Abstract
Our objective in this Danish population-based cohort study was to estimate the recurrence risk of isolated oral cleft (OC) for offspring of the unaffected co-twins of OC discordant twin pairs and to compare this risk to the recurrence risk in the offspring of the affected co-twin as well as to the risk in the background population.
During 1936–2004, 207 twin pairs were ascertained, among whom at least one twin had an OC. The index persons were twins discordant for OC who had children (N=117), and their offspring (N=239). The participants were ascertained by linkage between The Danish Facial Cleft Database, The Danish Twin Registry and The Danish Civil Registration System. In the study OC recurrence risk for offspring of the affected and unaffected twin and relative risk were compared to the background prevalence. We found that among 110 children of the 54 OC affected twins, two (1.8%) children had OC corresponding to a significantly increased relative risk (RR = 10; 95% CI 1.2 to 35) when compared to the frequency in the background population. Among the 129 children of the 63 unaffected twins, three (2.3%) children were affected, corresponding to a significantly increased relative risk (RR = 13; 95% CI 2.6 to 36) when compared the background prevalence. We concluded that in OC discordant twin pairs similar increased recurrence risks were found among offspring of both OC affected and OC unaffected twins. This provides further evidence for a genetic component in cleft etiology and is useful information for genetic counseling of twin pairs discordant for clefting.
Keywords: recurrence risk, oral cleft, cleft lip and palate, twins, genetics
INTRODUCTION
Nonsyndromic oral clefts (OCs) are among the most common congenital malformations and have substantial implications for the affected individuals and their families. The etiology is multifactorial with both genetic and environmental factors playing an important role [Vieira, 2008]. Most individuals born with OC have unaffected parents, even though family data suggest a very high heritability (greater than 70%) [Christensen et al., 1993a]. This genetic influence entails an increased relative risk ranging from 15 times higher for first-degree relatives to two times higher for third-degree relatives compared to the risk of the background population [Grosen et al., 2010].
Twins with OC provide unique research possibilities. While it has been speculated that twinning might disturb the normal process of development of the lip and palate in the fetus during early pregnancy, there is no compelling evidence for an effect of twinning on the risk of OC [Christensen et al., 1993a; Christensen et al., 1993b; Christensen et al., 1996a; Mitchell et al., 1997]. In addition, twinning may affect (or occur secondary to) epigenetic phenomena such as X inactivation or DNA methylation that could also play a role in developmental disruptions such as clefting [Kimani et al., 2007]. Finally, discordant twins (one twin affected) could arise from somatic genetic events such that the affected twin might be the only member of the pair carrying a specific risk allele [Kondo et al., 2002; Mansilla et al., 2005; Sakuntabhai et al., 1999].
The question of how best to counsel the unaffected twin in a twin pair discordant for OC was raised by Wyszynski et al. in case reports from 1996 and 2002 [Wyszynski et al., 1996; Wyszynski et al., 2002]. At that time no empirical data were available, but the authors speculated that the risk for the offspring of the unaffected co-twin in the pair would be three to ten times higher than the risk of the background population, i.e. potentially as high a risk as for the affected twin, but likely to be smaller since the co-twin was unaffected.
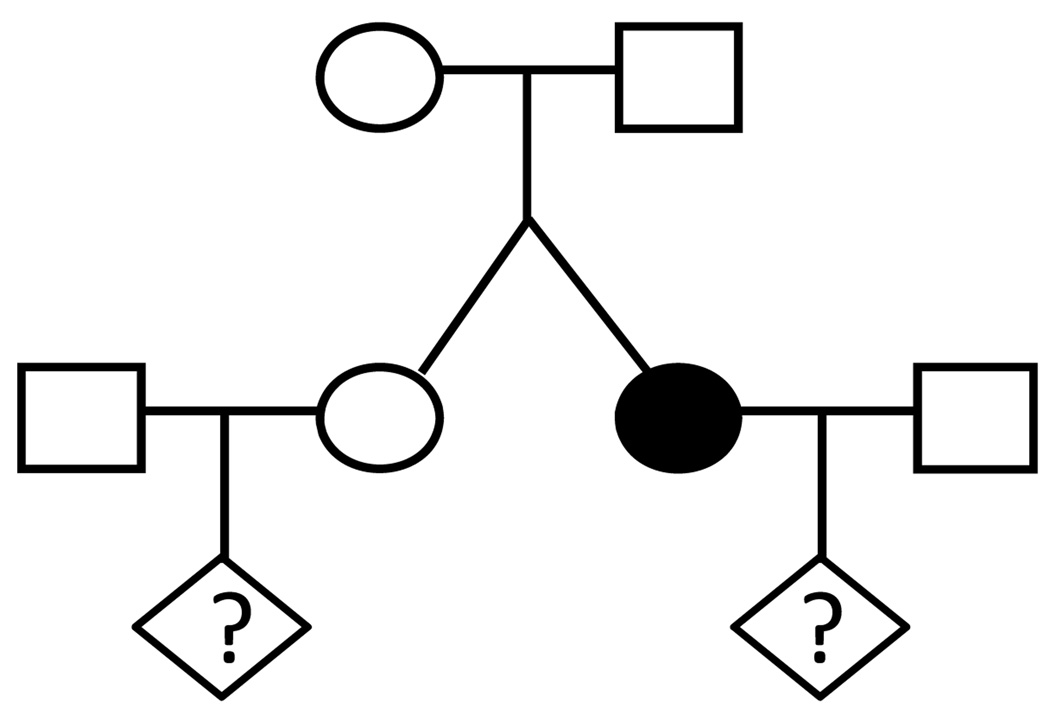
The aim of the present study was to use the large population-based registers available in Denmark to estimate the empiric recurrence risk for offspring of unaffected twins in twin pairs discordant for nonsyndromic OC and to compare this risk to the risk of the affected twins and the background population (Fig 1).
Fig 1.
Pedigree of a family with monozygotic/dizygotic twin girls discordant for oral clefting.
MATERIAL AND METHODS
The present study was a population-based cohort study based on record linkage between three nationwide registers in Denmark.
The Civil Registration System could identify all individuals who resided in Denmark at any time since its establishment in April, 1968. All individuals had a unique ten-digit personal identification number. The personal identification number included date of birth and information on sex, and contained a built-in check code disclosing invalid numbers. The register also contained a link to all first-degree relatives enabling a construction of pedigrees showing legal familial relationships (by matching individuals who share parental personal identification numbers).
The Danish Facial Cleft Database encompassed the birth cohorts from 1936 to 2005 and contained 10,025 live born individuals with OC in Denmark. For 9,146 (91%) individuals, a valid personal identification number was available. The nine percent without a personal identification number was OC individuals who either died before 1968 or could not be uniquely identified in order to be assigned a personal identification number. Both the registration and the treatment of individuals with OC have been centralized in Denmark since the 1930s and this has entailed a very high ascertainment for the cohorts under study. Clefts discovered later in a child’s life were also registered [Bille et al., 2005a]. Capture-recapture methods have indicated a 99% ascertainment for the sub-phenotype isolated cleft lip with or without cleft palate (CL/P) in the period 1983 to 1987 [Christensen et al., 1992]. Overt oral clefts could be classified into three groups in the Danish Facial Cleft Database: cleft lip (CL), cleft lip with cleft palate (CLP) and cleft palate only (CP). For bifid uvula the ascertainment was low and microforms of oral clefts, such as defects in the orbicularis oris muscle or dental anomalies, were not routinely registered in the Danish Facial Cleft Database. In the Danish Facial Cleft Database 876 (9.6%), individuals were registered with OC and another major anomaly and/or a recognized syndrome. The pattern of more anomalies associated with CP and fewer with CL and CLP was consistent with other cleft populations, but the overall rate was lower in Denmark. The OC association with anomalies/syndromes has previously been described in greater detail [Bille et al., 2005c; Bille et al., 2005a; Bille et al., 2005b; Christensen, 1999; Grosen et al., 2010].
The Danish Twin Registry included the Danish birth cohorts from 1870 to 2004, corresponding to more than 80,000 twin pairs. The twins were all born in Denmark, and they were ascertained independently of any disease. Before 1968 the ascertainment was about 90%, but since the establishment of the Civil Registration System it has been considered complete [Skytthe et al., 2002]. Zygosity determination has been made on same sex twins by use of questionnaires to determine the degree of similarity between co-twins and has been validated by comparison of blood type determinants and genetic markers. The misclassification rate was less than 5% [Bonnelykke et al., 1989; Christiansen et al., 2003], and about 75% of the twins had an assigned zygosity. In the Danish Twin Registry, 85% of the twins were registered with a personal identification number, and since 1968, 100% of the live born twins have been assigned a personal identification number which enables a linkage to the Civil Registration System, and hence linkage to the offspring of the twins.
Study population
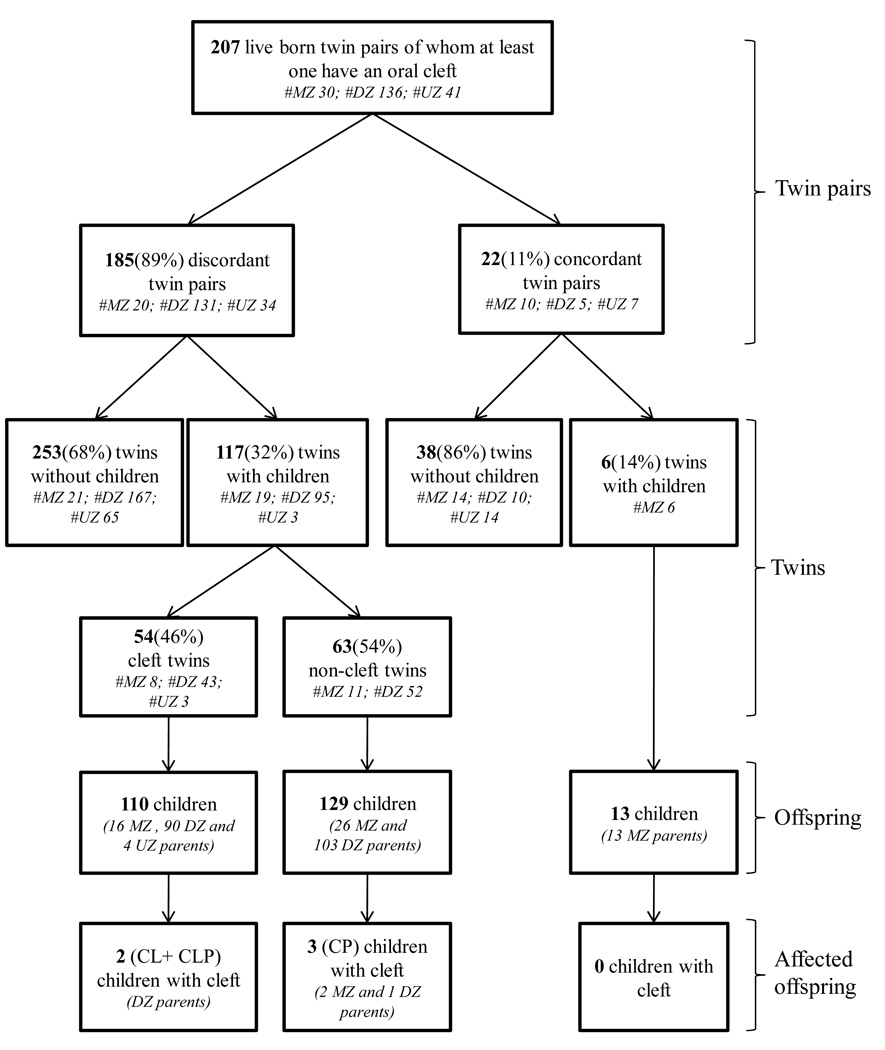
During 1936–2004, 207 mono- and dizygotic twin pairs were born in Denmark, among whom at least one twin was affected by an isolated OC (CL, CLP or CP). The index cases were twins discordant for isolated OC who had children (N=117), and their offspring (N=239) born from 1956 to 2005 (Fig 2). The population was restricted to all live born individuals with a valid personal identification number in the Civil Registration System to make it possible to identify these individuals in both the Danish Facial Cleft Database and the Danish Twin Registry. Only isolated OCs were included, that is, individuals with OC but with no other major anomalies or recognized syndromes; hence, unless specifically noted, all the results and discussion are for isolated OCs.
Fig 2.
Twin recurrence of isolated oral cleft, twins born from 1936 to 2004. MZ=monozygotic, DZ=dizygotic, UZ=unknown zygosity, CL=Cleft Lip, CLP=Cleft Lip and Palate, CP=Cleft Palate.
Technically, the Danish Facial Cleft Database and the Danish Twin Registry were linked by the personal identification number identifying twin pairs of whom at least one twin was affected with an isolated OC. Using the Civil Registration System offspring of the twins were identified and again linked to the Danish Facial Cleft Database to identify any offspring also affected by an isolated OC.
The recurrence risk for children of the affected twins was estimated by dividing the number of affected offspring by the total number of offspring. The risk was similar to the risk found when using the “singles” method described by Davie [1979] under complete ascertainment. For offspring of the unaffected twin in the discordant twin pairs, the same method was used when computing a pseudo-recurrence risk, even though an OC could technically not recur for an unaffected twin. The relative risks were compared to the prevalence in the background population born in the same time period, by dividing the recurrence risk for offspring by the population prevalence [Christensen et al., 1996b; Mitchell et al., 1996]. Fisher’s exact test was used to compare recurrence risk between affected and unaffected twins and the background population frequency. Stratification for type of OC was not possible for the recurrence risk estimates due to small sample size.
To describe our twin population (N=414 twins), the comparison between the likelihood of the unaffected and affected twins of becoming a parent and of the number of children born was performed using a Poisson regression. For the number of children born, the period of observation in adulthood for each twin was taken into account. We compared the age at first birth using a linear regression. Twin concordance, sex of the twins, zygosity, and OC were included as confounders when relevant. STATA 10.1 was used for all computations and the “cluster” option was used in all regression models to correct for the correlated nature of the twin data.
RESULTS
Table I shows the number of OC affected twins and unaffected co-twins according to phenotype and zygosity.
TABLE I.
Number of twins from twin pairs (N=207) with at least one twin affected by an isolated oral cleft
| Cleft twin population (1936–2004) | ||||
|---|---|---|---|---|
| Phenotype | Monozygotic (%) |
Dizygotic (%) |
Unknown zygosity (%) |
All zygosities |
| Cleft lip | 20 (27) | 39 (53) | 15 (20) | 74 |
| Cleft lip with cleft palate | 12 (12) | 68 (68) | 20 (20) | 100 |
| Cleft palate | 8 (14) | 34 (62) | 13 (24) | 55 |
| No oral cleft | 20 (11) | 131 (71) | 34 (18) | 185 |
| All | 60 (14) | 272 (66) | 82 (20) | 414 |
Of the 207 twin pairs included in the study, 185 twin pairs were discordant for OC and 22 pairs were concordant (Fig 2). Among the discordant twins, 117 (32%) twin individuals had reproduced compared to 6 (14%) twins among the concordant twins (p = 0.05).
Recurrence and relative risk for discordant twins
The population frequency of OC in Denmark from 1956 to 2005 was 1.8 per 1,000 live births. Among the 110 children of the 54 OC twins, two (1.8%) children had OC, corresponding to a significantly increased relative risk (RR = 10; 95% confidence interval (CI) 1.2 to 35) when compared to the frequency in the background population (Table II). Among the 129 children of the 63 unaffected twins, three (2.3%) children were affected, corresponding to a significantly increased relative risk (RR = 13; 95% CI 2.6 to 36) when compared to the background prevalence. Both estimates were in the same order of magnitude as the relative risk (RR = 19; 95% CI 17 to 22) for the recurrence risk in the general population compared to the background prevalence.
TABLE II.
Recurrence and relative risk of isolated oral cleft, Denmark 1956 – 2005
| Designation of Relationship | Number Affected (n) |
Total (N) |
Recurrence Risk (%) [95% confidence interval] |
Relative Risk*, [95% confidence interval] |
|---|---|---|---|---|
| Background population prevalence | 6,194 | 3,394,923 | 0.18 | Reference |
| Offspring of affected parents (background population) |
234 | 6,642 | 3.5 [ 3.1 ; 4.0 ] | 19 [ 17 ; 22 ] |
| Offspring of affected discordant twins |
2 | 110 | 1.8 [ 0.22 ; 6.4 ] | 10 [ 1.2 ; 35 ] |
| Offspring of non-affected discordant twins |
3 | 129 | 2.3 [ 0.48 ; 6.7 ] | 13 [ 2.6 ; 36 ] |
Significant if p < 0.05, in bold
Compared to the risk in the background population born in the same time period
The proportion of monozygotic parents to all offspring of the discordant twins and to the recurrent cases was computed from the numbers displayed in Figure 2. Of all the 117 discordant twins with children, 19 (16%) were monozygotic, but two of the five (40%) twins with children who also had an OC were monozygotic (p = 0.20).
Table III displays the OC recurrence risk and the relative risk for unaffected and affected twins, respectively, stratified for zygosity. The monozygotic affected twin parents had no affected offspring. The highest recurrence risk was seen for offspring of the monozygotic unaffected twins and the relative risk was significantly increased (RR = 42; 95% CI 5.3 to 140) when compared to the background population.
TABLE III.
Twin pairs discordant for isolated oral cleft. Zygosity stratification of recurrence risk and relative risk, Denmark 1956 to 2005
| Twin parents status | Recurrence | Recurrence risk (%) [95% confidence interval] |
Relative risk*, [95% confidence interval] |
||
|---|---|---|---|---|---|
| Zygosity | Oral Cleft | Number Affected (n) |
Total (N) | ||
| Monozygotic | No | 2 | 26 | 7.7 [ 0.95 ; 25 ] | 42 [ 5.3 ; 140 ] |
| Yes | 0 | 16 | 0 | 0 | |
| Dizygotic | No | 1 | 103 | 0.97 [ 0.025 ; 5.3 ] | 5.3 [ 0.17 ; 29 ] |
| Yes | 2 | 90 | 2.2 [ 0.27 ; 7.8 ] | 12 [ 1.7 ; 43 ] | |
Significant if p<0.05, in bold
Compared to the risk in the background population born in the same time period
When the OC recurrence risk for the unaffected monozygotic twins was compared to the OC recurrence risk for the unaffected but dizygotic twins, the relative risk was increased (RR = 7.9; 95% CI 0.75 to 85), although not statistically significant. For the dizygotic twins, the relative risk for the affected twins was increased (RR = 2.3; 95% CI 0.21 to 25) when compared to the unaffected dizygotic twins, but also statistically non-significant.
All of the 414 twins (affected and unaffected) were followed for roughly the same number of reproductive years (i.e. from age 15 to 45 for both male and female, no fathers were older than 45). The unaffected twins had a significantly increased likelihood of having children (odds ratio = 1.3; 95% CI 1.1 to 1.6) when compared to the OC affected twins. When adjusting for possible confounding variables (concordance and sex), the estimate showed the same direction but was no longer statistically significant. Having an OC had no effect on the number of children per parent (incidence rate ratio = 1.2 (95% CI 0.92 to 1.5)). The parental age of twins who reproduced (N=123) followed a Gaussian distribution and the distribution of the time under study was the same whether the twins were affected by OC or not. No difference in age at first birth was seen according to OC status or zygosity, but female twins were 20 (95% CI −0.21 to 40) months younger at birth of the first child compared to the male twins.
DISCUSSION
This national population-based cohort study found that the OC recurrence risk for offspring of twins discordant for OC was similar regardless of whether the twin was affected, i.e. the unaffected twin’s risk for an affected offspring was not significantly different from that of the affected twin. In addition, the recurrence risk for offspring of both the affected and unaffected twin from a twin pair discordant for OC was significantly increased compared to the background population frequency.
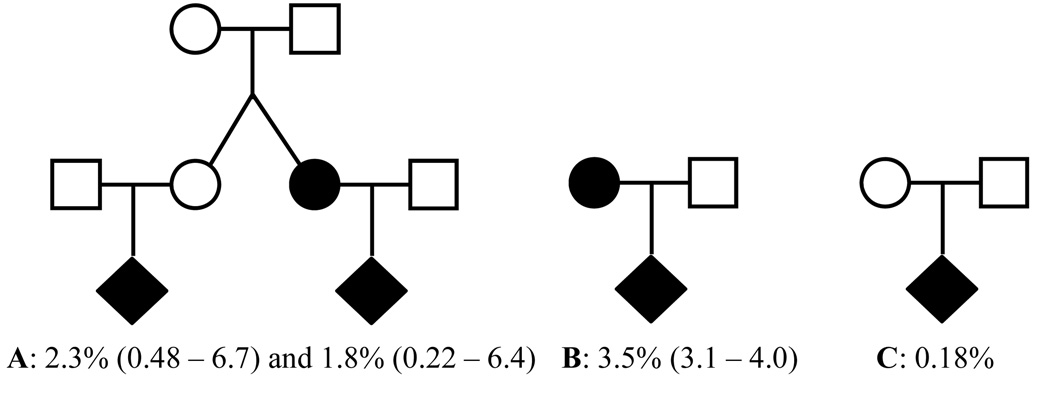
We have previously estimated recurrence risks for OC for more than 50,000 first-, second- and third-degree relatives by use of the same database, but for the 1952 to 2005 cohort [Grosen et al., 2010]. This study adds information on twin recurrence to be used in genetic counseling (Fig 3).
Fig. 3.
Pedigree with recurrence risk and 95% confidence intervals. A: Family with twin girls discordant for oral cleft, B: affected parents (twins and singletons), C: background population.
Monozygotic twins share 100% of their genes as opposed to 50% for dizygotic twins. Since the etiology of OC is mainly genetic, for a monozygotic twin pair discordant for OC, we would expect both twins to be carrying susceptibility genes for OC. The affected twin could have an additional novel mutation acquired after the division of the zygote. That mutation may have pushed this twin over the threshold of developing OC. When reproducing, both twins would pass on susceptibility genes, but since the one twin exceeded the threshold, we expected this twin’s risk to be the highest [Wyszynski et al., 1996; Wyszynski et al., 2002]. We observed the highest risk for offspring of the unaffected twins relative to the background population risk, although the confidence intervals were wide. This indicates that offspring of the unaffected co-twin of a discordant pair have an increased liability to OC and likely a similar liability as offspring of affected twins. Heritability studies suggest that OC has a strong genetic component [Christensen et al., 1993a; Murray, 2002]. The unaffected twins may therefore also have been carrying susceptibility genes for OC like the affected twins were most likely to have done. Another explanation could be that both twins had been exposed to an environmental factor while in the womb, which later increased the risk for their offspring when they reproduced.
In addition, we found similar OC recurrence risks for twin offspring and offspring to affected parents from the background population. It could indicate that the mechanism of clefting is the same whether the parent was a twin or not. A direct comparison of the OC occurrence among twins and singletons would be more suited to answer that question. Previous results have been ambiguous, but the majority found similar OC prevalence for twins and singletons [Christensen et al., 1993a; Christensen et al., 1993b; Christensen et al., 1996a; Mitchell et al., 1997; Nordstrom et al., 1996; Robert et al., 1996].
Our study of recurrence risk among twins is the largest to date owing to the long standing ascertainments in the Danish Facial Cleft Database and the Danish Twin Registry. The study covers a complete country with 70 years of follow up for the twins and 50 years for their offspring. Nonetheless, the study still lacks sufficient power to make valid conclusions when stratifying for zygosity and types of OC. We found that the overall recurrence risk was higher among offspring of the unaffected twins despite inclusion of the dizygotic twins. When we analyzed our results stratified by zygosity, we found that the highest risk was for offspring of the unaffected monozygotic twins and that risk was eight times higher than the risk for offspring of the unaffected dizygotic twins. Along with a tendency towards an increased proportion of monozygotic twin parent to recurrent cases compared to the proportion of monozygotic twin parents to all offspring, it was most likely the recurrence risk for offspring of monozygotic twins that drove the overall recurrence risk estimate for unaffected twins. These results add further evidence for a genetic etiology of oral clefting, but do not rule out yet unmeasured environmental factors. The power issue was critical as the event of clefting and twinning co-occurring in the time period observed was one in 45,000 live births in Denmark. Hence, when stratification was made for zygosity, caution should be taken when interpreting these results. Similar data from other Nordic countries could provide an additional source for obtaining estimates stratified for both zygosity and cleft phenotype.
Since the early work of Fogh-Andersen, the phenotypes CL/P and CP have been considered embryologically and epidemiologically distinct defects [Fogh-Andersen, 1942], and the two phenotypes rarely run in the same families. Accordingly, in the present study, the recurrence was of a similar type (CL/P or CP) except in one family which could represent an undiagnosed case (born in 1946) of van der Woude syndrome where both CL/P and CP occur. If this one family was excluded, the same overall results were obtained: the recurrence risk for offspring of unaffected twins was 1.6% (95% CI 0.19 % to 5.6 %) which did not differ from the recurrence risk among affected twins of 1.8% (p = 1.0), and a significantly increased relative risk (RR = 8.7; 95% CI 1.1 to 31) when compared to the background prevalence.
In the Danish Facial Cleft Database, ascertainment for cohorts born before 1954 depended mainly on surgical files [Christensen et al., 1992]. An individual with OC had to survive until the age of 2 months to be evaluated for surgery and hence be included in the later established Danish Facial Cleft Database. This survival bias was especially an issue for the twins and individuals with a severe OC and/or an associated anomaly/syndrome, who had an even higher neonatal mortality particularly before the availability of neonatal intensive care in the 1960s. Individuals with milder forms of CP that could easily be overlooked or might not need surgery were prone to selection bias [Bille et al., 2005a; Christensen, 1999]. Since 1954, when midwives in Denmark were required to report all types of OC discovered at birth or later in life directly to the National Institute for Defect of Speech, survival bias was reduced to a minimum, and ascertainment has been close to complete[Christensen et al., 1992]. This possible selection bias for the earlier cohorts could have resulted in a slight underestimation of the recurrence risk estimate, but comparison between the twins and the background prevalence should not be affected.
Previous studies have indicated a small effect of paternal age [Bille et al., 2005b] on OC occurrence, and our unpublished results have shown a slightly different reproductive history pattern for individuals affected by OC compared to the reproductive history pattern of the background population. For our twin population, the likelihood of having children was slightly decreased for individuals affected with OC. OC had no influence on parity for those having children or on the age of birth of the first child. All results were as expected from population figures from the complete Danish Facial Cleft population (unpublished results).
In 2002 Kondo et al reported on a pair of monozygotic twins discordant for van der Woude syndrome in which oral clefting is a major manifestation. They sequenced a large section on chromosome 1 that had been identified through genetic linkage studies and found a mutation in the IRF6 gene that is now known to be the cause of van der Woude syndrome. This strategy had been used for another Mendelian disorder [Sakuntabhai et al., 1999] and was subsequently applied to OC. These studies have used monozygotic twins discordant for isolated OC, but have failed to identify differences in genes of importance for oral clefting [Kimani et al., 2009; Mansilla et al., 2005], differences in copy number variations [Kimani et al., 2009; Mansilla et al., 2005] or in X-chromosome inactivation patterns [Kimani et al., 2007]. The major difference between the two disorders is that van der Woude syndrome is a monogenic disease whereas isolated OC is a multifactorial trait. Our results provide an additional explanation as to why this otherwise reasonable twin approach continues to fail. The unaffected twins were carrying a liability for oral clefting, e.g., susceptibility genes for oral clefting. They had, however, not reached the threshold for developing an overt cleft, either by chance or due to low penetrance or variable gene expression as seen for IRF6. Mutations in IRF6 can result in tooth agenesis for some individuals and isolated clefts or syndromic forms of clefts for other individuals [Vieira, 2008]. Likewise, the twins could have had a microform of OC such as a defect in the orbicularis oris muscle and hence not routinely registered. Several studies have shown that microforms of OC are seen more frequently among unaffected relatives, and a large multicenter study is currently exploring this finding in greater detail [Chatzistavrou et al., 2004; Marazita, 2007; Neiswanger et al., 2007; Weinberg et al., 2006; Weinberg et al., 2008a; Weinberg et al., 2008b]. Future studies including the microforms of OC can both increase the study population and diminish the risk of missing a true association between genes and OC occurrence.
In conclusion, this study benefits from the use of reliable Danish registers where selection bias was reduced to a minimum. It included an entire country for 70 years, but still sample size was a limiting factor. The similar increased recurrence risks found among offspring of both cleft-affected and cleft-unaffected discordant twins contribute further support for a genetic component in cleft etiology. Finally, this information is useful in the rare genetic counseling situation of twin pairs discordant for clefting.
ACKNOWLEDGMENTS
Ethical approval: This study was approved by the Danish Data Protection Agency (Case No 92/229 MC).
Funding: University of Southern Denmark, the Danish Graduate School in Public Health, the National Institute of Health, Grants Nos. R01 DE 11948 and DE08559. All contributors are independent of funding sources.
REFERENCES
- Bille C, Knudsen LB, Christensen K. Changing lifestyles and oral clefts occurrence in Denmark. Cleft Palate Craniofac.J. 2005a;42:255–259. doi: 10.1597/03-139.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bille C, Skytthe A, Vach W, Knudsen LB, Andersen AM, Murray JC, Christensen K. Parent's age and the risk of oral clefts. Epidemiology. 2005b;16:311–316. doi: 10.1097/01.ede.0000158745.84019.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bille C, Winther JF, Bautz A, Murray JC, Olsen J, Christensen K. Cancer risk in persons with oral cleft--a population-based study of 8,093 cases. Am J Epidemiol. 2005c;161:1047–1055. doi: 10.1093/aje/kwi132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelykke B, Hauge M, Holm N, Kristoffersen K, Gurtler H. Evaluation of zygosity diagnosis in twin pairs below age seven by means of a mailed questionnaire. Acta Genet Med Gemellol (Roma.) 1989;38:305–313. doi: 10.1017/s0001566000002713. [DOI] [PubMed] [Google Scholar]
- Chatzistavrou E, Ross RB, Tompson BD, Johnston MC. Predisposing factors to formation of cleft lip and palate: inherited craniofacial skeletal morphology. Cleft Palate Craniofac.J. 2004;41:613–621. doi: 10.1597/03-090.1. [DOI] [PubMed] [Google Scholar]
- Christensen K. The 20th century Danish facial cleft population--epidemiological and genetic-epidemiological studies. Cleft Palate Craniofac.J. 1999;36:96–104. doi: 10.1597/1545-1569_1999_036_0096_tcdfcp_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Christensen K, Fogh-Andersen P. Cleft lip (+/− cleft palate) in Danish twins, 1970–1990. Am J Med Genet. 1993a;47:910–916. doi: 10.1002/ajmg.1320470620. [DOI] [PubMed] [Google Scholar]
- Christensen K, Fogh-Andersen P. Isolated cleft palate in Danish multiple births, 1970–1990. Cleft Palate Craniofac.J. 1993b;30:469–474. doi: 10.1597/1545-1569_1993_030_0469_icpidm_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Christensen K, Fogh-Andersen P. Cleft-twin sets in Finland 1948–1987. Cleft Palate Craniofac.J. 1996a;33:530. [PubMed] [Google Scholar]
- Christensen K, Holm NV, Olsen J, Kock K, Fogh-Andersen P. Selection bias in genetic-epidemiological studies of cleft lip and palate. Am J Hum Genet. 1992;51:654–659. [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Mitchell LE. Familial recurrence-pattern analysis of nonsyndromic isolated cleft palate--a Danish Registry study. Am J Hum Genet. 1996b;58:182–190. [PMC free article] [PubMed] [Google Scholar]
- Christiansen L, Frederiksen H, Schousboe K, Skytthe A, von Wurmb-Schwark N, Christensen K, Kyvik K. Age- and sex-differences in the validity of questionnaire-based zygosity in twins. Twin Res. 2003;6:275–278. doi: 10.1375/136905203322296610. [DOI] [PubMed] [Google Scholar]
- Davie AM. The 'singles' method for segregation analysis under incomplete ascertainment. Ann Hum Genet. 1979;42:507–512. doi: 10.1111/j.1469-1809.1979.tb00683.x. [DOI] [PubMed] [Google Scholar]
- Fogh-Andersen P. Inheritance of Harelip and Cleft Palate. Arnold Busck: Nyt Nordisk Forlag; 1942. [Google Scholar]
- Grosen D, Chevrier C, Skytthe A, Bille C, Molsted K, Sivertsen A, Murray JC, Christensen K. A cohort study of recurrence patterns among more than 54,000 relatives of oral cleft cases in Denmark: support for the multifactorial threshold model of inheritance. J Med Genet. 2010;47:162–168. doi: 10.1136/jmg.2009.069385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimani JW, Shi M, ack-Hirsch S, Christensen K, Moretti-Ferreira D, Marazita ML, Field LL, Canady JW, Murray JC. X-chromosome inactivation patterns in monozygotic twins and sib pairs discordant for nonsyndromic cleft lip and/or palate. Am J Med Genet A. 2007;143A:3267–3272. doi: 10.1002/ajmg.a.32098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimani JW, Yoshiura K, Shi M, Jugessur A, Moretti-Ferreira D, Christensen K, Murray JC. Search for Genomic Alterations in Monozygotic Twins Discordant for Cleft Lip and/or Palate. Twin Res Hum Genet. 2009;12:462–468. doi: 10.1375/twin.12.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Schutte BC, Richardson RJ, Bjork BC, Knight AS, Watanabe Y, Howard E, de Lima RL, Daack-Hirsch S, Sander A, Donald-McGinn DM, Zackai EH, Lammer EJ, Aylsworth AS, Ardinger HH, Lidral AC, Pober BR, Moreno L, rcos-Burgos M, Valencia C, Houdayer C, Bahuau M, Moretti-Ferreira D, Richieri-Costa A, Dixon MJ, Murray JC. Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat Genet. 2002;32:285–289. doi: 10.1038/ng985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansilla MA, Kimani J, Mitchell LE, Christensen K, Boomsma DI, ack-Hirsch S, Nepomucena B, Wyszynski DF, Felix TM, Martin NG, Murray JC. Discordant MZ twins with cleft lip and palate: a model for identifying genes in complex traits. Twin Res Hum Genet. 2005;8:39–46. doi: 10.1375/1832427053435373. [DOI] [PubMed] [Google Scholar]
- Marazita ML. Subclinical features in non-syndromic cleft lip with or without cleft palate (CL/P): review of the evidence that subepithelial orbicularis oris muscle defects are part of an expanded phenotype for CL/P. Orthod Craniofac Res. 2007;10:82–87. doi: 10.1111/j.1601-6343.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- Mitchell LE, Christensen K. Analysis of the recurrence patterns for nonsyndromic cleft lip with or without cleft palate in the families of 3,073 Danish probands. Am J Med Genet. 1996;61:371–376. doi: 10.1002/(SICI)1096-8628(19960202)61:4<371::AID-AJMG12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Mitchell LE, Christensen K. Evaluation of family history data for Danish twins with nonsyndromic cleft lip with or without cleft palate. Am J Med Genet. 1997;72:120–121. doi: 10.1002/(sici)1096-8628(19971003)72:1<120::aid-ajmg25>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Murray JC. Gene/environment causes of cleft lip and/or palate. Clin Genet. 2002;61:248–256. doi: 10.1034/j.1399-0004.2002.610402.x. [DOI] [PubMed] [Google Scholar]
- Neiswanger K, Weinberg SM, Rogers CR, Brandon CA, Cooper ME, Bardi KM, Deleyiannis FW, Resick JM, Bowen A, Mooney MP, de Salamanca JE, Gonzalez B, Maher BS, Martin RA, Marazita ML. Orbicularis oris muscle defects as an expanded phenotypic feature in nonsyndromic cleft lip with or without cleft palate. Am J Med Genet A. 2007;143:1143–1149. doi: 10.1002/ajmg.a.31760. [DOI] [PubMed] [Google Scholar]
- Nordstrom RE, Laatikainen T, Juvonen TO, Ranta RE. Cleft-twin sets in Finland 1948–1987. Cleft Palate Craniofac.J. 1996;33:340–347. doi: 10.1597/1545-1569_1996_033_0340_ctsif_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Robert E, Kallen B, Harris J. The epidemiology of orofacial clefts. 1. Some general epidemiological characteristics. J Craniofac Genet Dev Biol. 1996;16:234–241. [PubMed] [Google Scholar]
- Sakuntabhai A, Ruiz-Perez V, Carter S, Jacobsen N, Burge S, Monk S, Smith M, Munro CS, O'Donovan M, Craddock N, Kucherlapati R, Rees JL, Owen M, Lathrop GM, Monaco AP, Strachan T, Hovnanian A. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21:271–277. doi: 10.1038/6784. [DOI] [PubMed] [Google Scholar]
- Skytthe A, Kyvik K, Holm NV, Vaupel JW, Christensen K. The Danish Twin Registry: 127 birth cohorts of twins. Twin Res. 2002;5:352–357. doi: 10.1375/136905202320906084. [DOI] [PubMed] [Google Scholar]
- Vieira AR. Unraveling human cleft lip and palate research. J Dent Res. 2008;87:119–125. doi: 10.1177/154405910808700202. [DOI] [PubMed] [Google Scholar]
- Weinberg SM, Brandon CA, McHenry TH, Neiswanger K, Deleyiannis FW, de Salamanca JE, Castilla EE, Czeizel AE, Vieira AR, Marazita ML. Rethinking isolated cleft palate: evidence of occult lip defects in a subset of cases. Am J Med Genet A. 2008a;146A:1670–1675. doi: 10.1002/ajmg.a.32291. [DOI] [PubMed] [Google Scholar]
- Weinberg SM, Maher BS, Marazita ML. Parental craniofacial morphology in cleft lip with or without cleft palate as determined by cephalometry: a meta-analysis. Orthod Craniofac Res. 2006;9:18–30. doi: 10.1111/j.1601-6343.2006.00339.x. [DOI] [PubMed] [Google Scholar]
- Weinberg SM, Neiswanger K, Richtsmeier JT, Maher BS, Mooney MP, Siegel MI, Marazita ML. Three-dimensional morphometric analysis of craniofacial shape in the unaffected relatives of individuals with nonsyndromic orofacial clefts: a possible marker for genetic susceptibility. Am J Med Genet A. 2008b;146A:409–420. doi: 10.1002/ajmg.a.32177. [DOI] [PubMed] [Google Scholar]
- Wyszynski DF, Beaty TH. Phenotypic discordance in a family with monozygotic twins and nonsyndromic cleft lip and palate: follow-up. Am J Med Genet. 2002;110:182–183. doi: 10.1002/ajmg.10443. [DOI] [PubMed] [Google Scholar]
- Wyszynski DF, Lewanda AF, Beaty TH. Phenotypic discordance in a family with monozygotic twins and non-syndromic cleft lip and palate. Am J Med Genet. 1996;66:468–470. doi: 10.1002/(SICI)1096-8628(19961230)66:4<468::AID-AJMG17>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]