MMSE=Mini Mental State Examination; BDI=Beck Depression Inventory; WMH=white matter hyperintensities; SCD=subclinical cerebrovascular disease
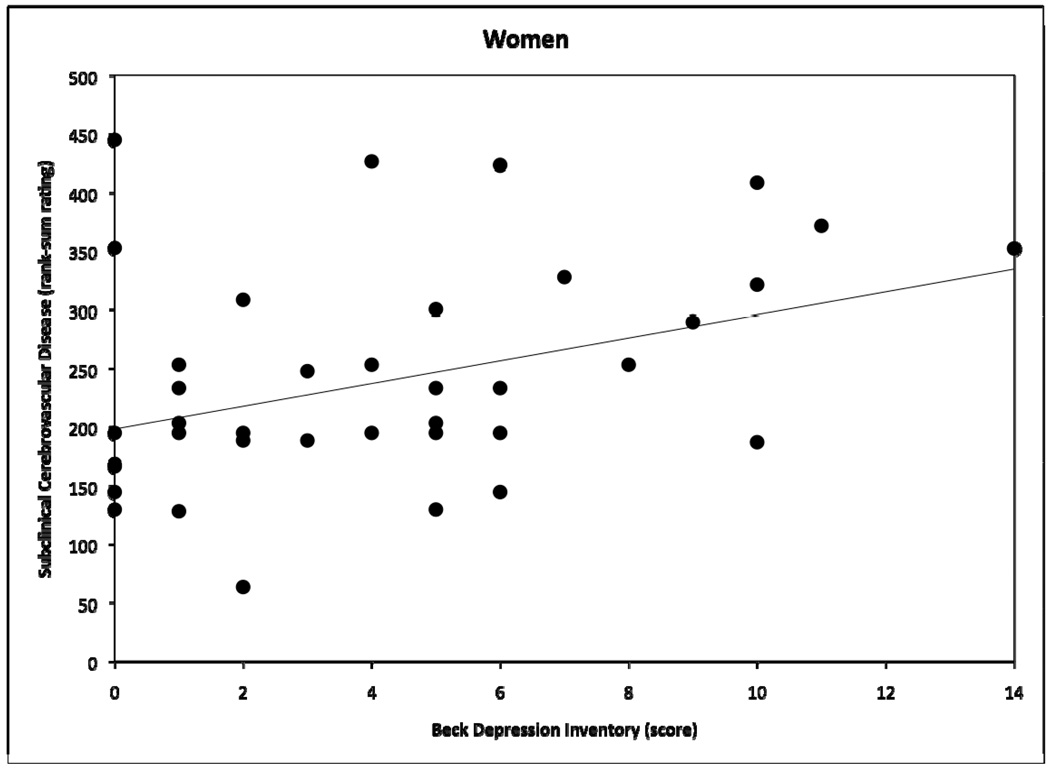
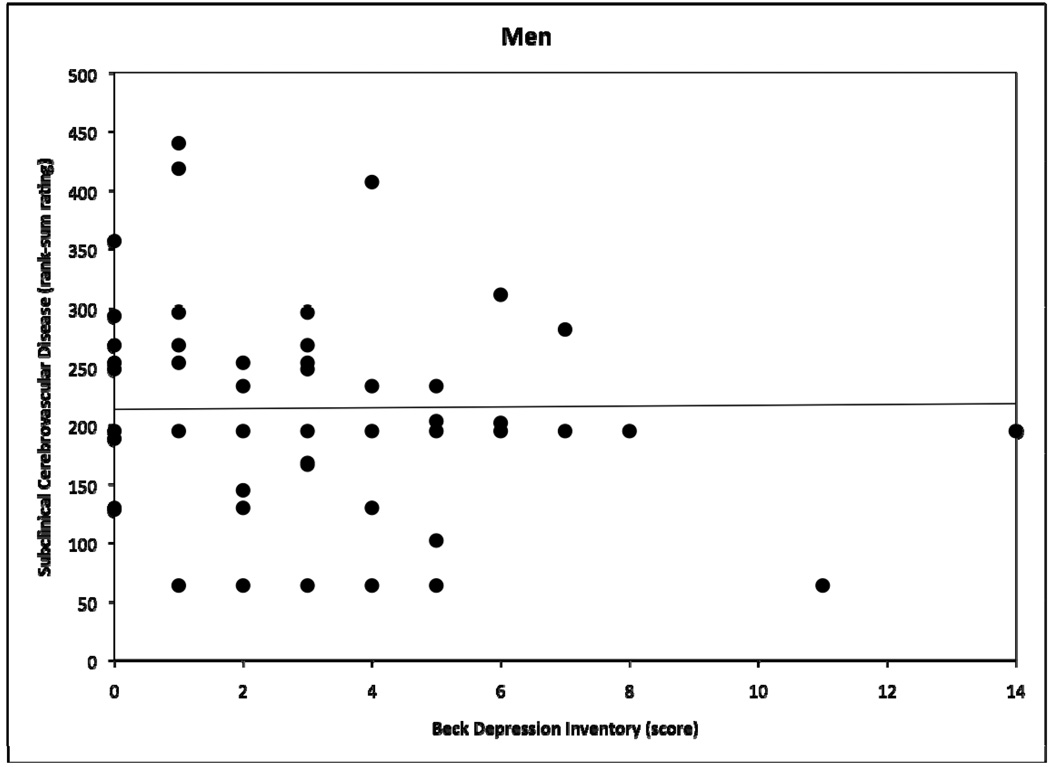
OLS GLM multiple regression analyses, adjusted for the aforementioned set of eight covariates, revealed a significant interactive effect of depressive symptoms × sex on SCD, but not brain atrophy. Age was significantly related to both SCD and brain atrophy. Table 2 shows coefficients for all terms in both models. Follow-up sex-stratified regression analyses showed that depressive symptoms and SCD were positively and strongly related among women (r sq=.17, b=13.2, t(32)=2.92 p<.01), but not men (r sq=.003, b=1.47, t(49)=0.42, p>.05). Figures 1 and 2 demonstrate raw BDI × SCD scatter plots for women and men, respectively. When BDI values were logarithmically transformed to attenuate positive skewness of the BDI score distribution, we observed no meaningful changes in these results. Inclusion of smoking status as a covariate also did not impact the results.
Table 2.
Coefficients from OLS Multiple Regression Models Relating Depressive Symptoms with Subclinical Cerebrovascular Disease and Brain Atrophy (n=101)
| Subclinical Cerebrovascular Disease |
Brain Atrophy | |||
|---|---|---|---|---|
| Variable | b† | SE | b† | SE |
| Intercept | 111 | 340 | −186 | 207 |
| Age | 3.74* | 1.64 | 5.80** | 1.00 |
| Sex | 26.3 | 33.1 | 18.7 | 20.1 |
| Education | −0.65 | 3.48 | 0.93 | 2.12 |
| SBP | −1.01 | 0.56 | 0.26 | 0.34 |
| BMI | −1.02 | 2.51 | 0.47 | 1.52 |
| VO2max | 0.88 | 1.95 | 0.81 | 1.19 |
| Glucose | 0.07 | 1.14 | 0.67 | 0.69 |
| Alcohol use | −1.21 | 2.83 | −0.19 | 1.72 |
| MMSE | −1.00 | 9.48 | −6.78 | 5.76 |
| BDI | 12.0** | 4.37 | −2.96 | 2.65 |
| BDI × sex | −11.7* | 5.36 | 2.30 | 3.26 |
p<.05,
p<.01;
Significance evaluated with t-tests (df=89);
SE=standard error; SBP=systolic blood pressure; BMI=body mass index; VO2max=maximal oxygen consumption; MMSE=Mini Mental State Examination; BDI=Beck Depression Inventory
Figure 1.
Scatter plot demonstrating relation between Beck Depression Inventory scores and subclinical cerebrovascular disease rank-sum ratings for women, with fitted regression line.
Figure 2.
Scatter plot demonstrating relation between Beck Depression Inventory scores and subclinical cerebrovascular disease rank-sum ratings for men, with fitted regression line.
Discussion
Our study is novel in its simultaneous examination of sex differences relative to a) the continuum of depressive symptoms associated with non-clinical depression and b) subclinical levels of cerebrovascular disease and brain atrophy among relatively healthy older adults. We found sex to be a significant effect modifier of the relation between depressive symptoms and SCD, such that depressive symptoms and SCD were significantly associated among women, but not men. Among women, depressive symptoms accounted for 17% of unique variance in SCD following adjustment for age, education, SBP, glucose, VO2max, BMI, alcohol use, and MMSE. This magnitude of an effect size is arguably clinically significant and is especially large for a psychological construct in the context of other key biomedical risk factors. Importantly, this finding does not appear to be explained by worse physical health or increased concurrent depressive symptoms among the women in the present sample. No associations between depressive symptoms and global brain atrophy were identified.
Clinical depression has repeatedly been linked with greater white matter hyperintensity burden in the elderly (3), and select studies have extended this finding to depressive symptoms among non-clinically depressed individuals (28). In fact, subclinical depressive symptoms have recently received increasing attention, as these symptoms are associated with similar clinical correlates as major depression, particularly in the elderly (29, 30). However, to our knowledge, sex differences have not been specifically examined in the context of subclinical depressive symptomatology and SCD. Previous studies have found that a similar distribution of men and women have “vascular” versus “nonvascular” depression (31, 32), but results from these studies cannot be directly compared to those from the present study because of discrepant sample characteristics (i.e., clinical versus non-clinical depression and cerebrovascular disease). Furthermore, these studies focused solely on presence or absence of a vascular etiology for depression, rather than depression as a putative risk factor for cerebrovascular disease.
Our significant finding of a relation between depressive symptoms and SCD among only women lends itself to several possible interpretations. In our sample, men and women displayed statistically equivalent depressive symptoms and SCD, yet depressive symptoms related to SCD only in women. Although not assessed directly here, chronic or repeated episodes of elevated depressive symptoms may predispose women to worse subclinical cerebrovascular health with aging. In other words, greater lifetime exposure to depressive symptoms among women may translate into poor later-life vascular brain outcomes, even subclinically, via the pathways mentioned previously (e.g., hypercortisolemia, atherosclerosis). Alternatively, women may be more susceptible to the affective consequences of poor cerebrovascular health. Depressive symptoms may simply not be as relevant to the development of SCD in men, despite higher base rates of cardiovascular disease among men, or men may be less vulnerable to cerebrovascular-related depressive symptomatology on a subclinical level.
This investigation offers several strengths. First, growing evidence underscores the importance of examining both subclinical depressive symptoms (33) and subclinical cerebrovascular disease (34), and our study combines both of these aims. Second, our sample particularly lends itself to examination of subclinical disease, given its reasonably stringent inclusion and exclusion criteria. Utilization of a relatively healthy community-dwelling sample limits confounding by the varied affective and cognitive consequences of clinical medical, neurologic, and psychiatric disease. Third, few studies to date have cited sex differences as a primary aim of analyses in this area. A number of studies identifying relations between depressive symptoms and SCD neither provide sex-stratified sample characteristics nor consider sex as an effect modifier. This omission imparts the possibility that underlying sex differences remained undetected in the presence of a significant main effect of depressive symptoms.
Limitations of this investigation include its cross-sectional design, reliance on a single measure of depressive symptoms, a relatively homogeneous sample, utilization of the Fazekas scale (as opposed to more recently developed measures), and unavailability of more specific regional measures of subclinical cerebrovascular disease and brain atrophy. Our reliance on self-reported psychiatric history as a study exclusion is a major limitation, as psychiatric conditions are likely to be underreported due to associated stigma. However, participants were able to engage in an extensive six- to seven-session protocol, which lessens the probability that undiagnosed psychiatric conditions were included in the sample. Lastly, although these data may offer viable hypotheses with respect to potential pathogenic mechanisms, they do not directly translate into treatment recommendations. Additional research is necessary to determine such implications.
Future research should also address whether our findings translate to other samples of relatively healthy older adults. Longitudinal examinations would be highly beneficial to establish temporal associations between depressive symptoms and SCD. Mechanistic work linking particular genetic, biomedical, and behavioral pathways with depressive symptoms and SCD is also much needed. At a minimum, future researchers should consider providing sex-stratified results to determine whether existing sex differences in depression and brain outcomes clarify our current understanding of associations among these variables.
Summary
In sum, depressive symptoms and SCD were strongly associated among our sample of community-dwelling, relatively healthy older women, but not men. Depressive symptoms and global brain atrophy were unrelated in both men and women. Depressive symptoms may play a greater role in the development of SCD among women due to greater cumulative lifetime exposure to depressive symptoms, or women may be more vulnerable than men to the affective effects of SCD. Regardless, women may be at greater risk for a critical interplay between depressive symptoms and SCD. Attention to this sex difference is important in light of the potential for different explanatory mechanisms, and thus different intervention targets, for men versus women presenting with depressive symptoms or SCD.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants R29 AG15112, 2RO1 AG015112, Bristol Myers Squibb Medical Imaging, Inc., NIH K24 AG00930, a VA Merit Grant, the Department of Veterans Affairs Baltimore Geriatric Research Education and Clinical Center, and the Geriatrics and Gerontology Education and Research Program of the University of Maryland, Baltimore.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous Presentation: Partial results from this paper were previously presented at the 30th Annual Meeting of the Society of Behavioral Medicine, Montreal Canada in April 2009.
Conflicts of Interest: The authors have no disclosures to report.
One exception was made to this inclusion criterion; one 54-year-old was enrolled who was soon turning 55.
References
- 1.Kales HC, Maixner DF, Mellow AM. Cerebrovascular disease and late-life depression. Am J Geriatr Psychiatry. 2005;13:88–98. doi: 10.1176/appi.ajgp.13.2.88. [DOI] [PubMed] [Google Scholar]
- 2.Williams LS. Depression and stroke: cause or consequence? Semin Neurol. 2005;25:396–409. doi: 10.1055/s-2005-923534. [DOI] [PubMed] [Google Scholar]
- 3.Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79:619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien JT, Firbank MJ, Krishnan MS, et al. White matter hyperintensities rather than lacunar infarcts are associated with depressive symptoms in older people: the LADIS study. Am J Geriatr Psychiatry. 2006;14:834–841. doi: 10.1097/01.JGP.0000214558.63358.94. [DOI] [PubMed] [Google Scholar]
- 5.Everson SA, Roberts RE, Goldberg DE, et al. Depressive symptoms and increased risk of stroke mortality over a 29-year period. Arch Intern Med. 1998;158:1133–1138. doi: 10.1001/archinte.158.10.1133. [DOI] [PubMed] [Google Scholar]
- 6.Soares JC, Mann JJ. The anatomy of mood disorders--review of structural neuroimaging studies. Biol Psychiatry. 1997;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 7.Beyer JL, Krishnan KR. Volumetric brain imaging findings in mood disorders. Bipolar Disord. 2002;4:89–104. doi: 10.1034/j.1399-5618.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60:1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos GS, Meyers BS, Young RC, et al. 'Vascular depression' hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin RC. Is vascular depression a distinct sub-type of depressive disorder? A review of causal evidence. Int J Geriatr Psychiatry. 2005;20:1–11. doi: 10.1002/gps.1255. [DOI] [PubMed] [Google Scholar]
- 11.Beyer JL. Managing depression in geriatric populations. Ann Clin Psychiatry. 2007;19:221–238. doi: 10.1080/10401230701653245. [DOI] [PubMed] [Google Scholar]
- 12.Lebowitz BD, Pearson JL, Schneider LS, et al. Diagnosis and treatment of depression in late life. Consensus statement update. JAMA. 1997;278:1186–1190. [PubMed] [Google Scholar]
- 13.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessler RC, McGonagle KA, Swartz M, et al. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 15.Grigoriadis S, Robinson GE. Gender issues in depression. Ann Clin Psychiatry. 2007;19:247–255. doi: 10.1080/10401230701653294. [DOI] [PubMed] [Google Scholar]
- 16.Mulsant BH, Ganguli M. Epidemiology and diagnosis of depression in late life. J Clin Psychiatry. 1999;60 Suppl 20:9–15. [PubMed] [Google Scholar]
- 17.Gur RC, Gunning-Dixon FM, Turetsky BI, et al. Brain region and sex differences in age association with brain volume: a quantitative MRI study of healthy young adults. Am J Geriatr Psychiatry. 2002;10:72–80. [PubMed] [Google Scholar]
- 18.Coffey CE, Lucke JF, Saxton JA, et al. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol. 1998;55:169–179. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- 19.Paradiso S, Robinson RG. Gender differences in poststroke depression. J Neuropsychiatry Clin Neurosci. 1998;10:41–47. doi: 10.1176/jnp.10.1.41. [DOI] [PubMed] [Google Scholar]
- 20.Lavretsky H, Lesser IM, Wohl M, et al. Relationship of age, age at onset, and sex to depression in older adults. Am J Geriatr Psychiatry. 1998;6:248–256. [PubMed] [Google Scholar]
- 21.Faul F, Erdfelder E, Lang AG, et al. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Beh Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J, editor. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 23.Beck AT. Beck Depression Inventory: Manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 24.Fazekas F, Niederkorn K, Schmidt R, et al. White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke. 1988;19:1285–1288. doi: 10.1161/01.str.19.10.1285. [DOI] [PubMed] [Google Scholar]
- 25.Price TR, Manolio TA, Kronmal RA, et al. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1997;28:1158–1164. doi: 10.1161/01.str.28.6.1158. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nebes RD, Vora IJ, Meltzer CC, et al. Relationship of deep white matter hyperintensities and apolipoprotein E genotype to depressive symptoms in older adults without clinical depression. Am J Psychiatry. 2001;158:878–884. doi: 10.1176/appi.ajp.158.6.878. [DOI] [PubMed] [Google Scholar]
- 29.Lavretsky H, Kumar A. Clinically significant non-major depression: old concepts, new insights. Am J Geriatr Psychiatry. 2002;10:239–255. [PubMed] [Google Scholar]
- 30.Lyness JM, Kim J, Tang W, et al. The clinical significance of subsyndromal depression in older primary care patients. Am J Geriatr Psychiatry. 2007;15:214–223. doi: 10.1097/01.JGP.0000235763.50230.83. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 32.Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562–565. doi: 10.1176/ajp.154.4.562. [DOI] [PubMed] [Google Scholar]
- 33.Lyness JM, Heo M, Datto CJ, et al. Outcomes of minor and subsyndromal depression among elderly patients in primary care settings. Ann Intern Med. 2006;144:496–504. doi: 10.7326/0003-4819-144-7-200604040-00008. [DOI] [PubMed] [Google Scholar]
- 34.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]