Abstract
Oseltamivir and peramivir are being considered for combination treatment of serious influenza virus infections in humans. Both compounds are influenza virus neuraminidase inhibitors, and since peramivir binds tighter to the enzyme than oseltamivir carboxylate (the active form of oseltamivir), the possibility exists that antagonistic interactions might result when using the two compounds together. To study this possibility, combination chemotherapy experiments were conducted in vitro and in mice infected with influenza A/NWS/33 (H1N1) virus. Treatment of infected MDCK cells was performed with combinations of oseltamivir carboxylate and peramivir at 0.32-100 μM for 3 days, followed by virus yield determinations. Additive drug interactions with a narrow region of synergy were found using the MacSynergy method. In a viral neuraminidase assay with combinations of inhibitors at 0.01-10 nM, no significant antagonistic or synergistic interactions were observed across the range of concentrations. Infected mice were treated twice-daily for 5 days starting 2 hours prior to virus challenge using drug doses of 0.05-0.4 mg/kg/day. Consistent and statistically significant increases in the numbers of survivors were seen when twice daily oral oseltamivir (0.4 mg/kg/day) was combined with twice daily intramuscular peramivir (0.1 and 0.2 mg/kg/day) compared to single drug treatments The data demonstrate that combinations of oseltamivir and peramivir perform better than suboptimal doses of each compound alone to treat influenza infections in mice. Treatment with these two compounds should be considered as an option.
Keywords: Drug combination, Oseltamivir carboxylate, Peramivir, Synergy, Antiviral
1. Introduction
Combinations of antiviral agents are being explored for the treatment of influenza virus infections as a means to improve efficacy and to help suppress the emergence of drug resistant viruses. The recent H1N1 pandemic starting in the spring of 2009 (Centers for Disease Control and Prevention, 2009) highlights the need for effective antiviral therapy in a largely naïve population. Three classes of anti-influenza virus compounds with different modes of action have been identified. They are viral neuraminidase inhibitors (i.e. oseltamivir carboxylate, zanamivir and peramivir), viral M2 channel blockers (i.e. amantadine and rimantadine), and viral RNA polymerase inhibitors (i.e. ribavirin, viramidine and T-705 [favipiravir]). Amantadine and rimantadine have been rendered largely ineffective due to the high prevalence of resistant viruses in nature (Cheung et al., 2006; Ilyushina et al., 2006; Deyde et al., 2007; Hata et al., 2007; Mossad, 2009). After many years of only low prevalence of oseltamivir resistance, a transmissible seasonal H1N1 virus variant carrying the H275Y resistance mutation emerged in 2007 in Europe and spread worldwide until early 2009 (Besselaar et al., 2008; Dharan et al., 2009; Meijer et al., 2009). This seasonal H1N1 virus disappeared during 2009, concomitantly with the emergence of the 2009 pandemic H1N1 virus, sensitive to oseltamivir (Wang et al., 2010). Other drug- resistant virus variants may continue to emerge in the future, requiring new treatment strategies that may include combination treatments. Studies have been reported using combinations of compounds from these different classes either as double drug (Galabov et al., 2006; Ilyushina et al., 2007, 2008; Smee et al., 2002, 2009) or, more recently, triple drug (Nguyen et al., 2009, 2010) combinations. Treatment of influenza virus infections with these combinations has generally resulted in additive to synergistic interactions.
Because oseltamivir carboxylate and peramivir are both viral neuraminidase inhibitors, the use of these two agents together would not be anticipated to produce synergistic interactions. Additivity would be more likely. Peramivir has a tighter binding affinity to the neuraminidase than oseltamivir carboxylate (Bantia et al., 2006). Oseltamivir and peramivir both bind to the neuraminidase active site. Contributions of active site residues to binding affinity for both compounds differ slightly, which results in a slightly different resistance profile. Compound specific resistance mutations have been observed, e.g. E119V in N2 neuraminidase conferred resistance to oseltamivir, but not peramivir, whereas E119D conferred higher resistance to peramivir (Mishin et al., 2005; Hurt et al., 2006). Other viral isolates resistant to both inhibitors have been identified (Gubareva et al., 2001; Memoli et al., 2010; Okomo-Adhiambo et al., 2010) Antivir Res 85:381-388. Most importantly, H275Y mutations in N1 neuraminidase confer resistance to both oseltamivir and peramivir.
A clear advantage to the use of oseltamivir to treat patients is its oral bioavailability (Li et al., 1998). Peramivir is orally active in mice (Bantia et al., 2001; Sidwell et al., 2001), but proved to be very poorly absorbable by this route in humans (Barroso et al., 2005; Bantia et al., 2006). For this reason, recent studies have focused on treatment with peramivir by intramuscular injection (Bantia et al., 2006; Boltz et al., 2008; Yun et al., 2008). Intravenous studies with peramivir in hospitalized patients are underway.
The purpose of the present investigation was to explore whether the use of oseltamivir combined with peramivir would prove to be adverse or beneficial in treating influenza virus infections. Studies were conducted in vitro and in mice infected with an oseltamivir- sensitive influenza A (H1N1) virus.
2. Materials and methods
2.1. Compounds
Oseltamivir carboxylate was kindly provided by Roche, Basel, Switzerland. Oseltamivir phosphate (as Tamiflu® capsules, here referred to merely as oseltamivir), the orally active prodrug form of oseltamivir carboxylate, was purchased from a local pharmacy. Peramivir was provided by Biocryst Pharmaceuticals (Birmingham, AL). Oseltamivir carboxylate and peramivir were dissolved in cell culture medium for in vitro studies. Oseltamivir was used for animal studies. Because oseltamivir was obtained from pharmaceutical capsules that also contained other ingredients as filler material besides the drug, the contents of entire capsules minus the shell were added to sterile water to make up the highest mg/kg/day dose of drug for oral administration. Lower doses of oseltamivir were made by dilution into sterile water. We recently demonstrated that oseltamivir from Tamiflu is as active as the pure ingredient, oseltamivir phosphate (Smee et al., 2010). The mg/kg/day doses of oseltamivir reported here represent the active form of the drug (75 mg in the capsule), not the prodrug form. Peramivir was prepared in sterile saline for intramuscular (i.m.) treatment of mice. The i.m. route for peramivir was selected based upon the published literature.
2.2. Virus
Influenza A/NWS/33 (H1N1) was originally obtained from Kenneth Cochran (University of Michigan, Ann Arbor). The virus was passaged 3 times in mice and one time in Madin-Darby canine kidney (MDCK) cells (obtained from the American Type Culture Collection, Manassas, VA). The virus pool was pre-titrated in MDCK cells and in mice prior to performing these studies to determine appropriate doses.
2.3. Cell culture antiviral studies
Antiviral activities of oseltamivir carboxylate and peramivir were determined in confluent cultures of MDCK cells. The assays were performed in 96-well microplates infected with approximately fifty 50% cell culture infectious doses (CCID50) of virus, by quantifying virus yield after three days in culture. The plates of samples were frozen at - 80°C. Medium from two microwells were later pooled and used to produce samples for titration. Virus yields at each inhibitor concentration were determined by titration of samples (in 10-fold dilution increments) on fresh monolayers of MDCK cells in 96-well microplates by endpoint dilution method (Reed and Muench, 1938) using four microwells per dilution. Microplates were examined at 3 and 6 days of infection for the presence or absence of viral cytopathology. Virus titers were expressed as log10 CCID50 per 0.1 ml.
2.4. Viral neuraminidase inhibition assay
The effects of compounds on viral neuraminidase activity were determined using a commercially available kit (NA-Star® Influenza Neuraminidase Inhibitor Resistance Detection Kit, Applied Biosystems, Foster City, CA) in 96-well solid white microplates following the Manufacturer's instructions and as has been reported (Smee et al., 2010). Compounds in half-log dilution increments were incubated with virus (as the source of neuraminidase). The amount of influenza A/NWS/33 (H1N1) virus in each microwell was approximately 500 cell culture infectious doses. Plates were pre-incubated for 10 min at 37°C prior to addition of chemiluminescent substrate. Following addition of substrate the plates were incubated for 30 min at 37°C. The neuraminidase activity was evaluated using a Centro LB 960 luminometer (Berthold Technologies, Oak Ridge, TN) for 0.5 sec immediately after addition of NA-Star® accelerator solution. Percentages of chemiluminescent counts at each compound concentration were based upon counts normalized to 100% under untreated conditions.
2.5. Animal experiment design
Female BALB/c mice (18-20 g, Charles River Labs, Wilmington, MA) were anesthetized by i.p. injection of ketamine (100 mg/kg) followed by intranasal infection with a 50-μl suspension of influenza virus; the infection inoculation of approximately 104.5 CCID50/mouse equaled three 50% mouse lethal challenge doses (MLD50). Compounds were administered p.o. (oseltamivir) by gavage or i.m. (peramivir) twice a day at 12-hour intervals for 5 days starting 2 hours before virus challenge. Placebo-treated mice received both p.o. and i.m. treatments. Ten drug-treated infected mice and 10 placebo-treated controls were observed daily for death through 21 days. Mice that died during the treatment phase were excluded from the total count. Body weights were determined every other day.
2.6. Drug combination analysis
Drug-drug interactions were analyzed by the three-dimensional model of Prichard and Shipman (1990), using the MacSynergy II software program. Theoretical additive interactions were calculated from the dose-response curves for each compound used individually. This additive surface was subtracted from the actual dose-response curve to give regions of non-additive interactions. These are expressed graphically and also reported as volumes of synergy or antagonism. The MacSynergy software automatically calculates volumes of synergy or antagonism for each three-dimensional plot of data. Synergy plots and volumes of synergy/antagonism were made at the 95% confidence limit. Descriptions of synergistic or antagonistic interactions using this computer model based upon volumes of synergy or antagonism have been defined for data represented as percentages (Ilyushina et al., 2008). Briefly, 0-25, 25-50, 50-100, and >100 μm2 unit % calculated values in either a positive or negative direction using MacSynergy software are defined as insignificant synergy or antagonism (indifference), minor synergy or antagonism, moderate synergy or antagonism, or strong synergy or antagonism, respectively. Definitions of what is considered weak, moderate, or strong antagonism or synergism have not been defined in the published literature for logarithmic data (such as viral titers).
2.7. Statistical analysis for animal studies
Survival curves comparisons of all groups were analyzed by the Mantel-Cox Log-rank test. Pairwise comparisons of differences in numbers of survivors were made by the two-tailed Fisher's exact test. The two-tailed Mann-Whitney U-test made pairwise comparisons of the mean day of death. The analysis of survival curves was made using Prism software, whereas the other tests were performed using InStat software, both from GraphPad, Inc. (San Diego, CA). Most comparisons were made between placebo and treated groups, and between combination treatment versus single drug treatment.
3. Results
3.1. Antiviral activity in cell culture
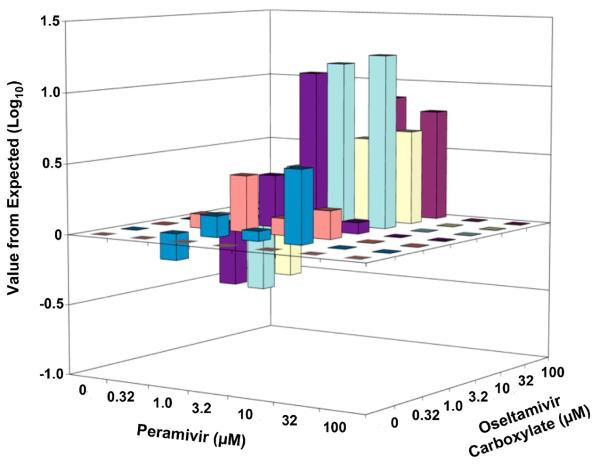
Oseltamivir carboxylate and peramivir were evaluated in combination for inhibition of virus yield in MDCK cell cultures using doses of 0.32 to 100 μM (Table 1). Oseltamivir carboxylate alone reduced virus yield by 4.4 log10 at 100 μM. Peramivir at 32 and 100 μM reduced virus yield by ≥ 5 log10 below the detection limit of the assay. Greater than 10-fold inhibition of virus titer from expected was found at three specific conditions, when 10 μM oseltamivir carboxylate was combined with either 3.2 or 10 μM peramivir, and using the combination of 3.2 μM of each inhibitor. A three-dimensional MacSynergy plot of the data showing values above and below expected are shown in Figure 1. A region of significant synergy was evident between 1 and 10 μM oseltamivir and 1 and 10 μM peramivir, giving a volume of synergy of 9.1. A region of minor antagonism occurred when 0.32 μM peramivir was combined with 3.2-32 μM oseltamivir carboxylate, for a calculated volume of antagonism of −1.7. The net effect across the entire surface was a volume of synergy of 7.4.
Table 1.
Combination of oseltamivir carboxylate and peramivir against influenza A/NWS/33 (H1N1) virus infections in MDCK cell culture.
| Oseltamivir Carboxylate (μM) |
Peramivir (μM) |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.32 | 1.0 | 3.2 | 10 | 32 | 100 | |
| 100 | 0.9 ± 1.0a | 0.7 ± 1.0 | 0.8 ± 1.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 32 | 2.2 ± 2.0 | 2.6 ± 2.4 | 2.2 ± 2.1 | 1.6 ± 1.8 | 0.8 ± 1.7 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 10 | 4.2 ± 1.0 | 4.6 ± 0.9 | 3.7 ± 0.9 | 2.6 ± 1.3 | 0.3 ± 0.6 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 3.2 | 4.5 ± 0.9 | 4.9 ± 0.9 | 4.0 ± 0.8 | 2.8 ± 0.6 | 1.4 ± 2.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 1.0 | 5.3 ± 0.6 | 4.8 ± 0.8 | 4.4 ± 0.7 | 3.9 ± 1.1 | 1.3 ± 1.8 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 0.32 | 5.2 ± 0.7 | 5.1 ± 0.8 | 4.6 ± 1.1 | 3.9 ± 1.4 | 1.0 ± 1.4 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 0 | 5.3 ± 0.6 | 5.2 ± 0.6 | 4.9 ± 1.0 | 4.0 ± 1.0 | 1.5 ± 2.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Values are mean virus titers (log10 CCID50/0.1 ml) ± SD for five independent experiments.
Figure 1.
Three-dimensional plot of the interaction of oseltamivir carboxylate and peramivir on influenza A/NWS/33 (H1N1) virus titers produced from MDCK cells. The MacSynergy plot was derived from the data in Table 1. The synergy or antagonism for the various combinations was significant at the 95% confidence limit.
The degree of synergy exhibited here for these logarithmic data is similar to that reported for other drug combinations (Nguyen et al., 2009).
3.2. Viral neuraminidase inhibition studies
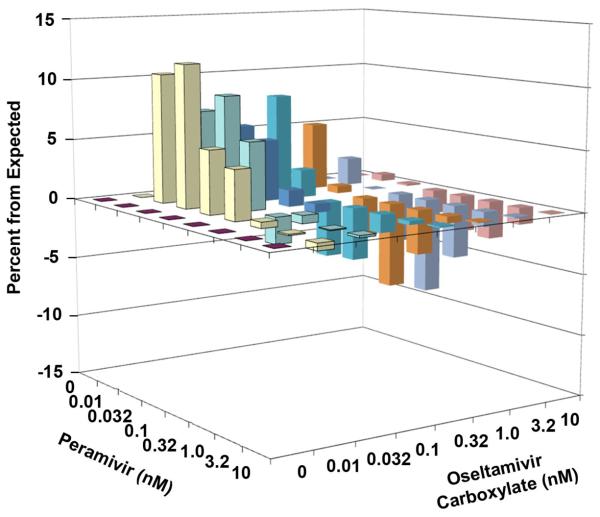
The effects of the combination of oseltamivir carboxylate and peramivir on neuraminidase activity are presented in Table 2. Minimal neuraminidase activity was evident in the presence of 10 nM oseltamivir carboxylate treatment or 1 to 10 nM peramivir treatment. The majority of the low-dose combinations (0.01 to 3.2 nM oseltamivir carboxylate combined with 0.01 to 0.32 nM peramivir) caused greater inhibition than either compound used alone. Higher concentrations of each inhibitor used in combination (0.32 to 10 nM) caused less inhibition than expected. This was in a region where peramivir alone was highly inhibitory to enzymatic activity, with not much potential for further inhibition by a drug combination. The three-dimensional MacSynergy plot of the data is shown in Figure 2. The percentages of increase or decrease for the combinations were small. The low-dose combination region had a volume of synergy of 86 (moderate synergy), whereas the high-dose combination region had a volume of antagonism of −65 (moderate antagonism) for a net effect across the entire surface of 21 (indifference).
Table 2.
Combinations of oseltamivir carboxylate and peramivir against influenza A/NWS/33 (H1N1) neuraminidase.
| Oseltamivir Carboxylate (nM) |
Peramivir (nM) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.01 | 0.032 | 0.1 | 0.32 | 1.0 | 3.2 | 10 | |
| 10 | 7 ± 0.3a | 6 ± 0.5 | 6 ± 0.4 | 6 ± 0.9 | 5 ± 1.1 | 4 ± 0.4 | 2 ± 0.4 | 1 ± 0.2 |
| 3.2 | 18 ± 1.1 | 16 ± 1.3 | 15 ± 0.4 | 15 ± 1.3 | 12 ± 1.3 | 6 ± 0.2 | 2 ± 0.4 | 1 ± 0.1 |
| 1.0 | 39 ± 3.5 | 34 ± 2.8 | 33 ± 2.4 | 26 ± 2.7 | 15 ± 1.6 | 6 ± 0.6 | 2 ± 0.3 | 1 ± 0.2 |
| 0.32 | 65 ± 6.9 | 58 ± 6.5 | 55 ± 0.8 | 43 ± 4.4 | 18 ± 1.3 | 5 ± 0.5 | 2 ± 0.3 | 1 ± 0.2 |
| 0.1 | 88 ± 6.7 | 84 ± 4.6 | 72 ± 4.0 | 50 ± 3.6 | 18 ± 1.2 | 5 ± 0.5 | 2 ± 0.3 | 1 ± 0.2 |
| 0.032 | 99 ± 3.7 | 93 ± 9.1 | 78 ± 2.8 | 52 ± 7.1 | 23 ± 4.2 | 4 ± 1.0 | 2 ± 0.3 | 1 ± 0.3 |
| 0.01 | 103 ± 3.0 | 95 ± 4.1 | 79 ± 7.8 | 55 ± 6.8 | 18 ± 2.2 | 4 ± 0.2 | 2 ± 0.2 | 1 ± 0.9 |
| 0 | 100 ± 0.0 | 102 ± 5.1 | 87 ± 8.3 | 58 ± 6.6 | 21 ± 2.1 | 5 ± 0.4 | 2 ± 0.1 | 1 ± 0.2 |
Values are percentages of untreated control ± SD for three independent assays.
Figure 2.
Three-dimensional plot of the interaction of oseltamivir carboxylate and peramivir on influenza A/NWS/33 (H1N1) neuraminidase activity. The MacSynergy plot was derived from the data in Table 2. The synergy or antagonism for the various combinations was significant at the 95% confidence limit.
3.3. Animal chemotherapy experiments
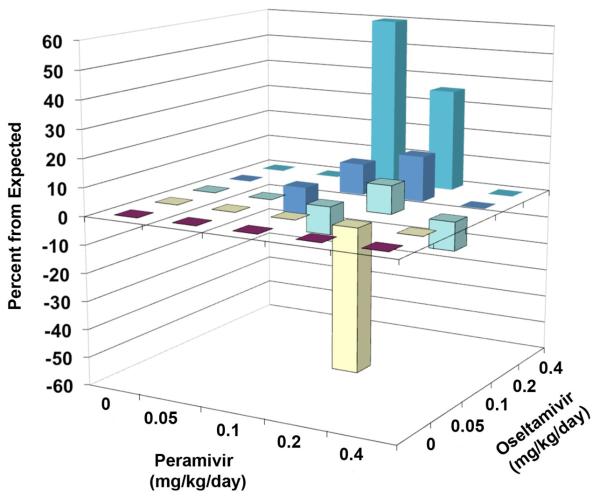
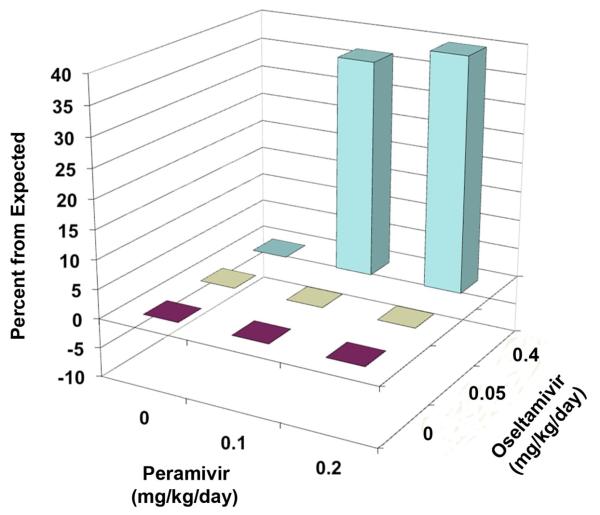
Results of combination treatment of a lethal infection in mice with various doses of oseltamivir and peramivir are reported in Table 3. Oseltamivir alone at 1 mg/kg/day protected 70% of mice from death, 0.4 and 0.2 mg/kg/day were 10% protective and lower doses showed no protection. Peramivir alone was 100% protective at 0.4 and 1 mg/kg/day, 60% protective at 0.2 mg/kg/day, 10% protective at 0.1 mg/kg/day, and not protective at lower doses. The 0.4 mg/kg/day dose of oseltamivir combined with 0.1 to 0.2 mg/kg/day doses of peramivir resulted in 80% and 100% protection, respectively. The effect at combination of 0.4 mg/kg/day oseltamivir plus 0.1 mg/kg/day peramivir (80% protection) substantially exceeded that observed by the sum of survivors of either compound alone (20% protection). The 0.2 mg/kg/day dose of oseltamivir combined with 0.1 or 0.2 mg/kg/day peramivir was 33% and 80% protective, respectively. The effect of treatment with 0.1 mg/kg/day oseltamivir combined with 0.2 mg/kg/day peramivir resulted in 70% survival. This was not significantly different from treatment with peramivir alone (60% survival). The 0.05 mg/kg/day dose of oseltamivir combined with 0.2 mg/kg/day peramivir produced fewer (10%) survivors than 0.2 mg/kg/day peramivir alone (60%). Although not statistically significant, this result either reflected a possibility of antagonism, or an outlier due to dose-effect variability. A MacSynergy plot of the data is shown in Figure 3, showing points of synergy (0.4 mg/kg/day oseltamivir plus 0.1 or 0.2 mg/kg/day peramivir) and antagonism (0.05 mg/kg/day oseltamivir plus 0.2 mg/kg/day peramivir). Volumes of synergy and antagonism for these results were 134 and −80, respectively, for a net volume of synergy across the entire surface of 54.
Table 3.
Survival results for the treatment of an influenza A/NWS/33 (H1N1) virus infection in mice with combinations of oseltamivir and peramivir. Intramuscular treatments with peramivir and oral treatments with oseltamivir were given twice a day for 5 days starting 2 hours prior to virus exposure.
| Survivors / Total (MDDa ± SD) |
||||||
|---|---|---|---|---|---|---|
| Peramivir, mg/kg/day |
||||||
| Oseltamivir Carboxylate, mg/kg/day |
0 | 0.05 | 0.1 | 0.2 | 0.4 | 1 |
| 1 | 7/10*** (14.1 ± 2.6**) |
- | - | - | - | - |
| 0.4 | 1/10 (11.0 ± 1.6***) |
1/9 (12.0 ± 2.1***) |
8/10***, ϕ (13.0 ± 4.2**) |
10/10*** | 10/10*** | - |
| 0.2 | 1/10 (9.8 ± 1.1**) |
0/10 (10.2 ± 0.6***) |
3/9* (16.5 ± 3.1***, ϕ) |
8/10*** (14.5 ± 2.1**) |
10/10*** | - |
| 0.1 | 0/10 (9.2 ± 0.6) |
0/10 (10.1 ± 0.9***) |
0/10 (11.3 ± 2.1***) |
7/10*** (12.3 ± 2.3**) |
9/10*** (10.0) |
- |
| 0.05 | 0/10 (9.2 ± 1.3) |
0/10 (10.2 ± 1.0***) |
1/10 (12.3 ± 3.9**) |
1/10ψ (12.3 ± 1.8***) |
10/10*** | - |
| 0 | 0/20 (8.7 ± 0.5) |
0/10 (9.4 ± 0.8*) |
1/10 (10.1 ± 1.5**) |
6/10*** (10.8 ± 1.5***) |
10/10*** | 10/10*** |
Mean day of death of mice that died prior to day 21 of the infection.
P<0.05,
P<0.01,
P<0.001, compared to placebo (oseltamivir - 0/peramivir - 0).
P<0.05, compared to either compound alone.
P= 0.0573 (not quite significant), compared to peramivir alone.
Figure 3.
Three-dimensional plot of the impact of oseltamivir and peramivir on the number of surviving mice from an influenza A/NWS/33 (H1N1) infection. The MacSynergy plot was derived from the data in Table 3. Two points of synergy and one point of antagonism were significant at the 95% confidence limit.
Mean day of death determinations for the experiment are also shown in Table 3. The majority of single drug treatments and combination chemotherapy doses significantly increased the mean day of death compared to the placebo group. Treatment with the drugs in combination resulted in longer delays in the time to death than either compound used alone, although most comparisons were not statistically significant.
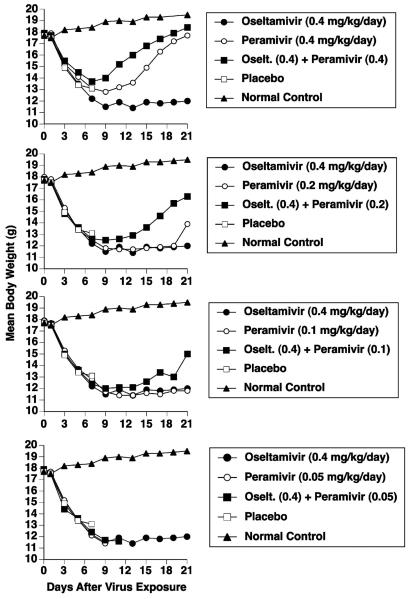
Oseltamivir treatment alone at 0.4 mg/kg/day did not prevent severe weight loss (or death) in 90% of the mice during the first 11 days of the infection, and the weight of the lone survivor remained low through day 21 (Figure 4). Improvement in body weight was seen when oseltamivir (0.4 mg/kg/day) was combined with peramivir (0.1 to 0.4 mg/kg/day). Combinations using lower doses of oseltamivir combined with peramivir did not provide additional benefits to body weight (data not shown).
Figure 4.
Effects of combination treatment of an influenza A/NWS/33 (H1N1) virus infection with oseltamivir (0.4 mg/kg/day) and peramivir (various doses) on mouse body weights. Intramuscular treatments with peramivir and p.o. treatments with oseltamivir were given twice a day for 5 days starting 2 hours prior to virus exposure. Body weights accompany the survival data of Table 3.
A second animal experiment was conducted to confirm the points of synergy (0.4 mg/kg/day of oseltamivir combined with 0.1 and 0.2 mg/kg/day of peramivir) and the single point of antagonism (0.05 mg/kg/day of oseltamivir combined with 0.2 mg/kg/day of peramivir) shown in Table 3 and Figure 3. A small number of doses were used, but group sizes were increased from 10 (first experiment, Table 3) to 20 mice each to obtain greater statistical power than in the first study. In this second experiment, treatment with oseltamivir alone at 0.4 mg/kg/day resulted in 45% survival compared to 5% in the placebo group (Table 4). This was substantially higher than observed in the first experiment (10% survival) for this dose. Treatment with peramivir alone at 0.2 mg/kg/day resulted in 10% survival compared to 5% in the placebo group. This was substantially lower than observed in the first experiment (60% survival) for this dose. Treatment results with 0.1 mg/kg/day peramivir were identical to placebo (5% survival). Combining 0.4 mg/kg/day of oseltamivir with 0.1 and 0.2 mg/kg/day of peramivir resulted in 80 and 90% survival, respectively. This level of protection in combination was similar to that observed in the first experiment (80% and 100% survival, respectively). Figure 5 is a MacSynergy plot of the results of the second animal experiment. The volume of synergy for this second experiment was 81, and there was no antagonism. The 0.05 mg/kg/day dose of oseltamivir alone was not different from placebo, similar to the result from the first experiment. When 0.05 mg/kg/day of oseltamivir was combined with peramivir (0.1 and 0.2 mg/kg/day), protection was identical to peramivir alone at these doses, thus no antagonism occurred. The prior study of peramivir alone at 0.2 mg/kg/day gave 60% protection (Table 3), compared to 10% protection (Table 4). Thus, there was variability in survival at this dose from one study to the next. Treatment with 0.4 mg/kg/day oseltamivir either alone or combined with peramivir significantly increased the mean day of death for mice that died (Table 4). Improvements in body weight for combination treatment were moderate compared to single drug treatments (data not shown).
Table 4.
Survival results for the combination treatment of an influenza A (H1N1) virus infection in mice using oseltamivir and peramivir at selected doses.
| Survivors / Total (MDDa ± SD) |
|||
|---|---|---|---|
| Compound (mg/kg/day) |
Peramivir (0) |
Peramivir (0.1) |
Peramivir (0.2) |
| Oseltamivir (0.4) |
9/20** (7.9 ± 1.1*) |
16/20***,ϕ (8.3 ± 0.5**) |
18/20***,ϕϕ (9.0 ± 0.0) |
| Oseltamivir (0.05) |
1/20 (6.4 ± 0.9) |
1/20 (7.2 ± 0.8) |
2/20 (7.8 ± 1.3*) |
| Oseltamivir (0) |
1/20 (7.1 ± 0.5) |
1/20 (7.0 ± 0.8) |
2/20 (7.5 ± 0.9) |
Mean day of death ± SD of mice during the infection period of 21 days.
P<0.05,
P<0.01,
P<0.001, compared to placebo.
P<0.05,
P<0.01, compared to oseltamivir alone.
Figure 5.
Three-dimensional plot of the impact of oseltamivir and peramivir on the number of surviving mice from an influenza A/NWS/33 (H1N1) virus infection. This second experiment used large group sizes for greater statistical power. The MacSynergy plot was derived from the data in Table 4. Two points of synergy were significant at the 95% confidence limit.
Toxicity evaluations for drug combinations in uninfected mice were not performed in conjunction with the infection studies. The doses used were quite low, and the survival and body weight data for the infection studies support the conclusion that these two agents used together at the highest combined doses were not toxic to the animals.
4. Discussion
The primary assumption that was made when beginning this research was that the combination of these two neuraminidase inhibitors, oseltamivir and peramivir, would lead to additive effects. This was based upon combining two compounds with the same mode of action. Synergy is generally expected for compounds having different modes of antiviral action. Because of the tighter binding of peramivir to the neuraminidase (Bantia et al., 2006), antagonistic interactions were also possible at specific ratios of the two compounds. To test these possibilities, we performed virus infection studies in cell culture, viral neuraminidase inhibition assays, and infections with influenza A (H1N1) virus in mice. Overall, the results showed additive to synergistic interactions when oseltamivir carboxylate and peramivir were combined. The small region of antagonism reported for the neuraminidase assay occurred where enzymatic activity was highly inhibited by peramivir alone, and the effect appeared inconsequential in terms of overall enzyme inhibition.
In cell culture assays, a narrow region of additivity to synergy occurred with two points depicted as antagonistic (Figure 1). Variability in virus titer at each data point for this assay was high (Table 1), which suggests a cautious interpretation of the data. The two points of antagonism may merely be due to biological variability. Conservatively, it is concluded that interactions between peramivir and oseltamivir in vitro were overall additive. Oseltamivir and peramivir are not particularly active in cell culture against this strain of virus, as has been previously reported (Smee et al., 2001, 2010), even though they are potent inhibitors of the specific viral neuraminidase (Bantia et al., 2006; Smee et al., 2010). The low potency of the inhibitor in cell culture is an artifact of replication of the virus in MDCK cells, where the binding of viral hemagglutinin to the cells is undoubtedly weak, reducing the need for viral neuraminidase activity in the cell culture system.
The viral neuraminidase assay had much less variability than the virus yield reduction assay. Less than 10% increase or decrease from expected was seen for the various combinations. Interaction was therefore overall additive. Importantly, combinations of the inhibitors did not produce antagonism.
Two independent combination studies were performed in mice under comparable infection and treatment conditions. Both animal studies demonstrated that a combination of oseltamivir and peramivir at suboptimal dose for each could result in overall effective protection from death. Oseltamivir dosed orally twice daily at 0.4 mg/kg/day alone protected 10% and 45% of mice respectively in the two studies. Peramivir dosed intramuscularly twice daily at 0.2 mg/kg/day alone protected 60% and 10% of mice respectively in the two studies. In combination, 100% and 90% of mice were protected. Therefore, despite apparent variability in efficacy at the suboptimal doses, the high protective effect in combination was reproducible. The first study also produced a result indicative of antagonism, when 0.05 mg/kg/day oseltamivir was combined with 0.2 mg/kg/day peramivir. This combination only protected 10% of animals from death, although oseltamivir alone was identical to placebo and peramivir alone at this dose protected 60% of animals from death in the same experiment. The second experiment using 2-fold larger groups failed to demonstrate this antagonism. In this experiment, 0.05 mg/kg/day oseltamivir remained identical to placebo, whereas 0.2mg/kg/day peramivir only protected 10% of animals from death and the combination also protected 10%. These results are consistent with higher variability at suboptimal doses. Variability in survival at particular low doses may shift mortality in one direction or another to skew results and conclusions. Thus, the repeated study was essential to gain a clearer picture of the drug interactions. Importantly, higher doses of both compounds have demonstrated ability to completely protect mice from death in this model (Smee et al., 2010). These experiments support the use of oseltamivir and peramivir in combination to treat serious human infections.
Besides providing added protection to mice in terms of morbidity (weight loss) and mortality during the infection when treated with suboptimal doses of the compounds, there is also the possibility that treatment with peramivir and oseltamivir in combination might delay the emergence of drug resistant viruses. This is because the overall exposure to neuraminidase inhibitor may be higher in combination and not all oseltamivir-resistant viruses are cross-resistant to peramivir (Mishin et al., 2005; Hurt et al., 2006), although some are, especially H1N1-H275Y viruses (Gubareva et al., 2001; Memoli et al., 2010). Cell culture studies showing inhibition of virus with drug combinations support the premise that combination chemotherapy will suppress the emergence of drug resistance (Ilyushina et al., 2006), although combinations of oseltamivir carboxylate and peramivir were not tested in their studies. Resistance development to either oseltamivir or peramivir was not assessed in the combination study described here.
In summary, these experiments demonstrated that combination treatment of influenza A (H1N1) virus infections in vitro and in mice with oseltamivir (or oseltamivir carboxylate) and peramivir did not lead to antagonism. Instead, particular dosage combinations produced additive to synergistic responses. The data suggest that treating humans with the combination may be beneficial.
Acknowledgements
This work was supported by contracts N01-AI-30048 and N01-AI-30063 (awarded to Southern Research Institute) from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA. The contents of this article do not necessarily reflect the position or policy of the government and no official endorsement should be inferred. The animal experiments were conducted in accordance with the approval of the Institutional Animal Care and Use Committee of Utah State University in the AAALAC-accredited Laboratory Animal Research Center. Work was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bantia S, Arnold CS, Parker CD, Upshaw R, Chand P. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antiviral Res. 2006;69:39–45. doi: 10.1016/j.antiviral.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Bantia S, Parker CD, Ananth SL, Horn LL, Andries K, Chand P, Kotian PL, Dehghani A, El-Kattan Y, Lin T, Hutchison TL, Montgomery JA, Kellog DL, Babu YS. Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob. Agents Chemother. 2001;45:1162–1167. doi: 10.1128/AAC.45.4.1162-1167.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso L, Treanor J, Gubareva L, Hayden FG. Efficacy and tolerability of the oral neuraminidase inhibitor peramivir in experimental human influenza: randomized, controlled trials for prophylaxis and treatment. Antivir. Ther. 2005;10:901–910. [PubMed] [Google Scholar]
- Besselaar TG, Naidoo D, Buys A, Gregory V, McAnerney J, Manamela JM, Blumberg L, Schoub BD. Widespread oseltamivir resistance in influenza A viruses (H1N1), South Africa. Emerg. Infect. Dis. 2008;14:1809–1810. doi: 10.3201/eid1411.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltz DA, Ilyushina NA, Arnold CS, Babu YS, Webster RG, Govorkova EA. Intramuscularly administered neuraminidase inhibitor peramivir is effective against lethal H5N1 influenza virus in mice. Antiviral Res. 2008;80:150–157. doi: 10.1016/j.antiviral.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Outbreak of swine-origin influenza A (H1N1) virus infection - Mexico, March-April 2009. MMWR Morb. Mortal. Wkly. Rep. 2009;58:467–470. [PubMed] [Google Scholar]
- Cheung CL, Rayner JM, Smith GJ, Wang P, Naipospos TS, Zhang J, Yuen KY, Webster RG, Peiris JS, Guan Y, Chen H. Distribution of amantadine-resistant H5N1 avian influenza variants in Asia. J. Infect. Dis. 2006;193:1626–1629. doi: 10.1086/504723. [DOI] [PubMed] [Google Scholar]
- Dharan NJ, Gubareva LV, Meyer JJ, Okomo-Adhiambo M, McClinton RC, Marshall SA, George K, Epperson S, Brammer L, Klimov AI, Bresee JS, Fry AM, Oseltamivir-Resistance Working Group Infections with oseltamivir-resistant influenza A (H1N1) virus in the United States. JAMA. 2009;301:1034–1041. doi: 10.1001/jama.2009.294. [DOI] [PubMed] [Google Scholar]
- Deyde VM, Xu X, Bright RA, Shaw M, Smith CB, Zhang Y, Shu Y, Gubareva LV, Cox NJ, Klimov AI. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 2007;196:249–257. doi: 10.1086/518936. [DOI] [PubMed] [Google Scholar]
- Galabov AS, Simeonova L, Gegova G. Rimantadine and oseltamivir demonstrate synergistic combination effect in an experimental infection with type 1 (H3N2) influenza virus in mice. Antivir. Chem. Chemother. 2006;17:251–258. doi: 10.1177/095632020601700502. [DOI] [PubMed] [Google Scholar]
- Gubareva LV, Webster RG, Hayden FG. Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrob. Agents Chemother. 2001;45:3403–3408. doi: 10.1128/AAC.45.12.3403-3408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata M, Tsuzuki M, Goto Y, Kumagai N, Harada M, Hashimoto M, Tanaka S, Sakae K, Kimura T, Minagawa H, Miyazaki Y. High frequency of amantadine-resistant influenza A (H3N2) viruses in the 2005-2006 season and rapid detection of amantadine-resistant influenza A (H3N2) viruses by MAMA-PCR. Jpn. J. Infect. Dis. 2007;60:202–204. [PubMed] [Google Scholar]
- Hurt AC, Iannello P, Jachno K, Komadina N, Hampson AW, Barr IG, McKimm- Breschkin JL. Neuraminidase inhibitor-resistant and -sensitive influenza B viruses isolated from an untreated human patient. Antimicrob. Agents Chemother. 2006;50:1872–1874. doi: 10.1128/AAC.50.5.1872-1874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyushina NA, Bovin NV, Webster RG, Govorkova EA. Combination chemotherapy, a potential strategy for reducing the emergence of drug-resistant influenza A variants. Antiviral Res. 2006;70:121–131. doi: 10.1016/j.antiviral.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Ilyushina NA, Hoffmann E, Salomon R, Webster RG, Govorkova EA. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antiviral Ther. 2007;12:363–370. [PubMed] [Google Scholar]
- Ilyushina NA, Hay A, Yilmaz N, Boon AC, Webster RG, Govorkova EA. Oseltamivir-ribavirin combination therapy for highly pathogenic H5N1 influenza virus infection in mice. Antimicrob. Agents Chemother. 2008;52:3889–3897. doi: 10.1128/AAC.01579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Escarpe PA, Eisenberg EJ, Cundy KC, Sweet C, Jakeman KJ, Merson J, Lew W, Williams M, Zhang L, Kim CU, Bischofberger N, Chen MS, Mendel DB. Identification of GS 4104 as an orally bioavailable prodrug of the influenza virus neuraminidase inhibitor GS 4071. Antimicrob. Agents Chemother. 1998;42:647–653. doi: 10.1128/aac.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer A, Lackenby A, Hungnes O, Lina B, van-der-Werf S, Schweiger B, Opp M, Paget J, van-de-Kassteele J, Hay A, Zambon M, European Influenza Surveillance Scheme Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007-08 season. Emerg. Infect. Dis. 2009;15:552–560. doi: 10.3201/eid1504.081280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memoli MJ, Hrabal RJ, Hassantoufighi A, Eichelberger MC, Taubenberger JK. Rapid selection of oseltamivir- and peramivir-resistant pandemic H1N1 virus during therapy in 2 immunocompromised hosts. Clin. Infect. Dis. 2010;50:1252–1255. doi: 10.1086/651605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishin VP, Hayden FG, Gubareva LV. Susceptibilities of antiviral-resistant influenza viruses to novel neuraminidase inhibitors. Antimicrob. Agents Chemother. 2005;49:4515–4520. doi: 10.1128/AAC.49.11.4515-4520.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossad SB. The resurgence of swine-origin influenza A (H1N1) Cleve. Clin. J. Med. 2009;76:337–343. doi: 10.3949/ccjm.76a.09047. [DOI] [PubMed] [Google Scholar]
- Nguyen JT, Hoopes JD, Le MH, Smee DF, Patick AK, Faix DJ, Blair PJ, de Jong MD, Prichard MN, Went GT. Triple combination of amantadine, ribavirin, and oseltamivir is highly active and synergistic against drug resistant influenza virus strains in vitro. PLoS One. 2010;5:e9332. doi: 10.1371/journal.pone.0009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JT, Hoopes JD, Smee DF, Prichard MN, Driebe EM, Engelthaler DM, Le MH, Keim PS, Spence RP, Went GT. Triple combination of oseltamivir, amantadine, and ribavirin displays synergistic activity against multiple influenza virus strains in vitro. Antimicrob. Agents Chemother. 2009;53:4115–4126. doi: 10.1128/AAC.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okomo-Adhiambo M, Nguyen HT, Sleeman K, Sheu TG, Deyde VM, Garten RJ, Xu X, Shaw MW, Klimov AI, Gubareva LV. Host cell selection of influenza neuraminidase variants: implications for drug resistance monitoring in A(H1N1) viruses. Antiviral Res. 2010;85:381–388. doi: 10.1016/j.antiviral.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Prichard MN, Shipman C., Jr. A three dimensional model to analyze drug-drug interactions. Antiviral Res. 1990;14:181–206. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–498. [Google Scholar]
- Sidwell RW, Smee DF, Huffman JH, Barnard DL, Bailey KW, Morrey JD, Babu YS. In vivo influenza virus-inhibitory effects of the cyclopentane neuraminidase inhibitor RJW-270201. Antimicrob. Agents Chemother. 2001;45:749–757. doi: 10.1128/AAC.45.3.749-757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Huffman JH, Morrison AC, Barnard DL, Sidwell RW. Cyclopentane neuraminidase inhibitors with potent in vitro anti-influenza virus activities. Antimicrob. Agents Chemother. 2001;45:743–748. doi: 10.1128/AAC.45.3.743-748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Bailey KW, Morrison AC, Sidwell RW. Combination treatment of influenza A virus infections in cell culture and in mice with the cyclopentane neuraminidase inhibitor RWJ-270201 and ribavirin. Chemotherapy. 2002;48:88–93. doi: 10.1159/000057668. [DOI] [PubMed] [Google Scholar]
- Smee DF, Hurst BL, Wong MH, Bailey KW, Morrey JD. Effects of double combinations of amantadine, oseltamivir, and ribavirin on influenza A (H5N1) virus infections in cell culture and in mice. Antimicrob. Agents Chemother. 2009;53:2120–2128. doi: 10.1128/AAC.01012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Hurst BL, Wong MH, Bailey KW, Tarbet EB, Morrey JD, Furuta Y. Effects of the combination of favipiravir (T-705) and oseltamivir on influenza A virus infections in mice. Antimicrob. Agents Chemother. 2010;54:126–133. doi: 10.1128/AAC.00933-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Dwyer DE, Blyth CC, Soedjono M, Shi H, Kesson A, Ratnamohan M, McPhie K, Cunningham AL, Saksena NK. Detection of the rapid emergence of the H275Y mutation associated with oseltamivir resistance in severe pandemic influenza virus A/H1N1 09 infections. Antiviral Res. 2010;87:16–21. doi: 10.1016/j.antiviral.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Yun NE, Linde NS, Zacks MA, Barr IG, Hurt AC, Smith JN, Dziuba N, Holbrook MR, Zhang L, Kilpatrick JM, Arnold CS, Paessler S. Injectable peramivir mitigates disease and promotes survival in ferrets and mice infected with the highly virulent influenza virus, A/Vietnam/1203/04 (H5N1) Virology. 2008;374:198–209. doi: 10.1016/j.virol.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]