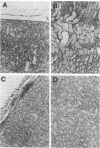
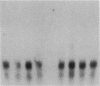
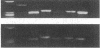
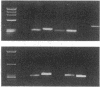
Abstract
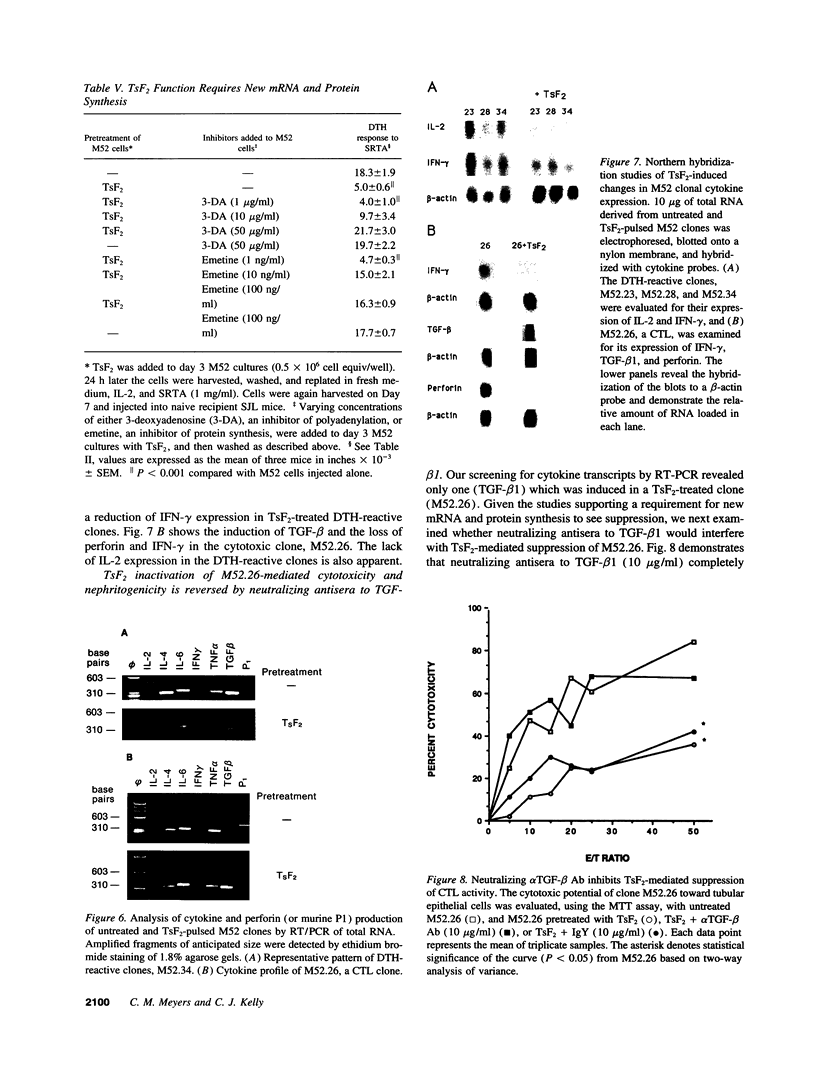
We have used a murine model of organ-specific autoimmunity to characterize therapeutic modalities capable of down-regulating the cellular limb of the autoimmune response. Murine interstitial nephritis is an autoimmune disease mediated by tubular antigen-specific CD8+ nephritogenic effector T cells which are delayed-type hypersensitivity (DTH) reactive and cytotoxic to renal epithelial cells. Previous studies have demonstrated that disease can be suppressed with experimentally induced populations of T cells (Ts1 and Ts2 cells) obtained after injection of tubular antigen-coupled splenocytes into syngeneic mice. As the target of Ts2 is the CD8+ effector T cell, we have evaluated its effects on nephritogenic effector T cell clones isolated from diseased animals. Our studies demonstrate that soluble proteins expressed by Ts2 cells (TsF2) specifically abrogate the DTH, cytotoxic, and nephritogenic potential of M52 cells, although T cell receptor and IL-2 receptor expression are unchanged in these unresponsive M52 clones. TsF2-induced inhibition is dependent on new mRNA and protein synthesis. In a cytotoxic clone, M52.26, exposure to TsF2 induces expression of TGF-beta 1 which is, in turn, required for inhibition of cytotoxicity and nephritogenicity. Our studies are consistent with TGF-beta 1 behaving, at least in some T cells, as a nonspecific final effector of clone-specific suppression.
Full text
PDF


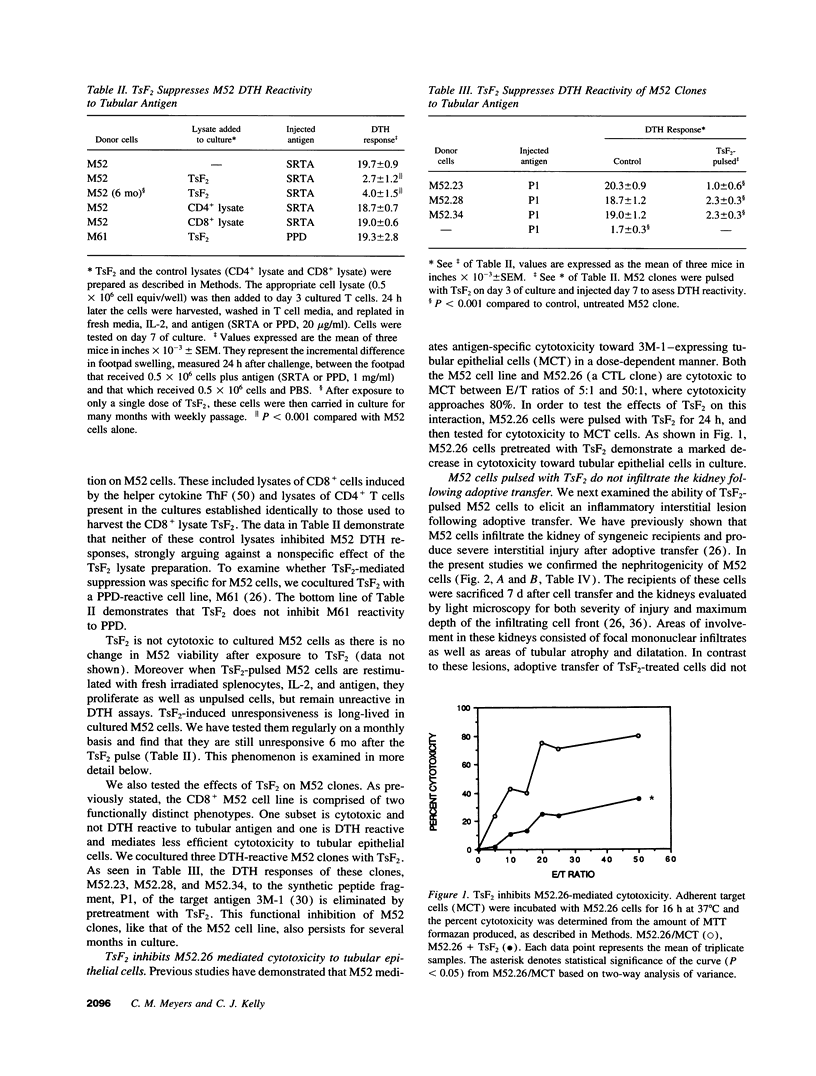
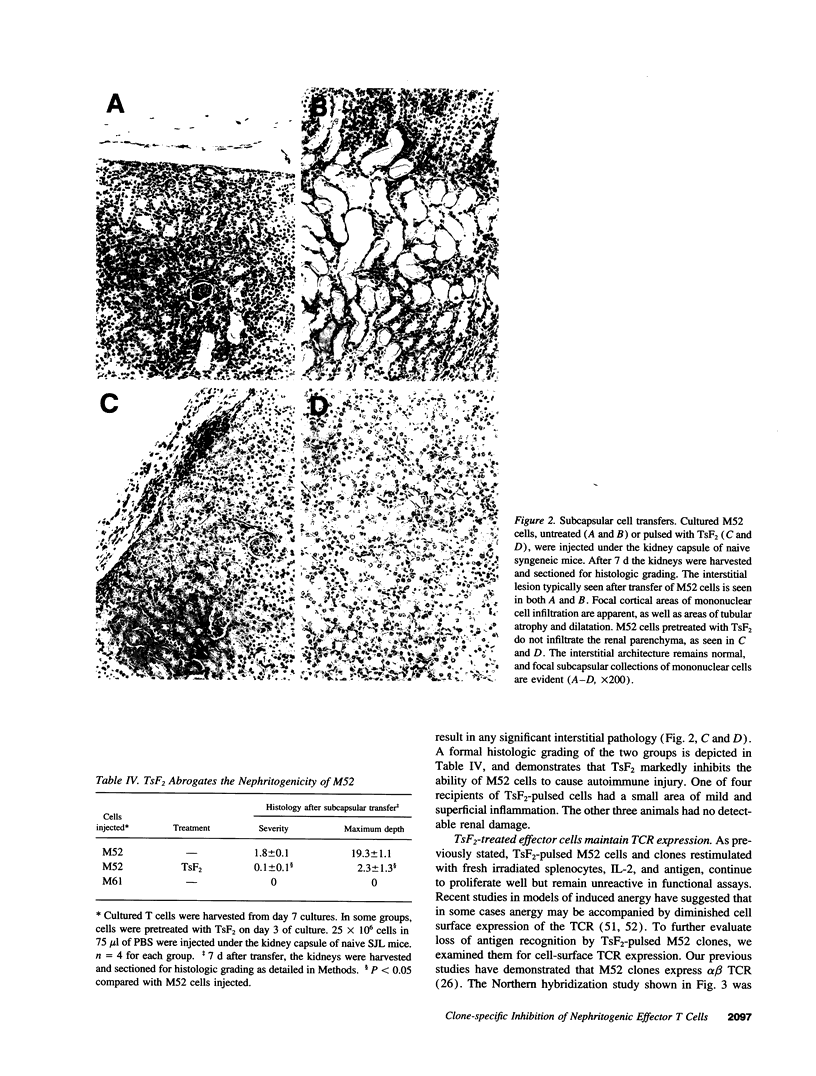
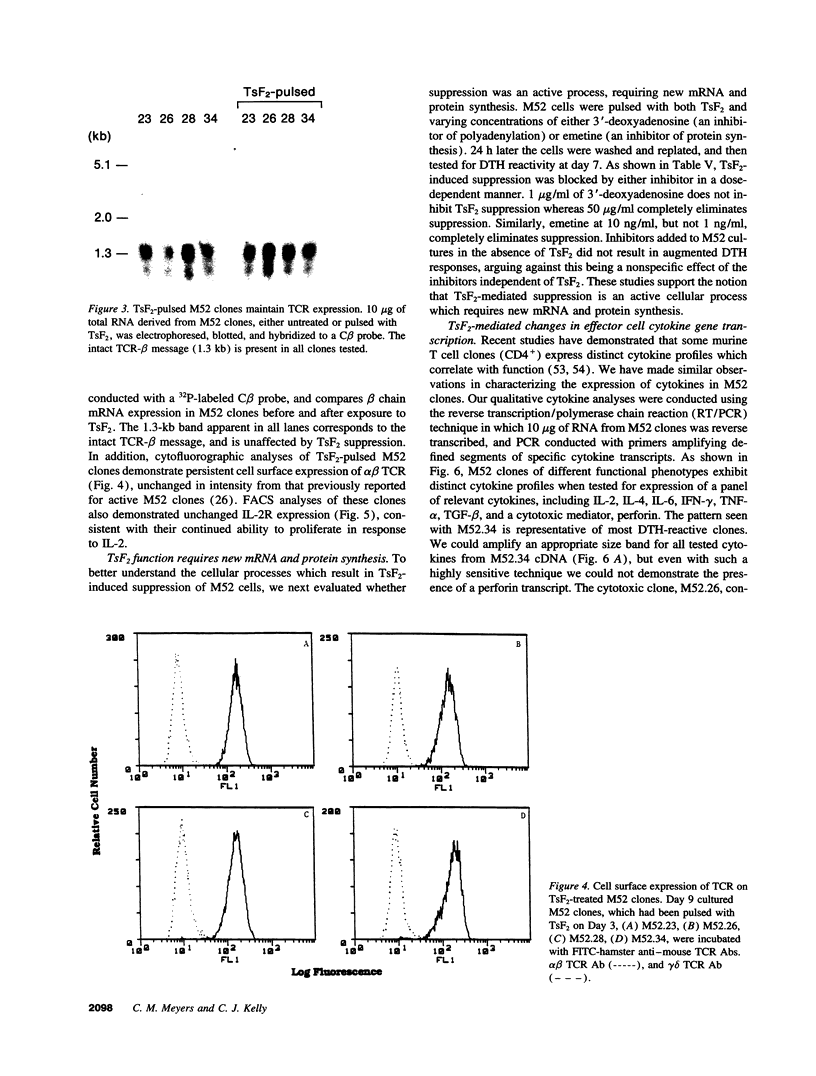
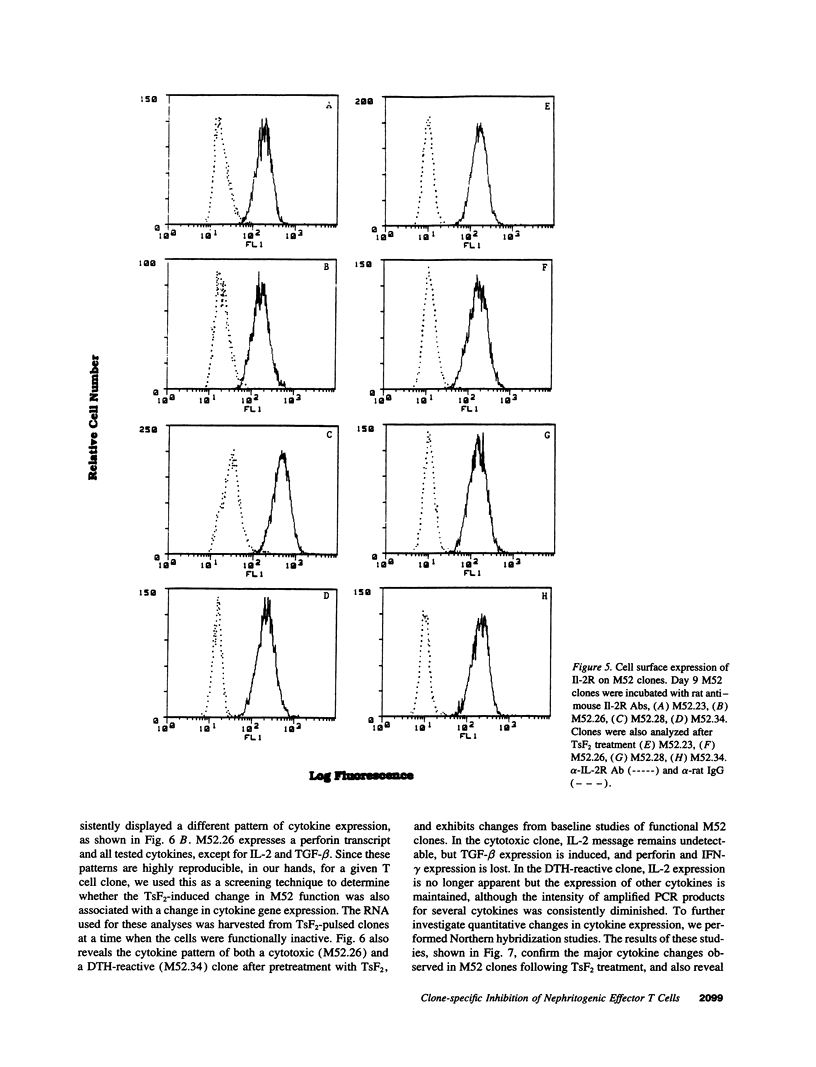




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Mitchell D. J., Timmermann L., Wraith D. C., Tausch G. S., Waldor M. K., Zamvil S. S., McDevitt H. O., Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988 Jul 15;54(2):263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- Alonso S., Minty A., Bourlet Y., Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23(1):11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- Boitard C., Bendelac A., Richard M. F., Carnaud C., Bach J. F. Prevention of diabetes in nonobese diabetic mice by anti-I-A monoclonal antibodies: transfer of protection by splenic T cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9719–9723. doi: 10.1073/pnas.85.24.9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H., Sharp G. C., Kyriakos M., Hayes N., Dunn C., Jepsen P., Sanders R. D. Suppression of experimental autoimmune thyroiditis in the guinea pig by pretreatment with thyroglobulin in incomplete Freund's adjuvant. Cell Immunol. 1978 Sep;39(2):289–296. doi: 10.1016/0008-8749(78)90104-1. [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H., Tompson J. G., Sharp G. C., Kyriakos M. Suppression of experimental autoimmune thyroiditis in guinea pigs by pretreatment with thyroglobulin-coupled spleen cells. Cell Immunol. 1980 May;51(2):408–413. doi: 10.1016/0008-8749(80)90272-5. [DOI] [PubMed] [Google Scholar]
- Brostoff S. W., Mason D. W. Experimental allergic encephalomyelitis: successful treatment in vivo with a monoclonal antibody that recognizes T helper cells. J Immunol. 1984 Oct;133(4):1938–1942. [PubMed] [Google Scholar]
- Burns F. R., Li X. B., Shen N., Offner H., Chou Y. K., Vandenbark A. A., Heber-Katz E. Both rat and mouse T cell receptors specific for the encephalitogenic determinant of myelin basic protein use similar V alpha and V beta chain genes even though the major histocompatibility complex and encephalitogenic determinants being recognized are different. J Exp Med. 1989 Jan 1;169(1):27–39. doi: 10.1084/jem.169.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clayman M. D., Martinez-Hernandez A., Michaud L., Alper R., Mann R., Kefalides N. A., Neilson E. G. Isolation and characterization of the nephritogenic antigen producing anti-tubular basement membrane disease. J Exp Med. 1985 Feb 1;161(2):290–305. doi: 10.1084/jem.161.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M., Kuchroo V. K., Whitters M. J., O'Hara R. M., Jr, Kelleher K., Kubo R. T., Dorf M. E. Expression of functional alpha beta T cell receptor gene rearrangements in suppressor T cell hybridomas correlates with antigen binding, but not with suppressor cell function. J Immunol. 1990 Nov 1;145(9):2809–2819. [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Goeddel D. V. The murine transforming growth factor-beta precursor. J Biol Chem. 1986 Apr 5;261(10):4377–4379. [PubMed] [Google Scholar]
- Devens B. H., Koontz A. W., Kapp J. A., Pierce C. W., Webb D. R. Involvement of two distinct regulatory T cell populations in the antigen-specific suppression of cytolytic T cell generation. J Immunol. 1991 Mar 1;146(5):1394–1401. [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Suppressor cells and immunoregulation. Annu Rev Immunol. 1984;2:127–157. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- Fransen L., Müller R., Marmenout A., Tavernier J., Van der Heyden J., Kawashima E., Chollet A., Tizard R., Van Heuverswyn H., Van Vliet A. Molecular cloning of mouse tumour necrosis factor cDNA and its eukaryotic expression. Nucleic Acids Res. 1985 Jun 25;13(12):4417–4429. doi: 10.1093/nar/13.12.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T., Lefrancois L. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. J Exp Med. 1989 Nov 1;170(5):1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. R., Bissonnette R., Zheng H. G., Onda T., Echeverri F., Mogil R. J., Steele J. K., Voralia M., Fotedar A. Immunoregulatory activity of the T-cell receptor alpha chain demonstrated by retroviral gene transfer. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8475–8479. doi: 10.1073/pnas.88.19.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. M., Reade J. L., Ware C. F. Rapid colorimetric assay for cell viability: application to the quantitation of cytotoxic and growth inhibitory lymphokines. J Immunol Methods. 1984 May 25;70(2):257–268. doi: 10.1016/0022-1759(84)90190-x. [DOI] [PubMed] [Google Scholar]
- Greene M. I., Nelles M. J., Sy M. S., Nisonoff A. Regulation of immunity to the azobenzenearsonate hapten. Adv Immunol. 1982;32:253–300. doi: 10.1016/s0065-2776(08)60723-3. [DOI] [PubMed] [Google Scholar]
- Haverty T. P., Kelly C. J., Hines W. H., Amenta P. S., Watanabe M., Harper R. A., Kefalides N. A., Neilson E. G. Characterization of a renal tubular epithelial cell line which secretes the autologous target antigen of autoimmune experimental interstitial nephritis. J Cell Biol. 1988 Oct;107(4):1359–1368. doi: 10.1083/jcb.107.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Germain R. N., Bevan M. J., Dorf M., Engel I., Fink P., Gascoigne N., Heber-Katz E., Kapp J., Kaufmann Y. Rearrangement and transcription of a T-cell receptor beta-chain gene in different T-cell subsets. Proc Natl Acad Sci U S A. 1985 Jan;82(2):531–535. doi: 10.1073/pnas.82.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines W. H., Mann R. A., Kelly C. J., Neilson E. G. Murine interstitial nephritis. IX. Induction of the nephritogenic effector T cell repertoire with an antigen-specific T cell cytokine. J Immunol. 1990 Jan 1;144(1):75–83. [PubMed] [Google Scholar]
- Hines W. H., Mann R. A., Neilson E. G. Murine interstitial nephritis. VIII. Characterization of Igh-V restriction determinants in the development of anti-idiotypic immunity using blocking antibodies. J Autoimmun. 1988 Apr;1(2):143–157. doi: 10.1016/0896-8411(88)90022-4. [DOI] [PubMed] [Google Scholar]
- Hisatsune T., Enomoto A., Nishijima K., Minai Y., Asano Y., Tada T., Kaminogawa S. CD8+ suppressor T cell clone capable of inhibiting the antigen- and anti-T cell receptor-induced proliferation of Th clones without cytolytic activity. J Immunol. 1990 Oct 15;145(8):2421–2426. [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell M. D., Winters S. T., Olee T., Powell H. C., Carlo D. J., Brostoff S. W. Vaccination against experimental allergic encephalomyelitis with T cell receptor peptides. Science. 1989 Nov 3;246(4930):668–670. doi: 10.1126/science.2814489. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Pardoll D. M., Mizuguchi J., Chused T. M., Schwartz R. H. Molecular events in the induction of a nonresponsive state in interleukin 2-producing helper T-lymphocyte clones. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5409–5413. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987 Feb 1;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpus W. J., Swanborg R. H. CD4+ suppressor cells differentially affect the production of IFN-gamma by effector cells of experimental autoimmune encephalomyelitis. J Immunol. 1989 Dec 1;143(11):3492–3497. [PubMed] [Google Scholar]
- Karpus W. J., Swanborg R. H. CD4+ suppressor cells inhibit the function of effector cells of experimental autoimmune encephalomyelitis through a mechanism involving transforming growth factor-beta. J Immunol. 1991 Feb 15;146(4):1163–1168. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley V. E., Gaulton G. N., Hattori M., Ikegami H., Eisenbarth G., Strom T. B. Anti-interleukin 2 receptor antibody suppresses murine diabetic insulitis and lupus nephritis. J Immunol. 1988 Jan 1;140(1):59–61. [PubMed] [Google Scholar]
- Kelly C. J., Clayman M. D., Neilson E. G. Immunoregulation in experimental interstitial nephritis: immunization with renal tubular antigen in incomplete Freund's adjuvant induces major histocompatibility complex-restricted, OX8+ suppressor T cells which are antigen-specific and inhibit the expression of disease. J Immunol. 1986 Feb 1;136(3):903–907. [PubMed] [Google Scholar]
- Kelly C. J., Neilson E. G. Contrasuppression in autoimmunity. Abnormal contrasuppression facilitates expression of nephritogenic effector T cells and interstitial nephritis in kdkd mice. J Exp Med. 1987 Jan 1;165(1):107–123. doi: 10.1084/jem.165.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C. J., Silvers W. K., Neilson E. G. Tolerance to parenchymal self. Regulatory role of major histocompatibility complex-restricted, OX8+ suppressor T cells specific for autologous renal tubular antigen in experimental interstitial nephritis. J Exp Med. 1985 Dec 1;162(6):1892–1903. doi: 10.1084/jem.162.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. K., Dal Canto M. C., Trotter J. L., Miller S. D. Specific immune regulation of chronic-relapsing experimental allergic encephalomyelitis in mice. J Immunol. 1988 Nov 1;141(9):2986–2993. [PubMed] [Google Scholar]
- Koide J., Engleman E. G. Differences in surface phenotype and mechanism of action between alloantigen-specific CD8+ cytotoxic and suppressor T cell clones. J Immunol. 1990 Jan 1;144(1):32–40. [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Kuchroo V. K., Byrne M. C., Atsumi Y., Greenfield E., Connolly J. B., Whitters M. J., O'Hara R. M., Jr, Collins M., Dorf M. E. T-cell receptor alpha chain plays a critical role in antigen-specific suppressor cell function. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8700–8704. doi: 10.1073/pnas.88.19.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lider O., Santos L. M., Lee C. S., Higgins P. J., Weiner H. L. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. II. Suppression of disease and in vitro immune responses is mediated by antigen-specific CD8+ T lymphocytes. J Immunol. 1989 Feb 1;142(3):748–752. [PubMed] [Google Scholar]
- Lowrey D. M., Aebischer T., Olsen K., Lichtenheld M., Rupp F., Hengartner H., Podack E. R. Cloning, analysis, and expression of murine perforin 1 cDNA, a component of cytolytic T-cell granules with homology to complement component C9. Proc Natl Acad Sci U S A. 1989 Jan;86(1):247–251. doi: 10.1073/pnas.86.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M., Minard K., Mjolsness S., Kronenberg M., Goverman J., Hunkapiller T., Prystowsky M. B., Yoshikai Y., Fitch F., Mak T. W. Mouse T cell antigen receptor: structure and organization of constant and joining gene segments encoding the beta polypeptide. Cell. 1984 Jul;37(3):1101–1110. doi: 10.1016/0092-8674(84)90444-6. [DOI] [PubMed] [Google Scholar]
- Mann R., Kelly C. J., Hines W. H., Clayman M. D., Blanchard N., Sun M. J., Neilson E. G. Effector T cell differentiation in experimental interstitial nephritis. I. The development and modulation of effector lymphocyte maturation by I-J+ regulatory T cells. J Immunol. 1987 Jun 15;138(12):4200–4208. [PubMed] [Google Scholar]
- Mann R., Neilson E. G. Murine interstitial nephritis. V. The auto-induction of antigen-specific Lyt-2+ suppressor T cells diminishes the expression of interstitial nephritis in mice with antitubular basement membrane disease. J Immunol. 1986 Feb 1;136(3):908–912. [PubMed] [Google Scholar]
- Meyers C. M., Kelly C. J. Effector mechanisms in organ-specific autoimmunity. I. Characterization of a CD8+ T cell line that mediates murine interstitial nephritis. J Clin Invest. 1991 Aug;88(2):408–416. doi: 10.1172/JCI115319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Neilson E. G., Kelly C. J., Clayman M. D., Hines W. H., Haverty T., Sun M. J., Blanchard N. Murine interstitial nephritis. VII. Suppression of renal injury after treatment with soluble suppressor factor TsF1. J Immunol. 1987 Sep 1;139(5):1518–1524. [PubMed] [Google Scholar]
- Neilson E. G., McCafferty E., Mann R., Michaud L., Clayman M. Murine interstitial nephritis. III. The selection of phenotypic (Lyt and L3T4) and idiotypic (RE-Id) T cell preferences by genes in Igh-1 and H-2K characterizes the cell-mediated potential for disease expression: susceptible mice provide a unique effector T cell repertoire in response to tubular antigen. J Immunol. 1985 Apr;134(4):2375–2382. [PubMed] [Google Scholar]
- Neilson E. G., McCafferty E., Mann R., Michaud L., Clayman M. Tubular antigen-derivatized cells induce a disease-protective, antigen-specific, and idiotype-specific suppressor T cell network restricted by I-J and Igh-V in mice with experimental interstitial nephritis. J Exp Med. 1985 Jul 1;162(1):215–230. doi: 10.1084/jem.162.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson E. G., McCafferty E., Phillips S. M., Clayman M. D., Kelly C. J. Antiidiotypic immunity in interstitial nephritis. II. Rats developing anti-tubular basement membrane disease fail to make an antiidiotypic regulatory response: the modulatory role of an RT7.1+, OX8- suppressor T cell mechanism. J Exp Med. 1984 Apr 1;159(4):1009–1026. doi: 10.1084/jem.159.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson E. G., Phillips S. M. Murine interstitial nephritis. I. Analysis of disease susceptibility and its relationship of pleiomorphic gene products defining both immune-response genes and a restrictive requirement for cytotoxic T cells at H-2K. J Exp Med. 1982 Apr 1;155(4):1075–1085. doi: 10.1084/jem.155.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson E. G., Sun M. J., Kelly C. J., Hines W. H., Haverty T. P., Clayman M. D., Cooke N. E. Molecular characterization of a major nephritogenic domain in the autoantigen of anti-tubular basement membrane disease. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):2006–2010. doi: 10.1073/pnas.88.5.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Ostergaard H. L., Clark W. R. Evidence for multiple lytic pathways used by cytotoxic T lymphocytes. J Immunol. 1989 Oct 1;143(7):2120–2126. [PubMed] [Google Scholar]
- Pankewycz O., Strom T. B., Rubin-Kelley V. E. Islet-infiltrating T cell clones from non-obese diabetic mice that promote or prevent accelerated onset diabetes. Eur J Immunol. 1991 Apr;21(4):873–879. doi: 10.1002/eji.1830210403. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Kupfer A. T-cell effector functions: mechanisms for delivery of cytotoxicity and help. Annu Rev Cell Biol. 1991;7:479–504. doi: 10.1146/annurev.cb.07.110191.002403. [DOI] [PubMed] [Google Scholar]
- Quill H., Schwartz R. H. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987 Jun 1;138(11):3704–3712. [PubMed] [Google Scholar]
- Rabin H., Hopkins R. F., 3rd, Ruscetti F. W., Neubauer R. H., Brown R. L., Kawakami T. G. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981 Nov;127(5):1852–1856. [PubMed] [Google Scholar]
- Racke M. K., Dhib-Jalbut S., Cannella B., Albert P. S., Raine C. S., McFarlin D. E. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-beta 1. J Immunol. 1991 May 1;146(9):3012–3017. [PubMed] [Google Scholar]
- Rocha B., von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991 Mar 8;251(4998):1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Ruegemer J. J., Ho S. N., Augustine J. A., Schlager J. W., Bell M. P., McKean D. J., Abraham R. T. Regulatory effects of transforming growth factor-beta on IL-2- and IL-4-dependent T cell-cycle progression. J Immunol. 1990 Mar 1;144(5):1767–1776. [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Schluesener H. J., Sobel R. A., Weiner H. L. Demyelinating experimental allergic encephalomyelitis (EAE) in the rat: treatment with a monoclonal antibody against activated T cells. J Neuroimmunol. 1988 Jul;18(4):341–351. doi: 10.1016/0165-5728(88)90055-0. [DOI] [PubMed] [Google Scholar]
- Schoen R. T., Greene M. I., Trentham D. E. Antigen-specific suppression of type II collagen-induced arthritis by collagen-coupled spleen cells. J Immunol. 1982 Feb;128(2):717–719. [PubMed] [Google Scholar]
- Schönrich G., Kalinke U., Momburg F., Malissen M., Schmitt-Verhulst A. M., Malissen B., Hämmerling G. J., Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991 Apr 19;65(2):293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- Sriram S., Roberts C. A. Treatment of established chronic relapsing experimental allergic encephalomyelitis with anti-L3T4 antibodies. J Immunol. 1986 Jun 15;136(12):4464–4469. [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Tada T., Ohzeki S., Utsumi K., Takiuchi H., Muramatsu M., Li X. F., Shimizu J., Fujiwara H., Hamaoka T. Transforming growth factor-beta-induced inhibition of T cell function. Susceptibility difference in T cells of various phenotypes and functions and its relevance to immunosuppression in the tumor-bearing state. J Immunol. 1991 Feb 1;146(3):1077–1082. [PubMed] [Google Scholar]
- Takata M., Maiti P. K., Kubo R. T., Chen Y. H., Holford-Strevens V., Rector E. S., Sehon A. H. Cloned suppressor T cells derived from mice tolerized with conjugates of antigen and monomethoxypolyethylene glycol. Relationship between monoclonal T suppressor factor and the T cell receptor. J Immunol. 1990 Nov 1;145(9):2846–2853. [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Szikora J. P., Renauld J. C., Van Roost E., Boon T., Simpson R. J. cDNA cloning of murine interleukin-HP1: homology with human interleukin 6. Eur J Immunol. 1988 Feb;18(2):193–197. doi: 10.1002/eji.1830180202. [DOI] [PubMed] [Google Scholar]
- Vandenbark A. A., Hashim G., Offner H. Immunization with a synthetic T-cell receptor V-region peptide protects against experimental autoimmune encephalomyelitis. Nature. 1989 Oct 12;341(6242):541–544. doi: 10.1038/341541a0. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wong H. L., Dougherty S., McCartney-Francis N., Wahl L. M., Ellingsworth L., Schmidt J. A., Hall G., Roberts A. B. Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation. J Immunol. 1988 May 1;140(9):3026–3032. [PubMed] [Google Scholar]
- Waldor M. K., Sriram S., Hardy R., Herzenberg L. A., Herzenberg L. A., Lanier L., Lim M., Steinman L. Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science. 1985 Jan 25;227(4685):415–417. doi: 10.1126/science.3155574. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]
- Yokota T., Arai N., Lee F., Rennick D., Mosmann T., Arai K. Use of a cDNA expression vector for isolation of mouse interleukin 2 cDNA clones: expression of T-cell growth-factor activity after transfection of monkey cells. Proc Natl Acad Sci U S A. 1985 Jan;82(1):68–72. doi: 10.1073/pnas.82.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. H., Peterson L. B., Wicker L. S., Persechini P. M., Young J. D. In vivo expression of perforin by CD8+ lymphocytes in autoimmune disease. Studies on spontaneous and adoptively transferred diabetes in nonobese diabetic mice. J Immunol. 1989 Dec 15;143(12):3994–3999. [PubMed] [Google Scholar]
- Zaller D. M., Osman G., Kanagawa O., Hood L. Prevention and treatment of murine experimental allergic encephalomyelitis with T cell receptor V beta-specific antibodies. J Exp Med. 1990 Jun 1;171(6):1943–1955. doi: 10.1084/jem.171.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Sahai B. M., Kilgannon P., Fotedar A., Green D. R. Specific inhibition of cell-surface T-cell receptor expression by antisense oligodeoxynucleotides and its effect on the production of an antigen-specific regulatory T-cell factor. Proc Natl Acad Sci U S A. 1989 May;86(10):3758–3762. doi: 10.1073/pnas.86.10.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubler R. H., Lowenthal J. W., Erard F., Hashimoto N., Devos R., MacDonald H. R. Activated B cells express receptors for, and proliferate in response to, pure interleukin 2. J Exp Med. 1984 Oct 1;160(4):1170–1183. doi: 10.1084/jem.160.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]